Strains from the United States are more virulent and have greater inflammatory potential.
Keywords: Lyme borreliosis, Borrelia burgdorferi sensu stricto, bacteria, B. afzelii, B. garinii, genotype, RNA intergenic spacer type, outer surface protein C, multilocus sequencing typing, erythema migrans, clinical features, inflammatory potential, Slovenia, Europe, United States
Abstract
Borrelia burgdorferi sensu stricto isolates from patients with erythema migrans in Europe and the United States were compared by genotype, clinical features of infection, and inflammatory potential. Analysis of outer surface protein C and multilocus sequence typing showed that strains from these 2 regions represent distinct genotypes. Clinical features of infection with B. burgdorferi in Slovenia were similar to infection with B. afzelii or B. garinii, the other 2 Borrelia spp. that cause disease in Europe, whereas B. burgdorferi strains from the United States were associated with more severe disease. Moreover, B. burgdorferi strains from the United States induced peripheral blood mononuclear cells to secrete higher levels of cytokines and chemokines associated with innate and Th1-adaptive immune responses, whereas strains from Europe induced greater Th17-associated responses. Thus, strains of the same B. burgdorferi species from Europe and the United States represent distinct clonal lineages that vary in virulence and inflammatory potential.
Incidence of Lyme borreliosis, the most common vectorborne disease in the Northern Hemisphere, is increasing. This disease usually begins with an expanding skin lesion, erythema migrans, which is often accompanied by nonspecific symptoms, such as headache, fatigue, myalgias, and arthralgias. If not treated, this infection can disseminate to the nervous system, heart, or joints (1,2).
Lyme borreliosis is caused primarily by 3 species of the Borrelia burgdorferi sensu lato complex: B. afzelii, B. garinii, and B. burgdorferi sensu stricto (hereafter referred to as B. burgdorferi) (3). Variations in geographic distribution and clinical manifestations of this disease have been noted for each species. In Europe, infection is predominantly with B. afzelii, which usually remains localized to the skin, and B. garinii, which is usually associated with nervous system involvement (1). B. burgdorferi infection is rare in Europe; little is known about its clinical course there. In the United States, B. burgdorferi is the sole agent of Lyme borreliosis; in the northeastern United States, it is particularly arthritogenic (1,2). For all 3 species, the first sign of infection is often an erythema migrans lesion. However, B. burgdorferi infection in the United States is associated with a greater number of disease-associated symptoms and more frequent hematogenous dissemination than B. afzelii or B. garinii infection in Europe (4–7).
Clinical manifestations of Lyme borreliosis are believed to result from host immune response to the spirochete. Erythema migrans lesions of B. burgdorferi–infected patients in the United States have higher levels of mRNA for cytokines and chemokines associated with innate and Th1-adaptive immune responses than lesions from patients in Austria infected with B. afzelii (4). B. burgdorferi–infected patients in the United States also have higher levels of cytokines and chemokines in serum, and isolates from these patients induce macrophages to secrete more interleukin-6 (IL-6), IL-8, IL-10, chemokine ligand 3 (CCL3), CCL4, and tumor necrosis factor (TNF) than B. afzelii or B. garinii from patients in Slovenia (8).
These differences in inflammatory potential between B. burgdorferi from the United States and B. afzelii and B. garinii from Europe might account, in part, for differences in clinical manifestations of Lyme borreliosis. However, little is known about virulence and inflammatory capacity of B. burgdorferi in Europe, or how it compares with B. burgdorferi in the United States. In this study, we compared infection with B. burgdorferi in Europe and the United States by genotype, clinical manifestations, and inflammatory potential.
Methods
Patients and Strains
Twenty-nine B. burgdorferi isolates were cultured from Lyme borreliosis patients in Slovenia (Central Europe) at the Institute of Microbiology and Immunology (Ljubljana, Slovenia). Isolates were identified as B. burgdorferi by using MluI large restriction fragment patterns (9). Twenty-four isolates were obtained from skin (19 from erythema migrans, 4 from acrodermatitis chronica atrophicans, 1 from borrelial lymphocytoma), and 5 from cerebrospinal fluid of patients with Lyme neuroborreliosis. These 29 strains represent all available patient-derived B. burgdorferi isolates collected during a 20-year period (1994–2013) at the Institute of Microbiology and Immunology. Clinical information and demographic data were obtained for same 29 patients at the Lyme Borreliosis Outpatient Clinic at the University Medical Center Ljubljana. The study was approved by Slovenian National Medical Ethics Committee (no.133/06/13).
In a study of patients with Lyme borreliosis in the United States (Rhode Island and Connecticut), 91 B. burgdorferi isolates were cultured from erythema migrans lesions (10). One isolate had a mixed genotype and was not included in our study. All patients met the Centers for Disease Control and Prevention (Atlanta, GA, USA) criteria for erythema migrans. The Human Investigation Committee at Tufts Medical Center and Massachusetts General Hospital approved this study.
Characterization of Strains
We genotyped Borrelia strains by using outer surface protein C (OspC), ribosomal RNA intergenic spacer type (RST), and multilocus sequence typing (MLST). OspC type was determined by using seminested PCR and sequencing. RST was determined by using nested PCR and restriction fragment length polymorphism analysis (11,12). OspC and RST genotypes were determined for all 29 B. burgdorferi isolates from Slovenia and 90 from the United States.
For MLST analysis, we amplified 8 chromosomal housekeeping genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) by using nested PCR, sequenced in both directions (13), and analyzed by using CLC Main Workbench (http://www.clcbio.com/products/clc-main-workbench/). MLST analysis included 29 isolates from Slovenia and a representative subset of 41 isolates comprising the most common B. burgdorferi subtypes from the northeastern United States. Because of the prevalence of OspC type B among isolates from Slovenia, this analysis also included all 11 OspC type B isolates from the United States in our collection. New sequence types were submitted to the MLST database (http://pubmlst.org/borrelia/).
Phylogenetic Analysis
We constructed a minimum spanning tree by using BioNumerics version 7.1 software (Applied Maths, Austin, TX, USA). Comparison of strains from different regions included MLSTs of human B. burgdorferi isolates in the Borrelia MLST database. Phylogenetic trees of concatenated sequences of housekeeping genes were constructed by using MrBayes software (14).
Comparison of Clinical Findings
Demographic and clinical findings were available for 14 of 19 erythema migrans patients with B. burgdorferi infection from Slovenia and 90 erythema migrans patients with B. burgdorferi infection from the northeastern United States. Findings for B. burgdorferi infection we compared findings for 200 patients in Slovenia with B. afzelii infection and 116 with B. garinii infection; all had culture-positive erythema migrans.
Inflammatory Potential of B. burgdorferi Isolates
We assessed the inflammatory capacity of B. burgdorferi strains by stimulating peripheral blood mononuclear cells (PBMC) with 29 B. burgdorferi isolates and determining levels of cytokines and chemokines in culture supernatants. These isolates included 14 B. burgdorferi isolates from erythema migrans lesions of patients from Slovenia for whom detailed clinical information was available and 15 representative B. burgdorferi isolates (5 each of RST1, RST2, and RST3) from patients in the United States. For cell culture experiments, 29 low-passage (<5) isolates were grown to mid-to-late log phase in complete Barbour-Stoenner-Kelly medium (Sigma-Aldrich, St. Louis, MO, USA) (15). Numbers of spirochetes in each culture were determined by optical density using a standard curve (8).
Human PBMC were obtained from 4 healthy donors at the Massachusetts General Hospital Blood-Component Laboratory, and PBMC were isolated from leukopaks by centrifugation in lymphocyte separation medium (MP Biomedicals, Santa Ana, CA, USA). Cells were cultured overnight in RPMI 1640 medium containing 10% human serum in 96-well plates (2 × 105 cells/well) at 37°C in 5% CO2. To keep host factors constant, we stimulated PBMC from each healthy donor with each of 29 patient-derived B. burgdorferi isolates (multiplicity of infection = 25) in 4 independent experiments for 7 days (16).
We assessed protein levels of 22 cytokines and chemokines associated with innate and adaptive immune responses (innate: TNF, IL-1β, IL-6, IL-10, granulocyte–macrophage colony-stimulating factor, IL-8, IFN-α, CCL2, CCL3, and CCL19; adaptive-Th1: IFN-γ, IL-12p40, IL-12p70, cysteine-X-cysteine motif cytokine ligand 9 (CXCL9), and CXCL10; adaptive-Th17: IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, and IL-27) in culture supernatants by using bead-based Luminex (EMD-Millipore, Darmstadt, Germany) multiplex assays. We averaged results from 4 experiments for analysis.
Statistical Analysis
We assessed differences between groups by using the Mann-Whitney rank-sum test and differences for categorical data by using the Fisher exact test (SigmaPlot-12.5; http://www.sigmaplot.com/products/sigmaplot/produpdates/prod-updates18.php0). p values <0.05 were considered significant.
Results
B. burgdorferi Genotypes
To examine genotypic characteristics of B. burgdorferi from Europe and the United States, we assessed 29 patient-derived isolates from Slovenia and 90 isolates from the United States by using the 2 most common typing systems, RST and OspC, which show strong linkage disequilibrium. Of 29 isolates from Slovenia, 21 (72%) were RST1 and 8 (28%) were RST3; none were RST2 (Figure 1; Table 1). Of 90 isolates from the United States, 38 (42%) were RST1, 39 (43%) were RST2, and 13 (14%) were RST3, a distribution consistent with those of other studies (6,10,17,18).
Figure 1.
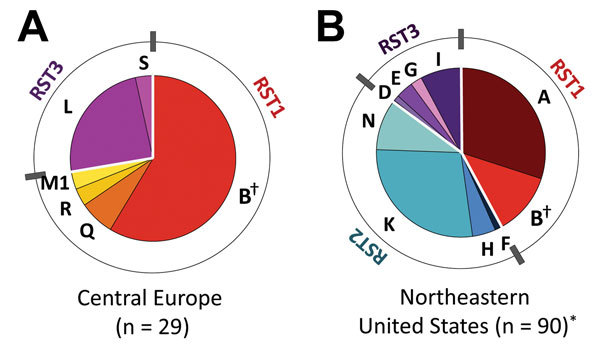
Distribution of Borrelia burgdorferi sensu stricto strains by outer surface protein C (OspC) and ribosomal RNA intergenic spacer type (RST). A) 29 isolates from patients with Lyme borreliosis in cental Europe (Slovenia). B) 90 isolates from patients with erythema migrans in the northeastern United States. OspC types are indicated by letters, and RSTs are indicated by colors. Red, RST1; blue, RST2; purple, RST3. *Based on previously published data (10). †Denotes OspC genotype (OspC type B) found in central Europe and the United States.
Table 1. Characterization of Borrelia burgdorferi sensu stricto strains, by RST and OspC, from patients in Slovenia and the United States*.
| Genotype | Slovenia, n = 29, no. (%) | United States, n = 90†, no. (%) |
|---|---|---|
| RST1 | 21 (72) | 38 (42) |
| OspC type A | 0 | 27 (30) |
| OspC type B‡ | 17 (58) | 11 (12) |
| OspC type Q | 2 (7) | 0 |
| OspC type R | 1 (3) | 0 |
| OspC type M1 | 1 (3) | 0 |
| RST2 | 0 | 39 (43) |
| OspC type F | 0 | 1 (1) |
| OspC type H | 0 | 4 (4) |
| OspC type K | 0 | 25 (28) |
| OspC type N | 0 | 9 (10) |
| RST3 | 8 (28) | 13 (14) |
| OspC type D | 0 | 1 (1) |
| OspC type E | 0 | 3 (3) |
| OspC type G | 0 | 2 (2) |
| OspC type I | 0 | 7 (8) |
| OspC type L | 7 (24) | 0 |
| OspC type S | 1 (3) | 0 |
*RST, ribosomal RNA intergenic sequence type; OspC, outer surface protein C. †Based on previously published data (10). ‡Found in central Europe and the United States.
OspC typing showed that OspC type B (RST1) was the only OspC type found among isolates from Europe and the United States. Other OspC types were found exclusively in Slovenia (Q, R, L, S) or the United States (A, F, K, N, D, E, G, I). The most common B. burgdorferi strains in Slovenia were RST1-OspC type B (58%) and RST3-OspC type L (24%), whereas the most common strains in the northeastern United States were RST1-OspC type A (30%) and RST2-OspC type K (28%).
MLST analysis (Table 2) included 29 isolates from Slovenia and 41 isolates from the United States, which comprised the major OspC types (A, K, and I) in the northeastern United States and all 11 OspC type B strains in our collection. Analysis identified 15 sequence types (STs). There was no overlap in STs from Europe and the United States, which demonstrated that strains of the same Borrelia species, including OspC type B strains, from the 2 regions were distinct genotypes.
Table 2. Characterization of Borrelia burgdorferi sensu stricto strains, by MLST genotyping, from patients in Slovenia and United States*.
| Genotype | No. (%) patients |
|
|---|---|---|
| Slovenia, n = 29 | United States, n = 41† | |
| RST1 | 21 | 21 |
| OspC type A | 0 | 10 |
| MLST ST1 | 0 | 10 (24) |
| OspC type B | 17 | 11 |
| MLST ST20 | 6 (20) | 0 |
| MLST ST314 | 6 (20) | 0 |
| MLST ST545‡ | 5 (17) | 0 |
| MLST ST59 | 0 | 10 (24) |
| MLST ST7 | 0 | 1 (2) |
| OspC type Q | 2 | 0 |
| MLST ST20 | 1 (3) | 0 |
| MLST ST546‡ | 1 (3) | 0 |
| OspC type R | 1 | 0 |
| MLST ST20 | 1 (3) | 0 |
| OspC type M1 | 1 | 0 |
| MLST ST20 |
1 (3) |
0 |
| RST2 | 0 | 10 |
| OspC type F | 0 | 1 |
| MLST ST8 | 0 | 1 (2) |
| OspC type K | 0 | 7 |
| MLST ST3 | 0 | 6 (15) |
| MLST ST9 | 0 | 1 (2) |
| OspC type N | 0 | 2 |
| MLST ST9 |
0 |
2 (5) |
| RST3 | 8 | 10 |
| OspC type D | 0 | 1 |
| MLST ST38 | 0 | 1 (2) |
| OspC type E | 0 | 2 |
| MLST ST19 | 0 | 2 (5) |
| OspC type G | 0 | 2 |
| MLST ST14 | 0 | 2 (5) |
| OspC type I | 0 | 5 |
| MLST ST16 | 0 | 5 (12) |
| OspC type L | 7 | 0 |
| MLST ST24 | 7 (24) | 0 |
| OspC type S | 1 | 0 |
| MLST ST24 | 1 (3) | 0 |
*MLST, multilocus sequence typing; RST, ribosomal RNA intergenic sequence type; OspC, outer surface protein C; ST, sequence type. †The 41 isolates from the United States were selected from a larger cohort that were representative of the most common RST/OspC subtypes in the northeastern United States. These isolates included all available OspC type B strains in our collection. ‡New ST.
Isolates from Slovenia represented 3 previously described STs and 2 new STs (ST545 and ST546) not in the MLST database. Strains from the United States represented a more heterogeneous group of 10 STs, all of which were reported previously in the MLST database. Three STs (ST9, ST20, and ST24) each comprised >1 OspC type. This result might be explained by greater propensity for horizontal transfer of genetic information at ospC gene loci, which are apparent recombination hotspots (19), whereas the 8 loci used in MLST analysis have lower rates of genetic recombination.
Minimum spanning tree and phylogenetic analyses of 70 B. burgdorferi strains underscored differences between strains in the 2 regions (Figure 2). Strains from Europe formed 2 clonal complexes (CC20 and CC24), which were separated by 3 and 6 alleles, respectively, from most the closely related strains in the United States.
Figure 2.
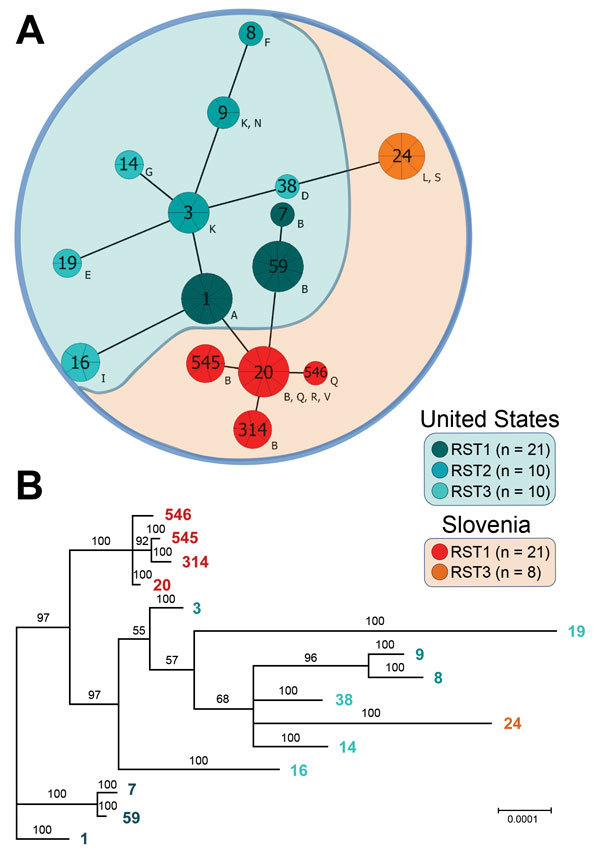
Phylogenetic analysis of Borrelia burgdorferi sensu stricto strains from central Europe (Slovenia) and the United States. A) Minimum spanning tree analysis of 70 isolates included in this study. Sequence types (STs) are indicated by numbers, and outer surface protein types are indicated by letters. Sizes of circles indicate ST sample sizes. Lengths of lines connecting STs indicate extent of variation (order of certainty) (no. locus variants). STs connected by the shortest black line are single-locus variants. Letters outside circles indicate OspC types. RST, ribosomal RNA intergenic spacer. B) Bayesian consensus tree resulting from simultaneous analysis of concatenated sequences of 8 housekeeping genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA). Values at nodes indicate Bayesian posterior probabilities (proportion of sampled trees containing the taxon bipartition). Scale bar indicates nucleotide substitutions per site.
Comparison of B. burgdorferi with B. afzelii or B. garinii (Figure 3) showed deeper branching among different Borrelia species but shallower branching within the same species, which demonstrated greater genetic discordance among species. Nevertheless, genotypic differences between B. burgdorferi in Europe and the United States, and closer clustering of strains within each geographic region, suggest divergence of B. burgdorferi on the 2 continents.
Figure 3.
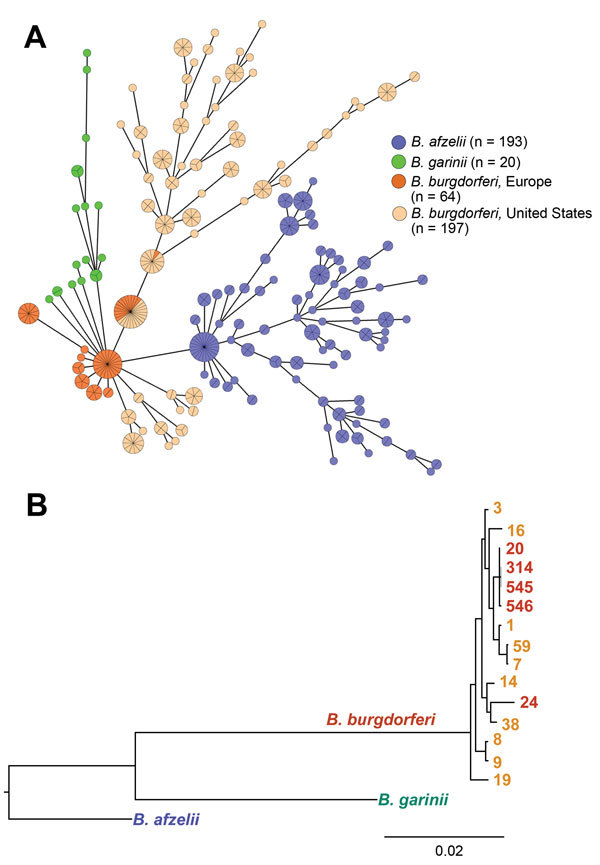
Phylogenetic comparison of 3 major pathogenic Borrelia species (Borrelia afzelii, B. garinii, and B. burgdorferi sensu stricto) that cause Lyme borreliosis. A) Minimum spanning tree analysis of 474 B. burgdorferi sensu lato human isolates. Analysis included 404 previously published datasets available in the multilocus sequence typing database (http://pubmlst.org/borrelia/) as of May 5, 2015, and 70 B. burgdorferi sensu stricto isolates from this study. Circles and numbers indicate specific sequence types (STs). Sizes of circles indicate MLST sample size and colors indicate origin of isolates. Lengths of lines connecting STs indicate order of certainty. STs connected by the shortest line are single locus-variants. B) Bayesian consensus tree resulting from simultaneous analysis of concatenated sequences of housekeeping genes of 70 B. burgdorferi sensu stricto isolates included in this study, representative strains of B. afzelii (http://pubmlst.org/borrelia/id:1546), and B. garinii (http://pubmlst.org/ borrelia/id:1829). Values at nodes indicate Bayesian posterior probabilities (proportion of sampled trees containing the taxon bipartition). Scale bar indicates nucleotide substitutions per site.
Clinical Findings for Patients with Erythema Migrans
We compared 14 B. burgdorferi–infected patients in Slovenia for whom detailed clinical information was available with 90 patients in the United States and found that patients from the United States had significantly shorter duration of erythema migrans at diagnosis (4 vs. 7 days; p = 0.02), greater frequency of associated symptoms (78% vs. 29%; p<0.001), and greater number of associated symptoms (4 vs. 0 symptoms; p = 0.005) (Table 3). B. burgdorferi from the United States differed from B. afzelii and B. garinii for most clinical parameters measured. In contrast, clinical features of B. burgdorferi infection in Slovenia were similar to those for B. afzelii or B. garinii infections (Table 3), despite substantial genotypic differences among species (Figure 3).
Table 3. Clinical characteristics of erythema migrans patients infected with Borrelia afzelii, B. garinii, or B. burgdorferi sensu stricto in Slovenia and B. burgdorferi sensu stricto in the United States*.
| Characteristic |
Slovenia | United States,
B. burgdorferi sensu stricto, n = 90† |
p value‡ |
| B. afzelii, n = 200† | B. garinii, n = 116† | B. burgdorferi sensu stricto, n = 14 | |||
|---|---|---|---|---|---|
| General | |||||
| Age, y | 50 (15–79) | 54 (20–83) | 50 (3–77) | 49 (15–82) | 0.6 |
| Female sex | 128 (64) | 61 (53) | 10 (71) | 44 (49) | 0.2 |
| EM duration at study entry, d | 9 (1–206) | 5 (1–61) | 7 (2–53) | 4 (1–30) | 0.02 |
| EM diameter, cm |
13 (5–86) |
18 (5–72) |
12 (5–38) |
11 (5–56) |
0.6 |
| Symptoms at study entry | |||||
| Symptoms/patient | 0 (0–6) | 0 (0–7) | 0 (0–6) | 4 (0–9) | 0.005 |
| Patients with symptoms | 66 (33) | 43 (37) | 4 (29) | 70 (78) | <0.001 |
| Fatigue | 51 (26) | 23 (20) | 3 (21) | 55 (61) | 0.01 |
| Arthralgia | 30 (15) | 21 (18) | 3 (21) | 42 (47) | 0.1 |
| Myalgia | 28 (14) | 22 (19) | 3 (21) | 28 (32) | 0.7 |
| Headache | 27 (14) | 21 (18) | 4 (29) | 45 (50) | 0.2 |
| Fever, chills | 12 (6) | 10 (9) | 2 (14) | 41 (46) | 0.05 |
| Neck stiffness | 9 (5) | 4 (3) | 0 | 43 (48) | 0.002 |
| Malaise | 21 (11) | 21 (18) | 2 (14) | 47 (52) | 0.01 |
*Values are median (range) or no. (%). EM, erythema migrans. †Based on previously published data: B. burgdorferi sensu stricto (10); B. afzelii (5); B. garinii (20). ‡For comparison of B. burgdorferi sensu stricto from Slovenia versus the United States. Values in bold indicate statistical significance.
Differences between Borrelia species from Europe and the United States were most apparent for symptomology of infection (Figure 4). In the United States, erythema migrans is typically associated with fever, neck stiffness, malaise, and fatigue. However, these symptoms were substantially less common in Slovenia. Thus, despite greater phylogenetic similarity with B. burgdorferi from the United States, infection with B. burgdorferi strains from Europe reflected more closely findings for 2 genetically discordant Borrelia species in Europe (B. afzelii and B. garinii) with which it shares an ecologic niche. In contrast, B. burgdorferi from the United States appeared to be more virulent.
Figure 4.
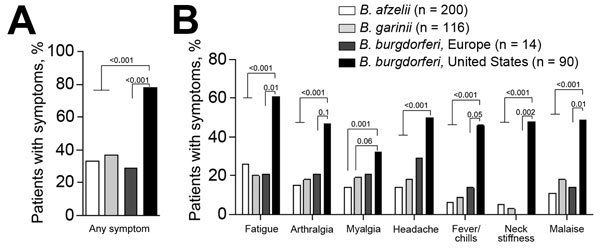
Frequency of symptoms in patients with erythema migrans infected with Borrelia afzelii, B. garinii, or B. burgdorferi sensu stricto from central Europe and B. burgdorferi sensu stricto from the United States. A) Any symptom, B) individual symptoms. Patients were assessed for 8 symptoms (fatigue, arthralgia, myalgia, headache, fever, chills, neck stiffness, or malaise). White bars indicate patients from Europe infected with B. afzelii, light grays bars indicate patients from Europe infected with B. garinii, dark gray bars indicate patients from Europe infected with B. burgdorferi sensu stricto, and black bars indicate patients from the United States infected with B. burgdorferi sensu stricto. Differences between strains were assessed by using the Fisher exact test. p values are indicated. There were no differences between B. afzelii, B. garinii, or B. burgdorferi sensu stricto from Slovenia.
Inflammatory Responses in PBMC Stimulated with B. burgdorferi
To determine whether B. burgdorferi from the United States and Europe vary in inflammatory potential, we assessed levels of 22 cytokines and chemokines in supernatants of healthy human PBMC stimulated with 14 B. burgdorferi isolates from patients in Slovenia and 15 representative isolates from patients in the United States (5 each RST1, RST2, and RST3), for whom detailed clinical information was available. All 29 isolates were tested by using PBMC from each of 4 healthy donors, and results from 4 experiments were averaged for analysis.
Isolates from the United States and Europe induced greater expression of most cytokines and chemokines tested compared with unstimulated controls (Figure 5). However, B. burgdorferi from the United States induced higher levels of several mediators associated with innate immune responses, including IL-1β, IL-8, IL-10, TNF, and CCL3, than did B. burgdorferi from Europe (Figure 5, panel A). A similar trend was observed for Th1-associated mediators (IL-12p40, INF-γ, INF-γ–inducible CXCL9, and CXCL10), which are strong chemoattractants for CD4+/CD8+ T-effector cells (Figure 5, panel B). In contrast, levels of several Th17 mediators, including IL-17A, IL-22, and IL-27, were higher in cells stimulated with B. burgdorferi from Europe. Because immune response seems to be critical in disease expression, these differences in inflammatory responses might contribute to differences in clinical features of Lyme borreliosis in Europe and the United States.
Figure 5.
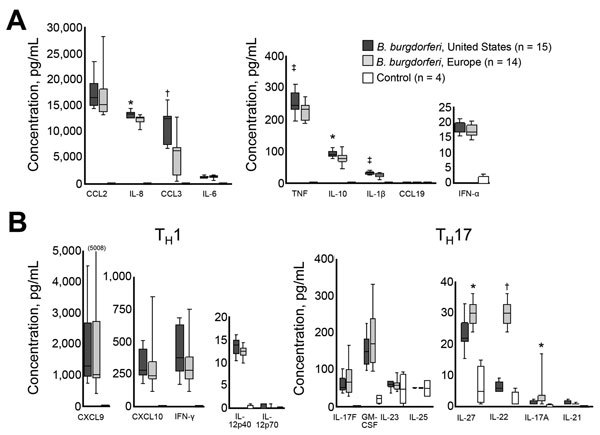
Inflammatory potential of Borrelia burgdorferi sensu stricto from Europe and the United States. Levels of 22 cytokines and chemokines associated with innate (A) or adaptive (B) immune responses. Innate responses: tumor necrosis factor (TNF), interleukin-1β (IL-1β), IL-6, IL-10, granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-8, chemokine ligand 2 (CCL2), CCL3, and CCL19. Adaptive immune responses: Th1: interferon-γ (IFN-γ), IFN-α, IL-12p40, IL-12p70, cysteine-X-cysteine motif chemokine ligand 9 (CXCL9), and CXCL10; Th17: IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, and IL-27. Immune responses were assessed in peripheral blood mononuclear cell culture supernatants after 7 days of stimulation with 14 B. burgdorferi sensu stricto isolates from Europe or 15 B. burgdorferi sensu stricto isolates from the United States (multiplicity of infection = 25) by using bead-based Luminex (EMD-Millipore, Darmstadt, Germany) multiplex assays. Each of 29 B. burgdorferi sensu stricto isolates was tested with peripheral blood mononuclear cells from each of 4 healthy donors in 4 independent experiments. Cytokine and chemokine values from the 4 experiments were averaged for analysis. Data are presented as box and whisker plots, boxes indicate interquartile ranges (1st and 3rd quartiles), lines inside boxes indicate median values, and error bars indicate 10th and 90th percentiles (value in parenthesis indicates the highest value). For comparison of B. burgdorferi sensu stricto isolates from Slovenia or the United States, *p<0.01, †p<0.001, ‡p<0.05.
Discussion
We compared infection with B. burgdorferi in Europe and the United States by genotype, clinical manifestations, and inflammatory potential. Strains in Europe differed from strains in the United States for all 3 parameters, which demonstrates transcontinental diversification of this species.
Although B. burgdorferi from Slovenia and the United States are depicted as the same species, they represent distinct clonal complexes that vary in capacity to induce host inflammatory immune responses and clinical features of disease. Clinical and immune characteristics of B. burgdorferi from Europe more closely resemble those of the phylogenetically distinct species B. afzelii and B. garinii from Europe, with which they share an ecological niche, than those of B. burgdorferi from the northeastern United States. These findings underscore divergence of B. burgdorferi strains on 2 continents. Moreover, data indicate a convergence of certain features among disparate Borrelia species within the same region, presumably through sharing of genetic information (21).
Three RST and >30 OspC genotypes have been identified in B. burgdorferi obtained from various sources (11,12), including 24 OspC subtypes that cause infection in humans (22). On the basis of 3 studies that evaluated genotypes of B. burgdorferi isolates that were obtained primarily from ticks from Europe or the United States (13,22,23), most OspC types were believed to be present exclusively in North America or Europe, whereas a few OspC types (A, B, K, E, L) were present in both continents (22–24).
Our study of B. burgdorferi from patients in Slovenia or the United States partially corroborates these findings and demonstrates that strains from these 2 regions are genetically distinct. However, of 15 OspC types identified, OspC type B was the only genotype found among isolates from both Slovenia and the United States. The high frequency of OspC type B (58%) in patients from Slovenia and absence of OspC types A, K, or E, which were reported in several countries in Europe (13,22,23), suggests that there might be regional variation in distribution of B. burgdorferi in different locations in Europe. Moreover, although not found in the northeastern United States, the high prevalence of OspC type L among patients in Slovenia and its recovery from patients in the midwestern United States (24) indicate that this strain also causes human disease.
Analysis of human of B. burgdorferi isolates by MLST confirmed that strains on the 2 continents represent different clonal complexes, which is consistent with previous findings in isolates from ticks (13,25). STs of the 29 isolates from Slovenia differed from STs of the 157 isolates from North America in the MLST database, which implies transcontinental diversification of B. burgdorferi. Moreover, Phylogenetic and minimum spanning tree analyses showed that isolates from Slovenia clustered in 2 distinct groups (ST20 and ST24), which suggests that strains in these groups evolved independently.
Although speculative, the data suggest that there might have been 2 independent divergence events for B. burgdorferi in Europe and the United States. One event involved migration of ST20, which is most closely related to RST1-OspC type A and B strains that are prevalent in the northeastern United States. A separate event involved ST24, which is closely related to OspC type L strains that were found in patients from the midwestern United States, but not in patients from the northeastern United States. However, these insights are based on a small sample size of isolates, all from humans. Genome analyses of larger numbers of isolates from various sources could provide better resolution of the geographic spread of B. burgdorferi (19).
Species determination is based on sequence homology of 16S rRNA, a highly conserved chromosomal region of the Borrelia genome that undergoes slow evolutionary change. However, ≈40% of borrelial DNA is located on plasmids, including highly polymorphic genes, such as ospC (26), which encode immunogenic proteins involved in spirochetal virulence. These genes are believed to be under considerable evolutionary pressure, and because of plasmid plasticity, there is evidence for gene recombination and lateral transfer of genetic information among strains (21). Thus, despite comprising the same species on the basis of 16S rRNA, B. burgdorferi from Europe and the United States appear divergent in expression of genes, such as ospC, that are responsible for immunogenicity and virulence. Consequently, strains defined as the same species might cause a spectrum of disease with different clinical features. Thus, species determination on the basis of genetically conserved regions of the genome might not adequately reflect the difference in virulence.
Researchers have reported differences in clinical features of infection with B. garinii and B. afzelii in Europe and B. burgdorferi in the northeastern United States (4,5,20,27–30). We extended these findings by showing differences in clinical features of erythema migrans caused by B. burgdorferi in Slovenia compared with B. burgdorferi in the United States. Despite substantial phylogenetic discordance among species, clinical features of infection with B. burgdorferi in Slovenia more closely resembled those of milder infections with B. afzelii and B. garinii, the 2 other Borrelia species that cause disease in Europe, than the more symptomatic infection associated with more closely phylogenetically related B. burgdorferi from the United States. These findings suggests sharing of genetic information among different Borrelia species.
Although we cannot exclude the possibility that host genetic or cultural differences might contribute to differences in clinical features of Lyme borreliosis in Slovenia and the United States, we do not believe that these differences are major factors. First, all study patients at both sites were of European descent and probably similar genetically. Second, evaluation of patients in both locations was similar and included assessment of objective measures, such as fever and erythema migrans diameter and duration, which would not be influenced by cultural differences in reporting symptoms. Third, median duration of erythema migrans in patients in the United States likely was shorter because these patients had more associated symptoms and sought treatment sooner than patients in Slovenia. In support of this interpretation, patients in Europe with B. garinii infection, which causes more pronounced itching and burning of erythema migrans lesions than other Borrelia species (29), had a similar duration of erythema migrans at study entry as patients in the United States. Thus, we believe that differences among Borrelia strains are the critical factor in explaining differences in clinical features of Lyme borreliosis on the 2 continents.
Our analysis in this study focused on clinical features of erythema migrans. However, distinctions in disease pathogenesis between B. burgdorferi strains from Slovenia and the United States are probably not limited to early disease. Of 29 B. burgdorferi isolates from Slovenia, 1 was obtained from a patient with borrelial lymphocytoma, and 4 were obtained from patients with acrodermatitis chronica atrophicans, a late disease manifestation. These clinical manifestations are rarely, if ever, seen in the northeastern United States, where late in the disease, B. burgdorferi is commonly associated with development of arthritis, which occurs rarely in Europe. These observations are consistent with a recent report suggesting higher frequency of Lyme neuroborreliosis after B. burgdorferi infection in Europe than in the United States (25). Thus, clinical differences between B. burgdorferi in Europe and the United States probably involve manifestations other than those associated with early infection.
Although there is a range of inflammatory potential for a given Borrelia RST strain (31), we previously showed in vivo and in vitro that B. burgdorferi from the northeastern United States induced greater inflammatory responses than B. afzelii and B. garinii from Europe (4,8). In this study, we demonstrated in cell culture that B. burgdorferi from Europe and the United States vary in inflammatory potential. Strains from the United States induced higher levels of cytokines and chemokines associated with innate immune responses and showed a similar trend for Th1-associated mediators. In contrast, strains from Europe induced higher levels of several Th17-associated cytokines.
The functional consequence of these differential immune responses is not known. Th17-associated mediators are detected in only a subset of patients with Lyme borreliosis and might not be as effective in spirochetal killing as innate or Th1-adaptive responses (32). However, because Lyme borreliosis patients are given antibiotic drugs, it is not known whether the natural history of the disease would be different in patients with predominantly Th1 or Th17 responses to the spirochete.
In addition to divergence of B. burgdorferi between Europe and the United States, emerging evidence suggests regional strain variation on each continent. A study of B. burgdorferi from humans in the midwestern and northeastern United States reported differences among isolates at these locations (24). In the northeastern United States, OspC types A (≈30%) and K (≈30%) are most common, whereas there is greater diversity in the midwestern United States, and OspC type H (≈20%) is most common. Although not assessed systematically, we believe that Lyme borreliosis is a generally milder disease in the midwestern United States. Similarly, the predominance of OspC type B and L strains in human isolates from Slovenia and absence of OspC type A and K strains found in other regions of Europe suggests region-specific diversification of strains. It will be useful to determine whether there are regional variations in Lyme borreliosis in Europe and Asia, where B. afzelii and B. garinii predominate. Greater knowledge of regional differences in infection might help clinicians in diagnosis and treatment specific for their region.
In conclusion, B. burgdorferi from Europe and the United States represent distinct genotypes that vary in inflammatory potential and clinical manifestations of Lyme borreliosis. Despite greater genetic discordance Borrelia species, clinical features of B. burgdorferi infection in Europe appear similar to those for B. afzelii or B. garinii infection, the most prevalent Borrelia species in Europe, indicating that strains within a particular regional environment, under similar evolutionary pressures, accrue similar characteristics as other strains that share the same ecologic niche.
Acknowledgments
K.S., F.S., and A.C.S. designed the study; T.C. and K.S. performed experiments and analyzed data; F.S., D.S., and A.C.S provided patient samples and clinical information; E.R.-S., A.C.S., and G.M. provided Borrelia isolates; E.R.-S., and G.M. provided assistance with culture and evaluation of isolates; and K.S., F.S., A.C.S., and T.C. wrote the article. All authors participated in developing, reviewing, and approving the article.
This study was supported by grants from the Slovenian Research Agency (P3-0296 and J3-3636 to F.S.), the National Institutes of Health (K01-AR-062098 to K.S. and RO1 AI-101175), the Centers for Disease Control and Prevention (CCU110 291), and the Eshe Fund (to A.C.S.).
Biography
Dr. Cerar is a postdoctoral researcher in the Laboratory for Diagnostics of Borreliosis and Leptospirosis and the Laboratory for Molecular Bacteriology and Mycology, Faculty of Medicine, University of Ljubljana. Ljubljana, Slovenia. Her primary research interest is clinical microbiology with a special focus on Lyme borreliosis.
Footnotes
Suggested citation for this article: Cerar T, Strle F, Stupica D, Ruzic-Sabljic E, McHugh G, Steere AC, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis. 2016 May [date cited]. http://dx.doi.org/10.3201/eid2205.151806
These authors contributed equally to this article.
References
- 1.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- 2.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. 10.1056/NEJM200107123450207 [DOI] [PubMed] [Google Scholar]
- 3.Wang G, van Dam AP, Schwartz I, Dankert J. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin Microbiol Rev. 1999;12:633–53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, et al. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92 . 10.1086/524022 [DOI] [PubMed] [Google Scholar]
- 5.Strle F, Nadelman RB, Cimperman J, Nowakowski J, Picken RN, Schwartz I, et al. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York State and by Borrelia afzelii in Slovenia. Ann Intern Med. 1999;130:32–6 . 10.7326/0003-4819-130-1-199901050-00006 [DOI] [PubMed] [Google Scholar]
- 6.Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–5. 10.7326/0003-4819-142-9-200505030-00011 [DOI] [PubMed] [Google Scholar]
- 7.Strle F, Ruzic-Sabljic E, Logar M, Maraspin V, Lotric-Furlan S, Cimperman J, et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis. 2011;11:1253–8. 10.1089/vbz.2010.0230 [DOI] [PubMed] [Google Scholar]
- 8.Strle K, Drouin EE, Shen S, El Khoury J, McHugh G, Ruzic-Sabljic E, et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis. 2009;200:1936–43. 10.1086/648091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfaiza J, Postic D, Bellenger E, Baranton G, Girons IS. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–13. 10.1128/JCM.01077-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, et al. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margos G, Gatewood AG, Aanensen DM, Hanincova K, Terekhova D, Vollmer SA, et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2008;105:8730–5. 10.1073/pnas.0800323105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–5 . [PMC free article] [PubMed] [Google Scholar]
- 16.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–39. 10.1016/j.ajpath.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. 10.1086/592279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wormser GP, Liveris D, Nowakowski J, Nadelman RB, Cavaliere LF, McKenna D, et al. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J Infect Dis. 1999;180:720–5. 10.1086/314922 [DOI] [PubMed] [Google Scholar]
- 19.Seifert SN, Khatchikian CE, Zhou W, Brisson D. Evolution and population genomics of the Lyme borreliosis pathogen, Borrelia burgdorferi. Trends Genet. 2015;31:201–7. 10.1016/j.tig.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennet L, Fraenkel CJ, Garpmo U, Halling A, Ingman M, Ornstein K, et al. Clinical appearance of erythema migrans caused by Borrelia afzelii and Borrelia garinii: effect of the patient’s sex. Wien Klin Wochenschr. 2006;118:531–7. 10.1007/s00508-006-0659-1 [DOI] [PubMed] [Google Scholar]
- 21.Barbour AG, Travinsky B. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. MBio. 2010;1:e00153–10. 10.1128/mBio.00153-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudenko N, Golovchenko M, Honig V, Mallatova N, Krbkova L, Mikulasek P, et al. Detection of Borrelia burgdorferi sensu stricto ospC alleles associated with human Lyme borreliosis worldwide in non-human-biting tick Ixodes affinis and rodent hosts in southeastern United States. Appl Environ Microbiol. 2013;79:1444–53. 10.1128/AEM.02749-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu WG, Bruno JF, McCaig WD, Xu Y, Livey I, Schriefer ME, et al. Wide distribution of a high-virulence Borrelia burgdorferi clone in Europe and North America. Emerg Infect Dis. 2008;14:1097–104. 10.3201/eid/1407.070880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanincova K, Mukherjee P, Ogden NH, Margos G, Wormser GP, Reed KD, et al. Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS ONE. 2013;8:e73066 . 10.1371/journal.pone.0073066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungnick S, Margos G, Rieger M, Dzaferovic E, Bent SJ, Overzier E, et al. Borrelia burgdorferi sensu stricto and Borrelia afzelii: population structure and differential pathogenicity. Int J Med Microbiol. 2015;305:673–81. 10.1016/j.ijmm.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 26.Sadziene A, Wilske B, Ferdows MS, Barbour AG. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsson SA, Granlund H, Jansson C, Nyman D, Wahlberg P. Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis. 2003;35:31–3. 10.1080/0036554021000026978 [DOI] [PubMed] [Google Scholar]
- 28.Logar M, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J, Jurca T, et al. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–9. 10.1007/s15010-004-3042-z [DOI] [PubMed] [Google Scholar]
- 29.Strle F, Ruzic-Sabljic E, Logar M, Maraspin V, Lotric-Furlan S, Cimperman J, et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis. 2011;11:1253–8. 10.1089/vbz.2010.0230 [DOI] [PubMed] [Google Scholar]
- 30.Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis. 2006;43:704–10. 10.1086/506936 [DOI] [PubMed] [Google Scholar]
- 31.Mason LM, Herkes EA, Krupna-Gaylord MA, Oei A, Van der Poll T, Wormser GP, et al. Borrelia burgdorferi clinical isolates induce human innate immune responses that are not dependent on genotype. Immunobiology. 2015;220:1141–50. 10.1016/j.imbio.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 32.Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post-Lyme disease symptoms following erythema migrans. Clin Infect Dis. 2014;58:372–80. 10.1093/cid/cit735 [DOI] [PMC free article] [PubMed] [Google Scholar]


