Abstract
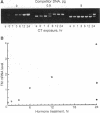
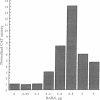
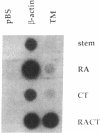
Thrombomodulin (TM) expression was investigated during differentiation of F9 embryonal carcinoma cells into primitive or parietal endoderm. Exposure of F9 cells to retinoic acid (RA) triggers differentiation into primitive endoderm and induces the appearance of barely detectable amounts of TM mRNA, whereas treatment with dibutyryl cAMP plus theophylline (CT) augments the levels of TM mRNA to a 4-fold greater extent than RA. Exposure of F9 cells to RA plus CT initiates differentiation into parietal endoderm and synergistically increases the levels of TM mRNA by 10- to 12-fold compared with CT. The time-dependent establishment of cooperativity between RA and CT appears to be secondary to RA-induced differentiation to primitive endoderm. The above alterations in TM mRNA levels occur by a transcriptional mechanism as judged by nuclear run-on experiments. Transient gene expression experiments show that the human TM promoter is transactivated by coexpression of the human RA receptor beta. Thus, the mechanism of induction of TM expression in F9 cells undergoing differentiation to parietal endoderm appears to be similar, but not identical, to that noted for other late response genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. D., Strewler G. J., Nissenson R. A. Transcriptional activation of Gs alpha expression by retinoic acid and parathyroid hormone-related protein in F9 teratocarcinoma cells. J Biol Chem. 1990 Nov 25;265(33):20081–20084. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Darrow A. L., Rickles R. J., Pecorino L. T., Strickland S. Transcription factor Sp1 is important for retinoic acid-induced expression of the tissue plasminogen activator gene during F9 teratocarcinoma cell differentiation. Mol Cell Biol. 1990 Nov;10(11):5883–5893. doi: 10.1128/mcb.10.11.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman W. A., Kumada T., Sadler J. E., Majerus P. W. The structure and function of mouse thrombomodulin. Phorbol myristate acetate stimulates degradation and synthesis of thrombomodulin without affecting mRNA levels in hemangioma cells. J Biol Chem. 1988 Oct 25;263(30):15815–15822. [PubMed] [Google Scholar]
- Dong J. M., Li F., Chiu J. F. Induction of F9 cell differentiation by transient exposure to retinoic acid. Biochem Biophys Res Commun. 1990 Jul 16;170(1):147–152. doi: 10.1016/0006-291x(90)91252-n. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Galvin-Parton P. A., Watkins D. C., Malbon C. C. Retinoic acid modulation of transmembrane signaling. Analysis in F9 teratocarcinoma cells. J Biol Chem. 1990 Oct 15;265(29):17771–17779. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., Aoki N. Up-regulation of thrombomodulin in human umbilical vein endothelial cells in vitro. J Biochem. 1990 Nov;108(5):839–845. doi: 10.1093/oxfordjournals.jbchem.a123290. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Imada M., Imada S., Iwasaki H., Kume A., Yamaguchi H., Moore E. E. Fetomodulin: marker surface protein of fetal development which is modulatable by cyclic AMP. Dev Biol. 1987 Aug;122(2):483–491. doi: 10.1016/0012-1606(87)90312-5. [DOI] [PubMed] [Google Scholar]
- Imada S., Imada M. Increase of a surface glycoprotein by cyclic AMP in Chinese hamster ovary cells. Dependence on cell-cell interaction. J Biol Chem. 1982 Aug 10;257(15):9108–9113. [PubMed] [Google Scholar]
- Imada S., Yamaguchi H., Imada M. Differential expression of fetomodulin and tissue plasminogen activator to characterize parietal endoderm differentiation of F9 embryonal carcinoma cells. Dev Biol. 1990 Oct;141(2):426–430. doi: 10.1016/0012-1606(90)90397-2. [DOI] [PubMed] [Google Scholar]
- Imada S., Yamaguchi H., Nagumo M., Katayanagi S., Iwasaki H., Imada M. Identification of fetomodulin, a surface marker protein of fetal development, as thrombomodulin by gene cloning and functional assays. Dev Biol. 1990 Jul;140(1):113–122. doi: 10.1016/0012-1606(90)90058-q. [DOI] [PubMed] [Google Scholar]
- Ito T., Ogura M., Morishita Y., Takamatsu J., Maruyama I., Yamamoto S., Ogawa K., Saito H. Enhanced expression of thrombomodulin by intracellular cyclic AMP-increasing agents in two human megakaryoblastic leukemia cell lines. Thromb Res. 1990 Jun 15;58(6):615–624. doi: 10.1016/0049-3848(90)90307-x. [DOI] [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., Fritze L., Soff G., Rosenberg R. D. Human thrombomodulin gene is intron depleted: nucleic acid sequences of the cDNA and gene predict protein structure and suggest sites of regulatory control. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6425–6429. doi: 10.1073/pnas.84.18.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., VanDeWater L., Rosenberg R. D. Characterization of a thrombomodulin cDNA reveals structural similarity to the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8834–8838. doi: 10.1073/pnas.83.23.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy J., Summers W. P., Summers W. C. Post-transcriptional mechanisms of deregulation of MYC following conversion of a human B cell line by Epstein-Barr virus. EMBO J. 1989 Jul;8(7):1973–1980. doi: 10.1002/j.1460-2075.1989.tb03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Differentiation-responsive elements in the 5' region of the mouse tissue plasminogen activator gene confer two-stage regulation by retinoic acid and cyclic AMP in teratocarcinoma cells. Mol Cell Biol. 1989 Apr;9(4):1691–1704. doi: 10.1128/mcb.9.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988 Jan 25;263(3):1563–1569. [PubMed] [Google Scholar]
- Soff G. A., Jackman R. W., Rosenberg R. D. Expression of thrombomodulin by smooth muscle cells in culture: different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood. 1991 Feb 1;77(3):515–518. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Stenflo J., Dahlbäck B., Teodorsson B. Inactivation of human coagulation factor V by activated protein C. J Biol Chem. 1983 Feb 10;258(3):1914–1920. [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G., Mader S., Gold J. D., Leid M., Lutz Y., Gaub M. P., Chambon P., Gudas L. The late retinoic acid induction of laminin B1 gene transcription involves RAR binding to the responsive element. EMBO J. 1991 May;10(5):1149–1158. doi: 10.1002/j.1460-2075.1991.tb08055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Kelly J., Bowen-Pope D. F., Stiles C. D. Retinoic acid promotes transcription of the platelet-derived growth factor alpha-receptor gene. Mol Cell Biol. 1990 Dec;10(12):6781–6784. doi: 10.1128/mcb.10.12.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- de Thé H., Marchio A., Tiollais P., Dejean A. A novel steroid thyroid hormone receptor-related gene inappropriately expressed in human hepatocellular carcinoma. Nature. 1987 Dec 17;330(6149):667–670. doi: 10.1038/330667a0. [DOI] [PubMed] [Google Scholar]