Abstract
Bipolar disorder (BD) is associated with increased reactivity to rewards and heightened positive affectivity. It is less clear to what extent this heightened reward sensitivity is evident across contexts and what the associated neural mechanisms might be. The present investigation employed both a monetary and social incentive delay task among adults with remitted BD type I (N=24) and a healthy non-psychiatric control group (HC; N=25) using fMRI. Both whole-brain and region-of-interest analyses revealed elevated ventral and dorsal striatal reactivity across monetary and social reward receipt, but not anticipation, in the BD group. Post-hoc analyses further suggested that greater striatal reactivity to reward receipt across monetary and social reward tasks predicted decreased self-reported positive affect when anticipating subsequent rewards in the HC, but not BD, group. Results point toward elevated striatal reactivity to reward receipt as a potential neural mechanism of reward reactivity.
Keywords: Reward, Bipolar Disorder, Social, Monetary, Ventral Striatum
Recent empirical models suggest that abnormalities in reward sensitivity have important clinical health consequences, including increased risk-taking, substance use, and higher rates of onset of mood disorders (e.g., Alloy et al., 2012a; Gruber, Mauss & Tamir, 2011; Gruber & Purcell, 2015; Richardson, Freedlander, Katz, Dai, & Chen, 2014; Sugam & Carelli, 2013). Such possible negative consequences underscore the need to broaden our understanding of reward sensitivity, including the context in which it occurs and its neural correlates. A prime candidate population to begin to unpack disturbances in reward sensitivity is bipolar disorder (BD). BD is a chronic, severe, and often fatal psychiatric illness (Tondo, Isacsson, & Baldessarini, 2003; Woods, 2000) that is centrally characterized by increased reactivity to rewards and elevations in positive affect across contexts (Alloy, Abramson, Urosevic, Bender, & Wagner, 2009; Gruber, 2011a; Johnson, 2005; Nusslock et al., 2012; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008). The present investigation adopted a multi-modal approach to examine reward sensitivity in BD across neural and affective levels of analysis as well as across distinct categories of monetary and social reward, in comparison to a healthy non-psychiatric comparison group. This approach is well suited to inform broader models of psychopathology and its relationship to reward-related disturbances (e.g., Blum et al., 2000; Gruber, 2011b).
Several lines of evidence underscore the rationale for examining reward sensitivity in BD (Hasler, Drevets, Gould, Gottesman, & Manji, 2006; Gruber, 2011b). First, people with BD exhibit elevated reactivity to rewards, excessive pursuits aimed at obtaining rewards, and impairments in reward-related learning (Gruber, 2011b; Meyer, Johnson, & Winters, 2001; Pizzagalli, Goetz, Ostacher, Iosifescu, & Perlis, 2008). For example, individuals with BD self-report greater positive affect at the prospect of future rewards in their daily lives than healthy controls, even during periods of symptom remission (Meyer et al., 2001). Second, emerging evidence illustrates the importance of elevated reward sensitivity in the etiology and course of BD (Alloy et al., 2012a; Johnson, 2005). For example, increased self-reported reward sensitivity has been found to predict the onset of BD (Alloy et al., 2012a), and life events involving goal attainment for rewards predict increases in symptoms of mania over time (Johnson et al., 2000). Third, researchers report that those diagnosed with BD are further characterized by trouble regulating reward-relevant affective and behavioral responses. For example, inter-episode BD patients report trouble decreasing or down-regulating reward sensitivity (Farmer et al., 2006), and engage in maladaptive emotion regulation strategies that amplify reward-relevant responses (Gruber, Eidelman, Johnson, Smith & Harvey, 2011; Johnson, McKenzie, & McMurrich, 2008) compared with healthy controls. Moreover, heightened sensitivity to rewards has been associated with decreased ability to ‘coast’, or ease back on goal pursuit following reward receipt (Fulford, Johnson, Llabre, & Carver, 2010). Finally, increased reward sensitivity is associated with clinical severity and impairment in BD (e.g., Alloy et al., 2012b; Johnson et al., 2005). Taken together, these data suggest that heightened reward sensitivity is a core component involved in the etiology, phenomenology, and course of BD.
Only recently have researchers begun to explore the neural mechanisms of reward sensitivity in BD (e.g., Nusslock, Young, & Damme, 2014). For example, Bermpohl et al. (2010) reported increased neural reactivity in the left lateral orbitofrontal cortex--a region partially implicated in the assignment of reward value of stimuli (Diekhof, Falkai, & Gruber, 2011)--during anticipation of gains during a monetary incentive delay task among currently manic individuals as compared to a healthy control group. Linke and colleagues (2012) also reported greater activation in the left medial orbitofrontal cortex among remitted individuals with BD I than in healthy controls in response to monetary reward. Another study employing a card-guessing reward paradigm reported greater activation in the ventral striatum and orbitofrontal cortex during reward anticipation in a remitted BD group compared to healthy controls (Nusslock et al., 2012). Taken together, these results suggest that increased activation of reward-related striatal and orbitofrontal regions in response to reward-relevant stimuli may represent a potential neural mechanism of elevated reward reactivity in BD.
Although such findings are encouraging, there remain several critical domains upon which to expand this work. First, existing studies have focused almost exclusively on money as a proxy for rewards more broadly (e.g., Linke et al., 2012; Nusslock et al., 2012). Given that the types of rewards individuals encounter in their daily lives are frequently interpersonal in nature, it is critical to examine additional domains of reward, especially those that are interpersonal or socially rewarding. Extending work on reward sensitivity to examine the processing of social rewards in BD is especially important as BD is associated with sensitivity to positive social stimuli including misperceiving social cues from others in an overly positive manner (e.g., Dutra et al., 2014; Lembke & Ketter, 2002; Piff et al., 2012), as well as associated social functioning difficulties (Coryell, Scheftner, & Keller., 1993; Faglioni et al., 2005). Thus, it will be important to understand which abnormalities in reactivity to rewards occur across contexts, and which occur only in response to specific classes of rewards. Indeed, the identification of more specific neural mechanisms of reward sensitivity within and across contexts is a critical next step toward a more comprehensive understanding of BD. Insight into such neural mechanisms would pave the way for research into the specific neural signatures of reward-related symptoms of BD. Furthermore, delineating the neural correlates of reward-related symptoms may contribute to the development of treatments aimed at ameliorating specific symptoms by normalizing the corresponding aberrant neural processes. In this way, this work promises to enhance our current understanding of the biological aspects of BD, the relationships between neural, affective, and behavioral characteristics of the disorder, and the most salient targets for direct intervention on the neural processes that may contribute to its symptoms.
Second, there is a mixed literature suggesting the possibility of altered neural processes implicated in the anticipation of potential rewards (Bermpohl et al., 2010; Chase et al., 2013; Nusslock et al., 2012; though also see Bermpohl et al., 2010; Caseras et al., 2013). This has been an important initial focus given clinical models of BD that emphasize heightened reward anticipation and pursuit in BD (Hayden et al., 2008; Johnson, Eisner, & Carver, 2009). However, other empirical findings point more strongly towards receipt of actual rewards in the moment as the catalyst for upward spirals into overly ambitious goal pursuit and increases in manic symptom severity in BD (Alloy et al., 2012a; Johnson et al., 2000). Examination of neural reactivity to reward in BD has the potential to shed unique light on the brain-based mechanisms of reward-related behaviors and clinical mood symptoms.
Therefore, the goal of the present investigation is to elucidate mechanisms of reward anticipation and receipt in BD as compared with healthy adults. Importantly for the study of reward processing across contexts, we adopted a comprehensive approach that sampled both monetary and social reward types simultaneously, across both neural and behavioral levels of analysis. This approach enabled us to conduct two main sets of analyses. First, we examined effects of group (BD, HC) and reward type, as well as their interaction, during reward anticipation. Second, we examined these same effects during reward receipt. Within each set of analyses, we first focused on two main regions of interest (ROIs). ROIs included (1) the ventral striatum, given a robust body of evidence demonstrating the centrality of this region to reward processing (Berridge, Robinson, & Aldridge, 2009; Pecina & Berridge, 2013), and (2) the orbitofrontal cortex (OFC) given the important role for this region in modulating responses to reward and punishment (Ernst et al., 2013; Wrase et al., 2007) and some mixed findings implicating the OFC in reward anticipation in BD (e.g., Chase et al., 2013; Nusslock et al., 2012; though also see Bermpohl et al., 2010; Caseras et al., 2013).We then employed a whole-brain approach to examine other neural regions where groups differed significantly during anticipation and receipt of rewards.
Method
Participants
Participants were 28 adults diagnosed with BD type I, currently remitted, and 27 healthy controls (HC) who did not meet current or past criteria for any DSM-IV-TR Axis I disorder. Participants were recruited using online advertisements and flyers posted in New Haven, CT and surrounding communities. Exclusion criteria for both groups included history of severe head trauma, stroke, neurological disease, severe medical illness (e.g., autoimmune disorder, HIV/AIDS), medications affecting cerebral blood flow (e.g., blood pressure medications), MRI safety incompatibility (e.g., metal implants), left-handedness, and pregnancy. In addition, because addiction is associated with persistent changes in brain reward systems (Abler, Greenhouse, Ongur, Walter, & Heckers, 2008; Koob & LeMoal, 2005), we excluded participants with a history of substance dependence and substance abuse in the past 6 months.
Diagnostic Evaluation
All diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV; First, Spitzer, Gibbon, & Williams, 2007), administered by trained researchers. Approximately one-fourth (n=12; 24.49%) of interviews were rated by an independent reviewer (κmean=1.00).
Mood Symptoms
Current symptoms of mania were measured using the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978) and current symptoms of depression were measured using the Inventory of Depressive Symptomatology (IDS-C; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). Intra-class correlation coefficients (ICC; Shrout & Fleiss, 1979) for absolute agreement between the original interviewer and an independent rater for approximately one fifth of study participants were strong for both the IDS-C (n=11; ICC=1.00) and YMRS (n=10; ICC=0.96). Remitted mood status (i.e., neither manic, depressed, nor mixed mood state) for the BD group was verified according to SCID-IV mood module criteria for the past month and cutoff scores on the YMRS (≤ 7), and IDS-C (≤ 11) for the past week. The IDS-C and YMRS were administered on the day of the diagnostic interview, and re-administered again on the day of the scan to ensure that participants scored below cutoffs on both days.
Medication Assessment
At the baseline laboratory visit, participants reported use of psychiatric and other medications over the past month, recorded using the Somatotherapy Index (Bauer et al., 1997).
Cognitive Functioning
Cognitive functioning was assessed using the Mini Mental Status Examination, a brief objective measure of cognitive status and impairment (MMSE; Folstein, Folstein, & McHugh, 1975). Raw scores (range: 0 to 30) were calculated as the total number of trials correct. All participants exceeded the eligibility cutoff score (≥24; Folstein et al., 1975).
Executive Functioning
Executive functioning was measured using the letter-number sequencing subtest of the Wechsler Adult Intelligence Scale-IV (WAIS-IV; Pearson, 2008). Raw scores were calculated as the total number of trials correct, from which WAIS-IV age-normed scaled scores were used in final analyses.
Procedures
Participants completed two study sessions, including an initial baseline diagnostic visit and a second fMRI scanning session approximately 2.5 months apart (M=78.51 days, SD=65.79). Between the two visits, a second unrelated fMRI scanning session was conducted during which symptoms were also reassessed to ensure continuity of remitted mood status in the BD group.
Baseline diagnostic visit
At baseline, participants completed a diagnostic evaluation in the laboratory that included the SCID-IV, YMRS, IDS-C, Positive Qualities Questionnaire (see below), medication information and demographics (along with additional questionnaires not part of the current investigation).
Positive Qualities Questionnaire (PQQ)
The PQQ is a 10-item questionnaire designed for the present study to elicit self-reported information about perceived positive qualities. It was used to derive personalized social feedback for use in the Social Incentive Delay (SID) task, described below. Specifically, participants were asked to “describe some positive events in your life, as well as some positive personal qualities and beliefs”, and to respond to each question in a few sentences. PQQ items span several domains including personal values (e.g., “Name some values that you believe are very important, and describe why they are important to you”), personal qualities (e.g., “Describe a quality that makes you unique”), social relationships (e.g., “Describe a time when you felt love for someone else”), and achievement (e.g., “Describe one of your greatest accomplishments”; see Appendix 1). The PQQ was used as a means for generating information from which to draw the positive adjectives presented in the SID task. Specifically, content analysis was performed to match adjectives from a validated database of 555 positive adjectives (Anderson, 1968) with individual responses to items on the PQQ. In order to assess the relative intensity of each positive adjective, 134 community participants rated 100 of these adjectives for their positive value on a scale of 1 (not at all positive) to 5 (extremely positive) in an anonymous online survey. Following this, all adjectives were standardized and assigned percentile rankings based on their average positivity rating. These rankings were used to categorize positive adjectives into one of two categories: (1) Level 2 adjectives were defined as ‘highly positive’ and consisted of adjectives with the highest 50th percentile; (2) Level 1 adjectives were defined as ‘moderately positive’ and consisted of adjectives with percentile rankings in the lowest 50th percentile. Positive adjectives were selected for each of the two reward levels individually for each participant. Two raters (SD and a trained research assistant) separately made adjective selections for each participant, and then had a consensus meeting to discuss discrepancies until 100% agreement was achieved.
fMRI scanning visit
The fMRI visit included four parts; namely, a pre-scan assessment, pre-scan task training, fMRI task, and post-scan phase. During the pre-scan assessment, the YMRS and IDS-C were re-administered to ensure that participants were below symptom thresholds and met remitted symptom status (Tohen et al., 2009). Next, the presence of current substance use was assessed using the Medimpex Multi-Drug Urine Test (United Inc.) and participants who tested positive for cocaine, amphetamines, methamphetamines, opiates, or benzodiazepines were excluded unless prescribed by a physician (see medication status in Table 1). Next, for the pre-scan task training, participants were trained on both the MID and SID tasks on a laptop computer for approximately 30 minutes. Task training began with the experimenter explaining the task, as described above, as step-by-step instructions were presented on a laptop. Then, participants were asked to complete 45 practice trials for the MID and 45 practice trials for the SID task in order to ensure that they were familiar with the task. During this practice, for both MID and SID tasks, average reaction times for each participant were calculated, to be used to titrate the duration of the target presentation during the fMRI session in order to ensure even distribution (~50%) of win trials on the MID and SID tasks, following prior research (e.g., Pizzagalli et al., 2009). Specifically, half the targets were presented for one standard deviation longer than the participant’s average reaction time (predicted ‘win’ trials), while the other half were presented for one standard deviation shorter than the participant’s average reaction time (predicted ‘no-win’ trials). For the fMRI task, participants were then escorted to the scanner, where they completed four runs of each task lasting approximately seven minutes each, presented in random order. Each individual run consisted of 22–23 trials, totaling 90 MID and 90 SID trials, broken down into 18 neutral/no-win trials (20%), 36 low reward trials (40%), and 36 high reward trials (40%). During the post-scan phase, once the fMRI tasks were completed, participants completed post-task questionnaires (including manipulation check items) in a testing room outside of the scanner, received compensation, and were debriefed.
Table 1.
Demographic, Cognitive, and Clinical Characteristics
| BD (n=24) |
HC (n=25) |
Statistic | Effect Size | |
|---|---|---|---|---|
| Demographic | ||||
| Age (Yrs) | 31.38 (11.86) | 29.44 (8.84) | F=0.42 | ηp2=0.01 |
| Female (%) | 62.5% | 60.0% | χ2=0.03 | V=0.03 |
| Caucasian (%) | 95.8% | 84.0% | χ2=1.87 | V=0.20 |
| Education (Yrs) | 15.20 (2.00) | 16.16 (1.57) | F=3.47 | ηp2=0.07 |
| Employed (%) | 50.0% | 72.0% | χ2=2.50 | V=0.23 |
| Married (%) | 12.5% | 4.0% | χ2=1.18 | V=0.16 |
| Cognitive | ||||
| MMSE | 27.96 (1.76) | 28.68 (1.57) | F=0.15 | ηp2=0.05 |
| WAIS-IV Letter Number Task | 11.92 (2.56) | 12.08 (3.37) | F=0.15 | ηp2=0.003 |
| Clinical | ||||
| YMRS | 1.50 (1.72) | 1.04 (1.46) | F=1.02 | ηp2=0.17 |
| IDS-C | 3.58 (2.08) | 1.40 (1.47) | F=18.07* | ηp2=0.99 |
| GAF | 70.63 (10.75) | 88.96 (3.93) | F=63.84* | ηp2=0.58 |
| Age at Onset (Yrs) | 16.33 (7.14) | -- | -- | -- |
| Illness Duration (Yrs) | 14.78 (11.45) | -- | -- | -- |
| # Comorbid Disorders | 0.42 (0.65) | -- | -- | -- |
| # Depressive Episodes | 13.00 (19.13) | -- | -- | -- |
| # Manic Episodes | 16.69 (37.40) | -- | -- | -- |
| # Antidepressants | 0.33 (0.56) | -- | -- | -- |
| # Lithium | 0.20 (0.41) | -- | -- | -- |
| # Benzodiazepines | 0.13 (0.34) | -- | -- | -- |
| # Typical Neuroleptics | 0.00 (0.00) | -- | -- | -- |
| # Atypical Neuroleptics | 0.21 (0.41) | -- | -- | -- |
Note: BD=Bipolar disorder group; HC=Healthy control group; MMSE=Mini Mental State Exam; WAIS-IV Letter Number Task =Letter-Number Sequencing subtest of the Wechsler Adult Intelligence Scale-IV; YMRS=Young Mania Rating Scale; IDS-C=Inventory of Depressive Symptoms – Clinician Rated; Age at Onset=Age of BD Onset; # Manic Episodes=Number of Lifetime Manic Episodes; # Depressive Episodes=Number of Lifetime Major Depressive Episodes; # Comorbid Disorders=Number of Comorbid DSM-IV-TR Axis I Diagnoses; Mean values are displayed with standard deviations in parentheses where applicable. Clinical information collected at initial laboratory visit.
p<0.05 comparison of BD and HC groups.
Monetary and social incentive delay tasks
Participants completed the previously-validated Monetary Incentive Delay (MID) task, originally developed by Knutson, Westdorp, Kaiser, & Hommer, (2000), and a Social Incentive Delay (SID) task developed for the present study, the SID task (see Figure 1 for task schematics). Both the MID and SID tasks consisted of 90 trials each, yielding a total of 180 trials. During pre-scan task training, participants were given the following cover story for the SID task. Each participant was told that two highly trained experimenters in the laboratory had viewed video recordings of their interactions with the diagnostic interviewer at the laboratory session, reviewed their responses to the PQQ and other questionnaires, and created a list of the participant’s most positive qualities based on this information. Participants were told that the object of this task was to view the items on these experimenters’ lists of their best personal qualities. In addition, participants were told that each of these experimenters had given a ‘global evaluation’ of their personality, and that while both experimenters had given positive evaluations, one was extremely complimentary, giving them the highest possible evaluation of their personality. This experimenter became the ‘high reward’ experimenter, providing the more positive descriptive adjectives during the SID task. Participants were told that while the other experimenter’s evaluation was also positive, it was less positive than the first’s. This experimenter became the ‘low reward’ experimenter, providing the less desirable adjectives.
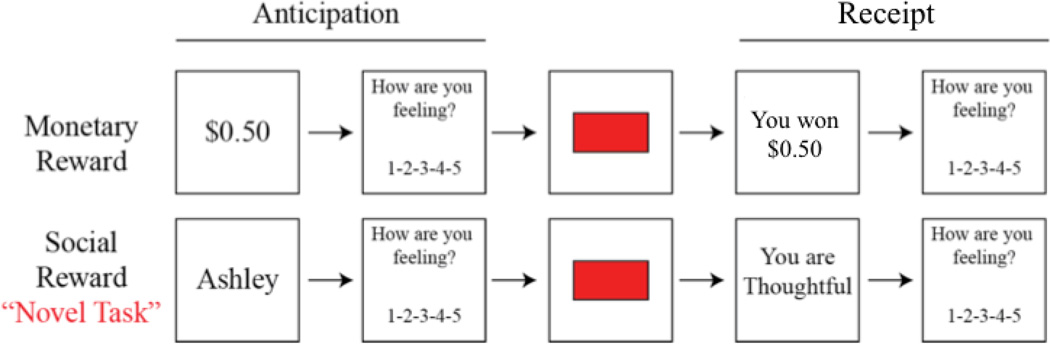
Figure 1.
Monetary Incentive Delay (MID) and Social Incentive Delay (SID) task schematics. First, participants saw a cue indicating either how much money was available (MID: e.g., $0.50), or whose feedback they were playing for (SID: e.g., Ashley) on that trial. Next, participants rated how they felt about the potential for winning the amount of money displayed in the cue, on a 1 (negative) to 5 (positive) scale. Next, a red box flashed briefly, and participants responded as quickly as possible. If they responded quickly enough, they would see a win outcome consisting either of monetary rewards (MID: e.g., You won $0.50) or praise (SID: e.g., “You are thoughtful). If they did not, a no-win outcome would appear. Finally, participants rated their affective responses to the outcome on a 1 (negative) to 5 (positive) scale.
Each task varied across three levels of reward (e.g., neutral, low reward, and high reward). Neutral MID trials are those in which participants could win “$0.00” (Ntrials=18; 20%), low reward trials in the MID are those in which participants could win $0.50 (Ntrials=36; 40%), and high reward trials are those in which participants could win $3.00 (Ntrials=36; 40%). Given that the task was designed for participants to win approximately 50% of the low-reward and high-reward trials, this design ensured that each type of reward receipt (i.e., no reward, low reward win, low reward no-win, high reward win, high reward no-win) occurred on approximately 20% of MID trials. Neutral SID trials are those in which anticipatory cues and receipt stated that no feedback was available (Ntrials=18; 20%), low reward trials in the SID are those in which participants can win “positive” feedback from an experimenter (Ntrials=36; 40%), and high reward trials are those in which participants can win “extremely positive” feedback from an experimenter (Ntrials=36; 40%). Assignment of confederate names “Keith” and “Ashley” to low-reward and high-reward conditions was randomized across participants.
Participants completed four runs of the MID task and four runs of the SID task, for a total of eight runs. Each individual run for both the MID and SID tasks consisted of approximately 22–23 trials, for a total of 90 trials for the MID task and 90 total trials for the SID task. The order in which the four MID and four SID task runs were presented was randomly selected for each participant at the time of testing using E-Prime 2.0. As can be seen in Figure 1, the temporal sequence of a single trial was as follows: first, participants viewed a 500ms cue, which indicated the potential reward value of that trial. Cues were either monetary in the MID task (e.g., $0.50) or the name of the experimenter providing the feedback (e.g., “Ashley”) in the SID task. Second, there was a variable duration inter-stimulus interval (ISI; jittered ~ 2.5s, range: 2.0–6.0s). Third, a red box (target) flashed briefly on the screen for a variable duration individually titrated to each participant’s average reaction time, as previously described (Pizzagalli et al., 2009). Fourth, participants responded as quickly as possible by pressing a response button to the target. Fifth, after an additional ISI (jittered ~ 2.5s, range: 2.0–6.0s), a reward receipt screen was presented for 1200ms to inform the participant as to whether (s)he had won that trial by responding to the target before it disappeared from the screen (e.g., “You won $3.00” for the MID or “You are thoughtful” for the SID). All trials were separated by an inter-trial interval of variable duration (ITI; jittered ~3s; range: 2.0–7.0s). To assess emotion during reward anticipation and reward receipt, participants self-reported their current feelings in response to the prompt, “How are you feeling?” on a Likert scale from 1 (negative) to 3 (neutral) to 5 (positive) immediately before reward receipt and following reward receipt.
Importantly, the MID and SID tasks were designed to be parallel in three main respects. First, the two tasks were designed to be parallel in the order and duration of presentation of each stimulus, as can be seen in Figure 1 and described above. Second, tasks were parallel in their three levels of reward magnitude. Third, tasks did not differ in the order of presentation. The order of the runs was randomly chosen for each participant by E-Prime 2.0.
Data Acquisition
Behavioral and self-report data
E-Prime 2.0 software (Psychology Software Tools, Inc.) was used for stimulus presentation and collection of self-report data. During the fMRI scan, stimuli were projected onto a screen behind the scanner, and participants viewed the stimuli via an angled mirror affixed to the head coil. Responses were made with the right hand using a five-button response box.
fMRI data
Data were collected on a Siemens TIM Trio 3T scanner (Siemens Medical Solutions, Erlangen, Germany). Functional images were acquired with a T2-weighted EPI BOLD sequence (TR=2000ms; TE=35ms; FOV=220mm; voxel dimensions 3.4×3.4×4.0mm; 28 slices). Structural images were obtained using a T1-weighted MPRAGE acquisition (TR=2530ms; TE=2.77 msec; FOV=256mm; voxel dimensions=1.0×1.0×1.0mm; 176 slices). fMRI data analysis was carried out using the fMRI Expert Analysis Tool (FEAT) Version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included non-brain removal, motion correction, spatial smoothing with a Gaussian kernel of full-width at half-maximum (FWHM) of 5mm, grand-mean intensity normalization, and high pass temporal filtering. Four BD and two HC participants were excluded from the final data analysis due to excessive motion during fMRI (>5mm movements during at least 4 of 8 runs), leaving a final sample of 24 BD and 25 HC participants. After pre-processing, second-level within-subject analyses were performed using a fixed effects model in FMRIB’s Local Analysis of Mixed Effects (FLAME) (Beckmann, Jenkinson, & Smith, 2003). Higher-level analyses, including between-group comparisons, were carried out using FMRIB’s Local Analysis of Mixed Effects (FLAME) stage 1 (Beckmann et al., 2003; Woolrich, 2004; Woolrich, 2008). Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z>2.3 and a (corrected) cluster significance threshold of p=0.05 (Worsley, 2001). The threshold to define contiguous voxel clusters was set at Z>2.3 (p<0.01) following standards in the field (Woo, Krishnan, & Wager, 2014) to balance minimization of family-wise error rates (i.e., identification of false positive clusters) with consideration of Type II errors. Figures were prepared using NeuroElf Software package (Neuroelf.Net, e.g., Kober, DeVito, DeLeone, Carroll, & Potenza, 2014).
Results
Demographic and Clinical Characteristics
As shown in Table 1, groups did not differ in age, gender, ethnicity, employment status, or years of education. Groups also did not differ on subthreshold mania symptoms. The BD group did score higher on low-level subthreshold depression symptoms, though both groups scored well below clinical thresholds for both mania and depressive symptoms. As expected, the BD group scored lower on global functioning than the HC group.
MID and SID Task Manipulation Checks
Prior to our main analyses, we conducted preliminary analyses of both the MID and SID tasks to ensure they were psychometrically comparable and elicited patterns of affective and behavioral responding consistent with previous studies using the MID (e.g., Knutson et al., 2000; Nielsen et al., 2008; Pizzagalli et al., 2009). This included examinations of self-reported affect, task difficulty, and perceived control of task outcomes, and the extent to which participants believed the task cover story.
For self-reported affect, we ran a 2 (Group: BD, HC) x 2 (Task: MID, SID) x 2 (Trial Type: Neutral, Reward) ANOVA on self-reported affect during reward anticipation, and a 2 (Group: BD, HC) x 2 (Task: MID, SID) x 2 (Receipt Outcome: Win, No-Win) ANOVA for self-reported affect during reward receipt (win/no-win). For anticipatory affect, a main effect of Trial Type emerged, reflecting more positive affective ratings for reward (M=3.69, SD=0.55) than neutral trials (M=2.87, SD=0.62), F(1,47)=97.39, p<.001, ηp2=0.67. In addition, there was a main effect of Task, reflecting more positive affect on MID (M=3.33, SD=0.63) than SID (M=3.22, SD=0.54) trials, F(1,47)=6.06, p=0.02, ηp2=0.11. In order to examine whether anticipation of social rewards in the SID task increased positive affect, a follow-up t-test examining self-reported anticipatory affect during reward vs. neutral trials on the SID task was conducted. Results revealed greater positive anticipatory affect for SID reward than SID neutral trials, t(48) = −7.30, p<.001. A Trial Type x Task interaction effect also emerged, reflecting a larger increase in affective ratings from neutral to reward cues in the MID (Neutral: M=2.83, SD=0.70; Reward: M=3.82, SD=0.56) than the SID (Neutral: M=2.90, SD=0.54; Reward: M=3.54; SD=0.54) task, F(1,47)=12.91, p=0.001, ηp2=0.22. A Group x Task interaction effect also emerged, reflecting a larger task-related discrepancy in affective ratings in the BD group (MID: M=3.43, SD=0.65; SID: M=3.22, SD=0.57) than the HC group (MID: M=3.23, SD=0.61; SID: M=3.22, SD=0.50), F(1,47)=4.66, p=0.04, ηp2=0.09. No additional effects emerged for the main effect of Group (p=0.45), Group x Trial Type interaction (p=0.36), or Group x Task x Trial Type interaction (p=0.20). For reward receipt, a main effect of Outcome emerged, reflecting more positive affective ratings for Win (M=4.13, SD=0.50) than No-Win outcomes (M=2.38, SD=0.82), F(1,47)=163.15, p<0.001, ηp2=0.78. A Task x Outcome interaction also emerged, reflecting a larger discrepancy in affective responses between Win and No-win outcomes in the MID (Win-No-Win M=1.94) than to the SID task (Win-No-Win M=1.55), F(1,47)=15.77, p<0.001, ηp2=0.25. No effects emerged for the main effect of Task (p=0.20), main effect of Group (p=.95), Group x Task interaction (p=0.26), Group x Outcome interaction (p=0.91), or Group x Task x Outcome interaction (p=0.54). These results are consistent with existing work using the MID task (e.g., Nielsen, Knutson, & Carstensen, 2008).
For task difficulty and agency, participants answered two self-report questions indexing perceived task difficulty (“I felt that I had the ability to perform well in the task”) and agency over task outcomes (“I felt that I had control over whether I won the trials”), each rated on a likert scale from 1 (strongly disagree) to 7 (strongly agree), at the end of the scan. In order to examine the extent to which groups and/or tasks may differ on these dimensions, we conducted Group (BD, HC) × Task (MID, SID) ANOVAs on participants’ responses to these items. Results revealed no main effects of Group (difficulty: p=0.07; agency: p=1.00), main effects of Task (difficulty: p=0.27; agency: p=0.61), or Group x Task interaction effects (difficulty: p=0.51; agency: p=0.61). Participants reported on average moderate agreement with the statements that they had they ability to perform well (M=4.79, SD=1.43) and reported control over winning the trials (M=4.33, SD=1.76) across both tasks.
After the scan, participants also rated the extent to which they believed the stated cover story or purpose of each task on a scale from 1 (not at all) to 5 (extremely). A Group x Task ANOVA on responses to this item revealed that participants generally believed the stated purposes of both tasks (MID: M=3.50, SD=1.11; SID: M=3.73, SD=1.13), and no main effects of Group (p=0.81) or Task (p=0.23), nor a Group x Task interaction effect emerged (p=0.24).
Reward Anticipation
In our first set of neuroimaging analyses, we examined group- and task-related differences in neural processing of reward anticipation. To this end, we first conducted ROI analyses focusing on the ventral striatum and OFC, using a [Reward>Neutral Cue] contrast. Masks for the right and left NAcc were created using the Harvard-Oxford subcortical structural atlas (see Supplementary Figure 1), and an OFC mask was created using the Harvard-Oxford cortical structural atlas (see Supplementary Figure 2). We extracted beta values from the right and left NAcc and OFC using FSL’s FeatQuery tool, and converted these to percent-signal-change values using FSLmaths. Results of a Group (BD, HC) × Hemisphere (Right, Left) × Task (MID, SID) ANOVA on NAcc activity during reward anticipation revealed a main effect of Task, with greater NAcc reactivity to MID (M=0.13, SD=0.15) than SID (M=0.03, SD=0.12) cues, F(1,47)=16.94, p<0.001,ηp2 =0.27. All other effects were nonsignificant, including main effects of Hemisphere (p=0.81) and Group (p=0.69), Hemisphere × Group interaction (p = 0.95), Task × Group interaction (p = 0.30), Hemisphere × Task interaction (p = 0.88), and Hemisphere × Task × Group interaction (p = 0.14). Results of a Group (BD, HC) × Task (MID, SID) ANOVA on OFC activity during reward anticipation also revealed a main effect of Task, reflecting greater OFC reactivity to monetary (M=0.05, SD=0.11) than social reward cues (M=-0.01, SD=0.10), F(1, 47)=8.76, p=.01, ηp2=0.16. No main effect of group (p=0.08) nor Group × Task interaction effect (p=0.79) emerged.
Next, we employed a whole-brain approach to identify additional regions of group- and task-related differences in reactivity to reward cues. Results of a Group (BD, HC) × Task (MID, SID) ANOVA revealed main effects of Group in several clusters including the orbitofrontal cortex, bilateral inferior frontal gyri and right lateral occipital cortex (see Table 2), reflecting greater activation in the HC than the BD group. No clusters emerged where the BD group showed greater activation than the HC group. In addition, main effects of Task emerged in a cluster encompassing bilateral occipital cortex regions including intracalcarine cortex and left occipital fusiform gyrus, reflecting greater activation during anticipation of monetary than social rewards (see Table 2). No clusters emerged reflecting greater activation during the SID than MID task. No Group × Task interaction effects emerged. Whole-brain and ROI results were comparable when including subthreshold depression symptoms (IDS-C scores), global functioning (GAF scores), presence vs. absence of antipsychotic medication, and presence vs. absence of comorbid anxiety as covariates1.
Table 2.
Neural Reactivity to Reward Anticipation (Without Covariates)
| Peak Coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of Activation | Laterality | x | y | z | Cluster | Max |
| Main Effects of Task | ||||||
| MID>SID | ||||||
| Occipital Cortex | R | 10 | −90 | −4 | 75167 | 7.83 |
| Occipital Pole | L | −8 | −92 | −4 | 7.5 | |
| Intracalcarine Cortex | R | 14 | −76 | 6 | 6.65 | |
| Intracalcarine Cortex | L | −10 | −84 | 4 | 6.59 | |
| Occipital Fusiform Gyrus | L | −16 | −80 | −16 | 6.58 | |
| Occipital Fusiform Gyrus | L | −24 | −80 | −12 | 6.54 | |
| Main Effects of Group | ||||||
| HC>BD | ||||||
| Orbitofrontal Cortex | L | −48 | 30 | −6 | 2662 | 4.34 |
| Inferior Frontal Gyrus | L | −50 | 34 | −6 | 4.19 | |
| Frontal Pole | L | −52 | 38 | 0 | 3.94 | |
| Middle Frontal Gyrus | L | −52 | 24 | 28 | 3.86 | |
| Inferior Frontal Gyrus | L | −40 | 18 | 18 | 3.75 | |
| Precentral Gyrus | L | −54 | 8 | 10 | 3.58 | |
| Inferior Frontal Gyrus | R | 62 | 18 | 12 | 2531 | 3.95 |
| Inferior Frontal Gyrus | R | 54 | 12 | 6 | 3.85 | |
| Inferior Frontal Gyrus | R | 54 | 12 | 12 | 3.81 | |
| Superior Frontal Gyrus | Midline | 0 | 10 | 56 | 3.79 | |
| Inferior Frontal Gyrus | R | 50 | 12 | 14 | 3.75 | |
| Inferior Frontal Gyrus | R | 60 | 26 | 2 | 3.74 | |
| Lateral Occipital Cortex | R | 20 | −66 | 50 | 1133 | 4.05 |
| Lateral Occipital Cortex | R | 36 | −86 | 24 | 3.71 | |
| Precuneus Cortex | R | 12 | −80 | 42 | 3.69 | |
| Lateral Occipital Cortex | R | 44 | −84 | 10 | 3.56 | |
| Lateral Occipital Cortex | R | 18 | −74 | 48 | 3.52 | |
| Occipital Pole | R | 34 | −90 | 18 | 3.34 | |
| Inferior Frontal Gyrus | R | 56 | 34 | 14 | 3.57 | |
| Frontal Pole | R | 46 | 60 | −6 | 3.52 | |
| Frontal Pole | R | 42 | 46 | −8 | 3.46 | |
| Frontal Pole | R | 54 | 38 | 14 | 3.43 | |
| Inferior Frontal Gyrus | R | 46 | 30 | 0 | 3.17 | |
| Frontal Pole | R | 38 | 42 | 8 | 3.06 | |
Note: Peak coordinates for main cluster and local maxima are reported in Montreal Neurological Institute (MNI) space (x, y, z). Results are cluster corrected at p<0.05.
Reward Receipt
In our second set of analyses, we examined group- and task-related differences during neural processing of reward receipt. To this end, we first conducted ROI analyses focusing on the ventral striatum and OFC, using a [Win>No Win Outcome] contrast. Masks for the right and left NAcc and OFC were the same as those used in Aim 1 (see Supplementary Figures 1 and 2). Also consistent with the procedures from Aim 1, we extracted beta values from each ROI using FSL’s FeatQuery tool, and converted these to percent-signal-change values using FSLmaths. Results of a Group (BD, HC) × Hemisphere (Right, Left) × Task (MID, SID) ANOVA on NAcc activity during reward receipt revealed a main effect of Task, reflecting greater NAcc reactivity to monetary (M=0.14, SD=0.19) than social (M=0.05, SD=0.16) reward receipt, F(1,47)=6.53, p=0.01, ηp2=0.12. A main effect of Group also emerged, reflecting greater NAcc reactivity to reward receipt in the BD (M=0.13, SD=0.15) than the HC (M=0.05, SD=0.19) group, F(1,47)=6.33, p=0.02, ηp2=0.12. All other effects were nonsignificant, including the main effect of Hemisphere (p = 0.81), Group × Hemisphere interaction (p = 0.07), Task × Group interaction (p = 0.62), Hemisphere × Task interaction (p = 0.06), and Hemisphere × Group × Task interaction (p = 0.45). Results of a Group (BD, HC) × Task (MID, SID) ANOVA on OFC activity during reward receipt also revealed a main effect of Task, reflecting greater OFC reactivity to social (M=0.08, SD=0.13) than monetary (M=-0.01, SD=0.11) rewards, F(1,47)=16.02, p<0.001, ηp2=0.25. No main effect of Group (p=0.07) nor Group × Task interaction (p=0.32) effects emerged.
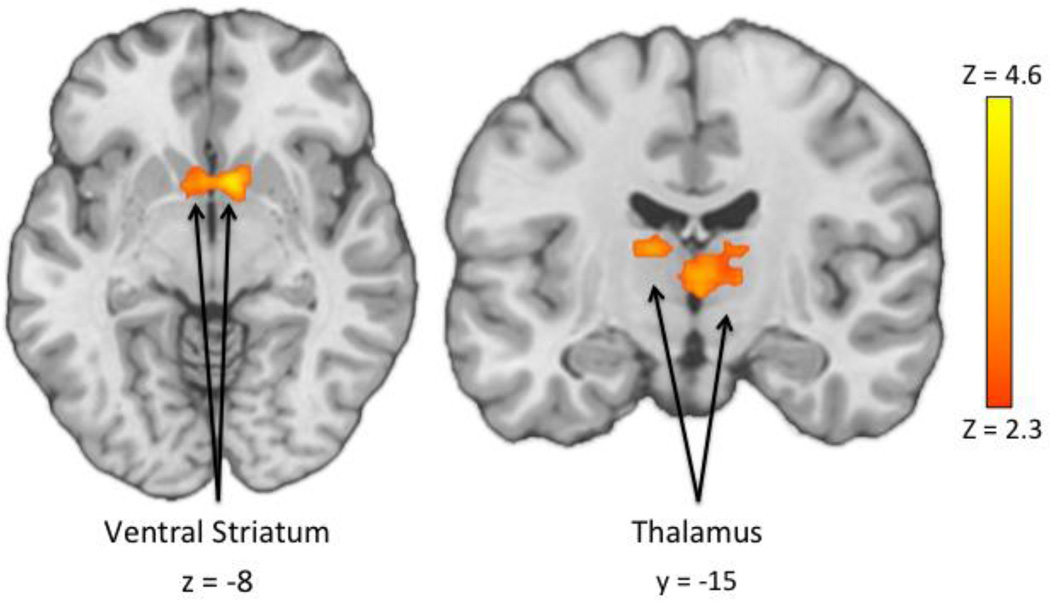
Next, we employed a whole-brain approach to identify additional regions of group- and task-related differences in reactivity to reward cues. Results of a Group (BD, HC) × Task (MID, SID) ANOVA during reward receipt revealed a main effect of Task reflecting greater reactivity to monetary than social rewards in two large clusters encompassing diffuse frontal and occipital cortical regions (see Table 3). In addition, three clusters emerged where reactivity was significantly greater to social than monetary reward receipt, including the left frontal pole, bilateral inferior frontal gyri and bilateral orbitofrontal cortex (see Table 3). In addition, a main effect of Group emerged in a striatal cluster wherein the BD group demonstrated significantly greater activation in the right NAcc, right caudate and bilateral thalamus than the HC group (see Figure 2 and Table 3). No clusters emerged where the HC group showed greater activation than the BD group. No significant Group × Task interaction effects emerged. Whole-brain and ROI results were comparable when including subthreshold depression symptoms (IDS-C scores), global functioning (GAF scores), presence vs. absence of antipsychotic medication, and presence vs. absence of comorbid anxiety as covariates1.
Table 3.
Neural Reactivity to Reward Receipt (Without Covariates)
| Peak Coordinates | ||||||
|---|---|---|---|---|---|---|
| Regions of Activation | Laterality | x | y | z | Cluster | Max Z |
| Main Effects of Task | ||||||
| MID>SID | ||||||
| Supramarginal Gyrus | R | 50 | −40 | 34 | 36380 | 6.56 |
| Lingual Gyrus | L | −10 | −82 | −14 | 6.37 | |
| Supramarginal Gyrus | R | 50 | −42 | 38 | 6.35 | |
| Lingual Gyrus | L | −8 | −84 | −8 | 6.24 | |
| Occipital Pole | L | −14 | −94 | 22 | 6.21 | |
| Occipital Fusiform Gyrus | L | −14 | −84 | −4 | 6.21 | |
| Middle Frontal Gyrus | R | 26 | 18 | 50 | 13044 | 7.05 |
| Middle Frontal Gyrus | R | 36 | 32 | 40 | 5.77 | |
| Middle Frontal Gyrus | R | 36 | 32 | 40 | 5.63 | |
| Superior Frontal Gyrus | L | −24 | 18 | 54 | 5.60 | |
| Frontal Pole | R | 32 | 54 | 14 | 5.60 | |
| Middle Frontal Gyrus | R | 40 | 30 | 34 | 5.38 | |
| SID>MID | ||||||
| Inferior Frontal Gyrus | L | −52 | 30 | 6 | 8631 | 7.24 |
| Orbitofrontal Cortex | L | −46 | 30 | −12 | 7.24 | |
| Inferior Frontal Gyrus | L | −50 | 28 | 2 | 7.21 | |
| Inferior Frontal Gyrus | L | −54 | 28 | 2 | 7.17 | |
| Orbitofrontal Cortex | L | −36 | 24 | −16 | 6.82 | |
| Frontal Pole | L | −50 | 36 | −14 | 6.71 | |
| Frontal Pole | L | −4 | 60 | 28 | 4267 | 7.18 |
| Superior Frontal Gyrus | L | −4 | 20 | 56 | 6.57 | |
| Frontal Pole | L | −8 | 54 | 36 | 5.93 | |
| Paracingulate Gyrus | L | −4 | 18 | 46 | 5.53 | |
| Superior Frontal Gyrus | L | −6 | 36 | 48 | 5.22 | |
| Superior Frontal Gyrus | L | −6 | 44 | 38 | 5.03 | |
| Orbitofrontal Cortex | R | 44 | 32 | −12 | 3660 | 6.55 |
| Inferior Frontal Gyrus | R | 58 | 26 | 2 | 5.22 | |
| Orbitofrontal Cortex | R | 34 | 16 | −22 | 5.08 | |
| Inferior Frontal Gyrus | R | 56 | 30 | −8 | 5.06 | |
| Temporal Pole | R | 56 | 8 | −26 | 4.87 | |
| Temporal Pole | R | 50 | 12 | −28 | 4.87 | |
| Main Effects of Group | ||||||
| BD>HC | ||||||
| Nucleus Accumbens | R | 8 | 8 | −8 | 1251 | 4.29 |
| Caudate Nucleus | R | 10 | 8 | 12 | 4.12 | |
| Thalamus | R | 4 | −10 | 2 | 3.94 | |
| Caudate Nucleus | R | 10 | 12 | 0 | 3.94 | |
| Thalamus | R | 2 | −6 | 2 | 3.71 | |
| Thalamus | L | −12 | −16 | 12 | 3.54 | |
Note: Peak coordinates for main cluster and local maxima are reported in Montreal Neurological Institute (MNI) space (x, y, z). Results are cluster corrected at p<0.05.
Figure 2.
Whole-brain results reflecting main effects of Group during reward receipt, using a BD (Win - No Win) > HC (Win - No Win) contrast. No significant regions emerged where HC > BD using the same contrast.
Exploratory Analyses: Examining Associations between Neural and Behavioral Data
On an exploratory basis, we examined associations between self-reported affect during the scan and ventral striatal reactivity to reward cues and outcomes. We first examined whether self-reported affect when anticipating rewards [Reward – Neutral Cue] would predict ventral striatal reactivity (% signal change values) to reward cues [Reward – Neutral Cue] and outcomes [Win – No-Win]. Linear regressions were conducted predicting ventral striatal reactivity to anticipatory reward cues, with output separated by groups. Results revealed no associations between self-reported anticipatory affect and right (MID BD: p=0.82, HC: p=0.89; SID BD: p=0.80; HC: p=0.83) or left (MID BD: p=0.57, HC: p=0.49; SID BD: p=0.63, HC: p=0.76) NAcc reactivity to reward cues. Similarly, no associations emerged between self-reported affect after reward outcome and right (MID BD: p=0.75, HC: p=0.30; SID BD: p=0.27, HC: p=0.80) or left (MID BD: p=0.85, HC: p=0.62; SID BD: p=0.26, HC: p=0.59) NAcc reactivity to reward outcomes.
Next we explored the possible implications of ventral striatal reward reactivity to reward outcomes for subsequent self-reported affect during reward anticipation on the next trial. This analysis was theoretically motivated by literature reporting sustained positive affect following reward receipt in BD (e.g., Alloy et al., 2012a; Fulford et al., 2010; Johnson et al., 2000). To this end, we examined whether ventral striatal responses to rewards would predict anticipatory affect on trials immediately following win outcomes (i.e., reward receipt). We examined percent signal change values reflecting right and left NAcc reactivity to rewards, using the main effect of Group [Win>No-Win] contrast from our main analyses, to predict anticipatory affect ratings for trials immediately following reward receipt. To create a difference score reflecting each individual’s average change in anticipatory affect immediately following rewards, we first subtracted each individual’s average anticipatory affective rating from their average anticipatory affective rating immediately following wins. In this analysis, we sought to examine the predictive power of NAcc reactivity to rewards for subsequent affect within each group, but also to examine the extent to which groups differed in this relationship. To achieve this, we first entered right and left NAcc percent signal change values into linear regressions predicting these values, separately for BD and HC groups. Results revealed that greater reactivity to reward receipt predicted less positive anticipatory affect on trials following successful reward receipt (i.e., Win) in the HC group, across both the right (b=-0.31, t=-2.80, p=0.01) and left NAcc (b=-0.28, t=-3.10, p=0.005). For the BD group, however, neither right (b=0.03, t=0.30, p=0.77) nor left (b=0.09, t=0.79, p=0.44) NAcc reactivity to rewards predicted affect on trials immediately following wins. Next, we compared the regression coefficients emerging from within-group regressions to examine whether the predictive power of NAcc reactivity to rewards for subsequent affect differed significantly between groups. When regression coefficients were compared, a group difference emerged such that NAcc reactivity to rewards predicted reduced subsequent anticipatory affect to a greater degree in the HC group than the BD group, across both right (b=0.34, t=2.17, p=0.04) and left (b=0.37, t=2.49, p=0.02).
Discussion
The current study makes three substantial contributions to the literature and our understanding of reward processing in BD. First, the SID task provides a means for future neuroimaging studies to expand the study of reward processing beyond monetary rewards to include ecologically-valid social rewards. Second, the finding of elevated striatal reactivity across monetary and social rewards in BD provides evidence of a domain-general process potentially related to clinical findings of enhanced reward sensitivity even among remitted individuals with BD, and is consistent with models of BD specifying persistence in elevated positive affect across contexts as a central feature of BD (Gruber, 2011b). In addition, this finding is also consistent with theoretical models emphasizing hypersensitivity to rewards as a central process implicated in BD (e.g., Alloy et al., 2012a; Johnson et al., 2005; Urosevic et al., 2008). Specifically, these models emphasize elevated sensitivity of the behavioral activation system (BAS; Carver & White, 1994), increasing these individuals’ propensity to respond to cues of potential reward, which may partially account for the heightened striatal reactivity observed to both monetary and social rewards reported in the present investigation. Third, the finding that striatal reactivity to rewards predicts reduced anticipatory positive affect in healthy individuals, but not in BD, provides evidence consistent with theories of difficulty coasting or easing back in appetitive behaviors following reward receipt in BD (Fulford et al., 2010). Each of these contributions are discussed in greater detail below.
Results suggested that the novel SID task was effective in eliciting increased positive affect during anticipation and receipt of socially rewarding positive feedback (i.e., praise). Given that the vast majority of existing neuroimaging studies on reward processing have employed solely monetary rewards, the addition of an ecologically-valid social reward task to the literature is a critical tool. Future studies employing this task could expand the knowledge base on reward processing across disorders, to better understand the extent to which previously identified mechanisms extend across multiple domains of reward. Establishment of the generalizability of findings from the MID task will be essential for mapping of neuroimaging findings onto findings from clinical and behavioral studies of reward-related behavior, which examine participants’ responses to diverse types of rewards in daily life (e.g., Collip et al., 2014; Geschwind et al., 2010). This may be particularly important for disorders in which abnormalities in social information processing and reward-related mechanisms are critically implicated (Demurie, Roeyers, Baeyes, & Sonuga-Barke, 2011; Kohls, Chevallier, Trojani, & Schultz, 2012).
Results of analyses examining potential group differences in neural reactivity to rewards revealed a main effect of Group reflecting elevated striatal reactivity to monetary and social reward receipt in the BD group. Importantly, these results remained consistent with and without covariates, and no Group x Task interaction effects emerged in striatal regions with or without covariates. The striatal regions identified as having elevated reactivity to rewards in the BD group, including the NAcc within the ventral striatum, have been primarily implicated in incentive motivation and reward (Berridge et al., 2009; Peciña & Berridge, 2013). Among healthy individuals, this region is activated largely prior to reward receipt, and is associated with reward anticipation (Abler, Walter, Erk, Kammerer, & Spitzer, 2006; Ikemoto & Panksepp, 1999; Knutson & Greer, 2008). Thus, this finding is consistent with a potential brain-based mechanism by which reward receipt may be associated with increased positive affect in BD (Farmer et al., 2006; Johnson et al., 2000). This finding also dovetails with existing models of positive emotion disturbance in BD across stimuli rather than in response to any particular affective cue (Gruber, 2011a; 2011b). In addition, this finding is generally consistent with theoretical models emphasizing hypersensitivity to rewards as a central process implicated in BD (e.g., Alloy et al., 2009, 2012; Nusslock et al., 2012; Urosevic et al., 2008). These models emphasize elevated sensitivity of the behavioral activation system (Carver & White, 1994), increasing these individuals’ propensity to respond to cues of potential reward.
Analyses examining group differences during reward anticipation revealed some unexpected results, including lower reactivity to reward cues in the bilateral inferior frontal gyrus and right lateral occipital cortex, and right precuneus of the BD group. Given the role of these regions in representing hand-object interactions (Johnson-Frey et al., 2003), object recognition (Grill-Spector, Kourtzi, & Kanwisher, 2001), and self-related mental representations (Cavanna & Trimble, 2006), one possibility is that our HC group was recruiting these regions for a coordinated representation of their upcoming button-press to a greater degree than the BD group. Further research would be needed to better understand this pattern, and examine the possibility of any functional or behavioral consequences.
This study failed to replicate previously published findings suggesting elevated striatal and orbitofrontal cortex activation during reward anticipation in a sample of 21 remitted BD patients and 20 healthy controls (Nusslock et al., 2012). In fact, with a slightly larger sample size we instead found an opposite pattern of findings suggestive of blunted OFC activation during reward anticipation in the BD group in our main analyses, though we note this effect was eliminated when covariates were included. On the one hand, this is somewhat surprising given behavioral data suggesting elevated affective sensitivity to potential reward cues in BD (Alloy & Abramson, 2010; Meyer, Johnson, & Winters, 2001). On the other hand, when examining the neuroimaging literature carefully, supportive data for group-relevant differences during reward anticipation have been mixed, with some research groups reporting elevated striatal and frontal cortical activation during reward anticipation (Chase et al., 2013; Nusslock et al., 2012), and others reporting similar findings to the present study which report either blunted anticipatory striatal activation or no group differences between BD and control participants in striatal activation during reward anticipation (Abler et al., 2008; Bermpohl et al., 2010). More generally, mixed findings on reward anticipation hasten the call for replication efforts in the field more broadly (e.g., Pashler & Wagenmakers, 2012) to identify robust patterns across studies of group-related differences in neural processing when anticipating future rewards. Whole-brain analyses also revealed task-related effects. During reward anticipation, the MID task elicited greater reactivity than the SID task in occipital cortex regions including occipital pole, intracalcarine cortex, and occipital fusiform gyrus. Given that our analyses of self-reported affect revealed greater positive affect when anticipating monetary than social rewards, and the role of these regions in motivated attention (Bradley et al., 2003), it is possible that these results are reflective of greater motivation to obtain monetary than social rewards. During reward receipt, group-related findings emerged in both directions. The SID task elicited greater activation in bilateral OFC clusters and inferior frontal gyri. These regions have been implicated in social information processing and emotion regulation, and may have been recruited to a greater extent in the SID task in order to process the social feedback and regulate resulting emotions (Beer, John, Scabini, & Knight, 2006; Grecucci, Giorgetta, Bonini, & Sanfey, 2013). Also during receipt, the MID task elicited greater activity than the SID task in prefrontal (e.g., middle frontal gyrus) and occipital (e.g., lingual gyrus, occipital fusiform gyrus) cortical regions, and may reflect enhanced motivated attention in the MID task (Bradley et al., 2003; Yamasaki, LaBar, & McCarthy, 2002).
Exploratory analyses further indicated that striatal reactivity to rewards inversely predicted anticipatory positive affect to subsequent reward cues, such that more reward-related neural reactivity was associated with attenuated subsequent positive anticipatory affect in HC, but not BD participants. This finding is consistent with clinical observations that healthy individuals respond to success or rewarding feedback by ‘coasting’ (Fulford et al., 2010), while those with BD demonstrate persistent or even increasing positive affect and reward pursuit (Johnson, 2005; Johnson et al., 2000). This finding provides potential initial evidence of a mechanism by which elevated striatal reactivity to rewards in BD may be directly related to the clinical presentation of the disorder. However, the current study was not explicitly designed to test an association between striatal reactivity to rewards and subsequent incentive motivation, and thus future studies more directly targeting this question are needed. Interestingly, exploratory analyses examining associations between positive affect in anticipation of reward and striatal reactivity to reward cues and outcomes revealed no significant results. Though it is perhaps surprising to see this lack of association, one potential explanation is that neural reactivity to reward cues and outcomes in the VS indexed motivation more closely than positive affect, which was probed in our self-report items. Future studies should probe self-reported motivation during the MID and SID tasks explicitly to explore this possibility.
These results should be interpreted within the confines of several caveats. First, our sample size was relatively small in our final data analysis. While this sample size is consistent with, or larger than, the majority of neuroimaging studies of individuals with severe psychopathology including adults diagnosed with BD (e.g., Delvecchio et al., 2012; Jardri, Pouchet, Pins, & Thomas, 2011; Nusslock et al., 2012), direct replication in future studies with larger sample sizes will help to ensure the generalizability of these results. Second, our BD sample was taking a variety of medications at the time of testing. While our analysis indicated that the main results remained consistent when taking into account use of antipsychotic medications, future studies should aim to recruit samples of individuals with BD on specific subclasses of medication. Third, our study did not include a punishment condition as a comparison alongside reward, and future studies including such a condition will be important for the development of a comprehensive understanding of reward and punishment processing in BD. Fourth, although our two tasks were developed to have comparable structures and demonstrated many comparable features, we acknowledge the possibility that results could be influenced by additional variables that cannot be ruled out. As such, the comparability of the MID and SID tasks’ difficulty and discriminability is an important question that awaits further work. Future studies examining various reward types using structurally similar tasks should aim to ensure that tasks are fully psychometrically parallel. Finally, without a clinical comparison group of individuals with abnormal reward processing (e.g., remitted major depressive disorder), we cannot be sure that these results are specific to BD. However, similar studies examining reward processing in MDD have found blunted striatal reactivity to rewards among these individuals (Pizzagalli et al., 2009), which is consistent with anhedonia and blunted incentive motivation. To build on these findings, future studies should employ this or similar paradigms across mood states among individuals with BD, and in other populations of psychiatric disorders characterized by dysfunctional reward processing such as MDD.
Future studies could also modify our design and methods to improve power, sensitivity and specificity of results. Additionally, future studies building on the current findings may choose to employ smaller voxel sizes. When selecting our voxel sizes, we took into consideration that increasing voxel size enhances sensitivity to BOLD signal but decreases spatial resolution (Amaro & Barker, 2006; Howseman et al., 1999). Given that the SID task is novel, we chose slightly larger voxel sizes than average in order to improve detection of BOLD responses elicited by the task. Future studies may choose to improve the spatial localization of task-related BOLD responses by reducing voxel size. A similar cost-benefit consideration was made in setting our motion-artifact threshold of 5mm. While this threshold allowed us to retain the maximal number of participants with usable data (after discarding four BD and two HC participants for excessive motion beyond our 5mm threshold), future studies could use more restrictive motion thresholds in order to reduce noise and improve spatial specificity of findings. Finally, future studies should examine prospectively whether striatal reactivity to rewards predicts changes in symptoms or behavior, consistent with those predicted by rewarding life events among individuals with BD.
Supplementary Material
General Scientific Summary (GSS): We examined neural reactivity to anticipation and receipt of monetary and social rewards among adults with bipolar disorder (BD; N=24) and a healthy control group. Results revealed elevated reactivity to reward receipt in the striatum, a region implicated in incentive sensitivity, in the BD group. These results may help to explain the elevated sensitivity to rewards observed clinically in BD.
Acknowledgments
This study was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (JG) and a CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), and NIH roadmap for Medical Research (JG and HK), and by grant K12-DA00167 from the National Institute of Drug Abuse (HK). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. This study was also supported by a grant from the American Psychological Foundation (SD) and Sigma Xi Grant-in-Aid of Research (SD). We thank Amanda Purcell, Maggie Mae Mell, Elizabeth Reeves, Franziska Goer, Cameron DeLeone, and John Purcell for their assistance with data collection and scoring.
Appendix 1. Positive Qualities Questionnaire
|
Instructions: This questionnaire will ask you to describe some positive events in your life, as well as some positive personal qualities and beliefs. Please respond to each question thoughtfully and honestly, with roughly 2–3 sentences in the space provided. | |
| 1. | Describe something you are very good at. |
| 2. | Describe a quality that makes you unique. |
| 3. | Name someone you admire, and describe the qualities you admire most about them. |
| 4. | Describe a time when you felt love for someone else. |
| 5. | Name some values that you believe are very important, and describe why they are important to you. |
| 6. | Describe something you hope to achieve in the future. |
| 7. | Describe a time when you felt hopeful about the future. |
| 8. | Describe one of your greatest accomplishments. |
| 9. | Describe a time when you felt content or at ease. |
| 10. | If we asked your friends and family about your best qualities, what might they say? |
Note. Each question was presented with an open-ended response box below so the participant could provide the appropriate length requested for each response.
Footnotes
In order to examine the role of potentially confounding variables in our results, we re-ran our analyses with four covariates. Covariates included (1) subthreshold depression symptoms on the day of the scan (IDS-C scores) and (2) scores on the Global Assessment of Functioning (GAF) measure, scored at the first laboratory visit, as groups differed significantly on these measures. In addition, (3) presence vs. absence of antipsychotic medication was included given existing evidence that this type of medication may influence neural reward processing (Abler, Erk, & Walter, 2007), and (4) presence vs. absence of one or more anxiety disorders (including generalized anxiety disorder, panic disorder, specific phobia, social phobia, obsessive-compulsive disorder, and post-traumatic stress disorder) given that a substantial subset of our BD sample (N=7; 29.17%) met criteria for one or more anxiety disorders which are common in (Freeman et al., 2002) and known to influence emotional responding (e.g., Mennin et al., 2005). No participants in our BD sample met criteria for comorbid somatoform disorders (including somatization disorder, pain disorder, hypochondriasis and body dysmorphic disorder) or eating disorders (including anorexia, bulimia, and binge eating disorder) precluding the need to include these diagnostic categories as additional covariates.
For reward anticipation, NAcc ROI analyses revealed that the main effect of Task, which was significant and reflected greater NAcc reactivity to monetary than social reward cues without covariates, was no longer present (p=0.83). All other effects were nonsignificant, consistent with results of this analysis without covariates. OFC ROI analyses revealed that again, the main effect of Task, which was significant and reflected greater OFC reactivity to monetary than social reward cues without covariates, was no longer present (p=0.42). All other effects were nonsignificant, consistent with results of this analysis without covariates. Whole-brain analysis results were generally consistent with those emerging from the same analysis without covariates (see Supplementary Table 1).
For reward receipt, NAcc ROI analyses revealed that the main effect of Task, which was significant and reflected greater NAcc reactivity to monetary than social reward outcomes without covariates, was no longer present (p=0.53). The main effect of Group, which had reflected significantly greater reactivity to reward outcomes in the BD than the HC group, shifted to become a trend-level effect when including covariates (p=0.054). OFC ROI analyses revealed that the main effect of Task, which had reflected greater OFC reactivity to monetary than social rewards, was no longer present (p=0.35). The main effect of Group, which had been a trend-level effect without covariates (p=0.07), emerged reflecting greater OFC reactivity to rewards in the BD (M=0.06, SD=0.12) than in the HC group (M=0.01, SD=0.12), F(1, 43)=5.13, p=0.03, ηp2=0.11.
Whole-brain analyses revealed Group × Task interaction effects which had not been present without covariates, reflecting a greater task-related difference in reactivity (MID>SID) in the HC group than in the BD group in the superior frontal gyrus, bilateral temporal poles, and midline frontal pole (see Supplementary Table 2). Main effects of Group reflecting greater striatal and thalamic reactivity to reward receipt in the BD than in the HC group remained consistent. Additional clusters also emerged, reflecting the same group difference (BD>HC) in diffuse cortical regions (see Supplementary Table 2). In addition, main effects of Task emerged reflecting greater activation in the MID than SID task in two clusters in frontal and occipital cortical regions, and greater activation in the SID than MID task in four including frontal cortical and thalamic regions (see Supplementary Table 2).
References
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. http://dx.doi.org/10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. http://dx.doi.org/10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. NeuroImage. 2006;31(2):790–795. doi: 10.1016/j.neuroimage.2006.01.001. http://dx.doi.org/10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. http://dx.doi.org/10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urosevic S, Bender RE, Wagner CA. Longitudinal predictors of bipolar spectrum disorders: A behavioral approach system (BAS) perspective. Clinical Psychology. 2009;16(2):206–226. doi: 10.1111/j.1468-2850.2009.01160.x. http://dx.doi.org/10.1111/j.1468-2850.2009.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, Abramson LY. High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology. 2012a;121:339–351. doi: 10.1037/a0025877. http://dx.doi.org/10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urosevic S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, Hogan M. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology. 2012b;121(1):16–27. doi: 10.1037/a0023973. http://dx.doi.org/10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro E, Barker GJ. Study design in fMRI: Basic Principles. Brain and Cognition. 2005;60:220–232. doi: 10.1016/j.bandc.2005.11.009. http://dx.doi.org/10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. http://dx.doi.org/10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Bauer MS, McBride L, Shea N, Gavin C, Holden F, Kendall S. Impact of an easy-access VA clinic-based program for patients with bipolar disorder. Psychiatric Services. 1997;48:491–496. doi: 10.1176/ps.48.4.491. http://dx.doi.org/10.1176/ps.48.4.491. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. http://dx.doi.org/10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: Integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience. 2003;18:871–879. doi: 10.1162/jocn.2006.18.6.871. http://dx.doi.org/10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, … Heinz A. Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping. 2010;31(7):958–969. doi: 10.1002/hbm.20909. http://dx.doi.org/10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: “liking”, “wanting”, and learning. Current Opinion in Pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. http://dx.doi.org/10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holer JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJH, Comings DE. The reward deficiency syndrome: A biogenic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. http://dx.doi.org/10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. http://dx.doi.org/10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. http://dx.doi.org/10.1037/0022-3514.67.2.319. [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: Differences between bipolar I and II disorders. American Journal of Psychiatry. 2013;170:533–541. doi: 10.1176/appi.ajp.2012.12020169. http://dx.doi.org/10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioral correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. http://dx.doi.org/10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders. 2013;15:839–854. doi: 10.1111/bdi.12132. http://dx.doi.org/10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collip D, Wigman JT, van Os J, Oorschot M, Jacobs N, Drom C, Thiery E, Peeters F, Wichers M, Myin-Germeys I. Positive emotions from social company in women with persisting subclinical psychosis: Lessons from daily life. Acta Psychiatrica Scandinavica. 2014;129:202–210. doi: 10.1111/acps.12151. http://dx.doi.org/10.1111/acps.12151. [DOI] [PubMed] [Google Scholar]
- Coryell W, Scheftner W, Keller M. The enduring psychosocial consequences of mania and depression. American Journal of Psychiatry. 1993;150:720–727. doi: 10.1176/ajp.150.5.720. http://dx.doi.org/10.1176/ajp.150.5.720. [DOI] [PubMed] [Google Scholar]
- Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, Hietala J, Lawrie SM, Martinot J-L, McIntosh AM, Meisenzahl E, Frangou S. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: A voxel-based meta-analysis of functional magnetic resonance imaging studies. European Neuropsychopharmacology. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. http://dx.doi.org/10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52:1164–1173. doi: 10.1111/j.1469-7610.2010.02374.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. The orbitofrontal cortex and its role in the assignment of behavioural significance. Neuropsychologia. 2011;49(5):984–991. doi: 10.1016/j.neuropsychologia.2011.01.032. http://dx.doi.org/10.1016/j.neuropsychologia.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Dutra SJ, West TV, Impett EA, Oveis C, Kogan A, Keltner D, Gruber J. Rose-colored glasses gone too far? Mania symptoms predict biased emotion experience and perception in couples. Motivation and Emotion. 2014;38:157–165. http://dx.doi.org/10.1007/s11031-013-9363-4. [Google Scholar]
- Ernst LH, Plichta MM, Lutz E, Zesewitz AK, Tupak SV, Dresler T, Ehlis A, Fallgatter AJ. Prefrontal activation patterns of automatic and regulated approach-avoidance reactions - A functional near-infared spectroscopy (fNIRS) study. Cortex. 2013;49:131–142. doi: 10.1016/j.cortex.2011.09.013. http://dx.doi.org/10.1016/j.cortex.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, Masalehdan A, Scott JA, Houck PR, Frank E. Functional impairment in the remission phase of bipolar disorder. Bipolar Disorders. 2005;7:281–285. doi: 10.1111/j.1399-5618.2005.00207.x. http://dx.doi.org/10.1111/j.1399-5618.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- Farmer A, Lam D, Sahakian B, Roiser J, Burke A, O’Neill N, McGuffin P. A pilot study of positive mood induction in euthymic bipolar subjects compared with healthy controls. Psychological Medicine. 2006;36(9):1213–1218. doi: 10.1017/S0033291706007835. http://dx.doi.org/10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-clinician version (SCID-CV), 1997. Washington, DC: American Psychiatric Association Press; 2007. [Google Scholar]
- Freeman MP, Freeman SA, McElroy SL. The comorbidity of bipolar and anxiety disorders: Prevalence, psychobiology, and treatment issues. Journal of Affective Disorders. 2002;68:1–23. doi: 10.1016/s0165-0327(00)00299-8. http://dx.doi.org/10.1016/S0165-0327(00)00299-8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. http://dx.doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: coasting is attenuated in bipolar disorder. Psychological Science. 2010;21(7):1021–1027. doi: 10.1177/0956797610373372. http://dx.doi.org/10.1177/0956797610373372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Jacobs N, Delespaul P, Derom C, Thiery E, van Os J, Wichers M. Meeting risk with resilience: high daily life reward experience preserves mental health. Acta Psychiatrica Scandinavica. 2010;122:129–138. doi: 10.1111/j.1600-0447.2009.01525.x. http://dx.doi.org/10.1111/j.1600-0447.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Bonini N, Sanfey A. Reappraising social emotions: The role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Frontiers in Human Neuroscience. 2013;7:1–12. doi: 10.3389/fnhum.2013.00523. http://dx.doi.org/10.3389/fnhum.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. http://dx.doi.org/10.1016/S0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clinical Psychology & Psychotherapy. 2011a;18(5):356–365. doi: 10.1002/cpp.776. http://dx.doi.org/10.1002/cpp.776. [DOI] [PubMed] [Google Scholar]
- Gruber J. Can feeling too good be bad? Positive emotion persistence (PEP) in bipolar disorder. Current Directions in Psychological Science. 2011b;20(4):217–221. http://dx.doi.org/10.1177/0963721411414632. [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: Rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology. 2011;120(4):956–961. doi: 10.1037/a0023667. http://dx.doi.org/10.1037/a0023667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Purcell J. The tides are changing: Unpacking the nature of positive emotion disturbance. In: Scott RA, Kosslyn SM, editors. Emerging Trends in the Social and Behavioral Sciences. Hoboken, NJ: John Wiley and Sons; in press. [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. http://dx.doi.org/10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Shekhar A, Nurnberger JL, O’Donnel BF, Jr, Hetrick WP. A multimethod investigation of the behavioral activation system in bipolar disorder. Journal of Abnormal Psychology. 2008;177:164–170. doi: 10.1037/0021-843X.117.1.164. http://dx.doi.org/10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Howseman AM, Grootoonk S, Porter DA, Ramdeen J, Holmes AP, Turner R. The effect of slice order and thickness on fMRI activation using multislice echo-planar imaging. Neuroimage. 1999;9:363–376. doi: 10.1006/nimg.1998.0418. http://dx.doi.org/10.1006/nimg.1998.0418. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research. Brain Research Reviews. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. http://dx.doi.org/10.1016/S0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. The American Journal of Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. http://dx.doi.org/10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: a review. Clinical Psychology Review. 2005;25(2):241–262. doi: 10.1016/j.cpr.2004.11.002. http://dx.doi.org/10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Annual Review of Clinical Psychology. 2012;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. http://dx.doi.org/10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, Carver CS. Elevated expectancies among persons diagnosed with bipolar disorder. British Journal of Clinical Psychology. 2009;48:217–222. doi: 10.1348/014466509X414655. http://dx.doi.org/10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, McKenzie G, McMurrich S. Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognitive Therapy and Research. 2008;32:702–713. doi: 10.1007/s10608-007-9158-6. http://dx.doi.org/10.1007/s10608-007-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, Keitner G. Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology. 2000;109(4):721–727. doi: 10.1037//0021-843x.109.4.721. http://dx.doi.org/10.1037/0021-843X.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39:1053–1058. doi: 10.1016/s0896-6273(03)00524-5. http://dx.doi.org/10.1016/S0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: Neural correlates and consequences for choice. Philosophical Transactions of the Royal Society. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. http://dx.doi.org/10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. http://dx.doi.org/10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: Relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–2298. doi: 10.1038/npp.2014.82. http://dx.doi.org/10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Chevallier C, Troiani V, Schultz RT. Social ‘wanting’ dysfunction in autism: Neurobiological underpinnings and treatment implications. Journal of Neurodevelopmental Disorders. 2012;4:10. doi: 10.1186/1866-1955-4-10. http://dx.doi.org/10.1186/1866-1955-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. http://dx.doi.org/10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. American Journal of Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. http://dx.doi.org/10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, … Wessa M. Increased medial orbitofrontal and amygdala activation: Evidence for a systems-level endophenotype of bipolar I disorder. American Journal of Psychiatry. 2012;169(3):316–325. doi: 10.1176/appi.ajp.2011.11050711. http://dx.doi.org/10.1176/appi.ajp.2011.11050711. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. http://dx.doi.org/10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23(3):133–143. doi: 10.1023/A:1010929402770. http://dx.doi.org/10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L, Knutson B, Carstensen LL. Affect dynamics, affective forecasting, and aging. Emotion. 2008;8:318–330. doi: 10.1037/1528-3542.8.3.318. http://dx.doi.org/10.1037/1528-3542.8.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, … Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14(3):249–260. doi: 10.1111/j.1399-5618.2012.01012.x. http://dx.doi.org/10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Young C, Damme K. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: Assessment and treatment implications. Behavioral Research and Therapy. 2014;62:74–87. doi: 10.1016/j.brat.2014.08.011. http://dx.doi.org/10.1016/j.brat.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H, Wagenmakers EJ. Editors’ introduction to the special section on replicability in psychological science: A crisis of confidence? Perspectives on Psychological Science. 2012;7(6):528–530. doi: 10.1177/1745691612465253. http://dx.doi.org/10.1177/1745691612465253. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: entire core and medial shell mapped as substrates for PIT enhancement. The European Journal of Neuroscience. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174. http://dx.doi.org/10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piff PK, Purcell A, Gruber J, Hertenstein MJ, Keltner D. Contact high: Mania proneness and positive perception of emotional touches. Cognition and Emotion. 2012;26:1116–1123. doi: 10.1080/02699931.2011.633987. http://dx.doi.org/10.1080/02699931.2011.633987. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biological Psychiatry. 2008;64(2):162–168. doi: 10.1016/j.biopsych.2007.12.001. http://dx.doi.org/10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. http://dx.doi.org/10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GB, Freedlander JM, Katz EC, Dai C, Chen C. Impulsivity links reward and threat sensitivities to substance use: A functional model. Frontiers in Psychology. 2014;5:1–37. doi: 10.3389/fpsyg.2014.01194. http://dx.doi.org/10.3389/fpsyg.2014.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. http://dx.doi.org/10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. http://dx.doi.org/10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. http://dx.doi.org/10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sugam JA, Carelli RM. Rolling the dice: The importance of mesolimbic dopamine signaling in risky decision making. Neuropsychopharmacology Reviews. 2013;38:248. doi: 10.1038/npp.2012.173. http://dx.doi.org/10.1038/npp.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Frank E, Bowden CL, Colom F, Ghaemi SN, Yatham LN, … Berk M. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disorders. 2009;11:453–473. doi: 10.1111/j.1399-5618.2009.00726.x. http://dx.doi.org/10.1111/j.1399-5618.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- Tondo L, Isacsson G, Baldessarini R. Suicidal behavior in bipolar disorder: risk and prevention. CNS Drugs. 2003;17:491–511. doi: 10.2165/00023210-200317070-00003. http://dx.doi.org/10.2165/00023210-200317070-00003. [DOI] [PubMed] [Google Scholar]
- Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. http://dx.doi.org/10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. http://dx.doi.org/10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. The economic burden of bipolar disease. Journal of Clinical Psychiatry. 2000;61(Supp 13):38–41. [PubMed] [Google Scholar]
- Woolrich M. Multilevel linear modeling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. http://dx.doi.org/10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. http://dx.doi.org/10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard PM, Matthews PM, Smith SM, editors. Functional MRI: An introduction to methods. New York, NY: Oxford University Press; 2001. http://dx.doi.org/10.1093/acprof:oso/9780192630711.003.0014. [Google Scholar]
- Wrase J, Kahnt T, Schlagenhaut F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. http://dx.doi.org/10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences, USA. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. http://dx.doi.org/10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. http://dx.doi.org/10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.