Abstract
The phagocytosis and destruction of pathogens and dead cells by macrophages is important for innate immunity and tissue maintenance. Multiple Rab family GTPases engage effector molecules to coordinate the early stages of phagocytosis, which include rapid changes in actin polymerization, membrane phospholipids, trafficking and the activation of receptors. Defining the spatiotemporal, sequential recruitment of these Rabs is critical for insights into how phagocytosis is initiated and coordinated. Here, we screened GFP-tagged Rabs expressed in fixed and live cells to identify and stratify those recruited to early phagocytic membranes at stages defined by phospholipid transitions. We propose a sequence of Rabs 35, 13, 8a, 8b, 27a, 10, and 31 that precedes and accompanies phagocytic cup closure, followed after closure by recruitment of endosomal Rabs 5a, 5b, 5c, 14, and 11. Reducing the expression of individual Rabs by siRNA knockdown, notably Rabs 35 and 13, disrupts phagocytosis prior to phagocytic cup closure, confirming a known role for Rab35 and revealing anew the involvement of Rab13. The results enhance our understanding of innate immune responses in macrophages by revealing the sequence of Rabs that initiates phagocytosis.
KEYWORDS: macrophage, phagocytosis, phosphoinositides, Rabs
Introduction
Phagocytosis is employed by cells to ingest large particles specifically for their destruction, including cellular debris, dead cells, foreign objects, and pathogens. Macrophages are ‘professional’ phagocytes that reside in most tissues and interstitial spaces of the body, where they are charged with maintaining surveillance for pathogens as part of the innate immune system.1 The macrophage cell surface is one of the most dynamic cellular environments known, housing a variety of cell surface protrusions and a multitude of trafficking pathways that sustain a high level of constitutive macropinocytosis, endocytosis, and exocytosis to allow induced trafficking through pathways such as phagocytosis. Initiating the engulfment and internalization of particles or pathogens during phagocytosis relies on the coordinated polymerization of cortical actin, the protrusion of pseudopod arms or ruffles, and changes in the phospholipid composition of the plasma membrane.2,3 The surface-membrane pseudopods or ruffles that go on to form phagocytic cups are often also the first contact points between the cell and external particles or pathogens. Thus a second key function for these early phagocytic membranes is to house the receptors and signaling molecules that discern and respond to the nature of particles or pathogens, and then prime the immune system accordingly.4 During phagocytosis, the particles or pathogens are internalized into a sealed phagosome, which is then modified through fusion of successive endosomes and eventually lysosomes to form the degradative environment of the phagolysosome that is critical for pathogen killing, antigen presentation, and waste disposal.1,5 Several pathogens that impact significantly on human health have learned to subvert the processes and machinery of phagocytosis, highlighting the need to better understand this key cellular pathway.5
The early stages of phagocytic cup formation are demarked by the accumulation of F-actin, followed by disassembly of the actin upon closure of the early phagosome.2 An accompanying event is the transition of membrane phosphoinositides (PI) from the resident phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) of the plasma membrane, which supports actin polymerization, to PI(3,4,5)P3 via the action of phosphoinositide 3-kinases (PI3Ks).3 The action of class I PI3Ks is also subsequently needed for phagosome closure.6 PI(3,4,5)P3 provides an important platform for protein sorting and for recruiting signaling molecules, and thus, this PI has regulatory roles in mediating many of the receptor-mediated cell responses that accompany phagocytosis including, cell proliferation, chemotaxis, differentiation, survival, metabolic changes, and immune responses.3,4,7 A well-characterized PI(3,4,5)P3-dependent signaling pathway universal to these processes is the recruitment of phosphoinositide-dependent kinase 1 (PDK1) for phosphorylation and activation of protein kinase B (Akt).3 Thus, the transitions of membrane phospholipid composition help to generate both the phagocytic process and associated receptor signaling.
Phagocytosis employs a vast and complex machinery of regulatory proteins. Members of multiple small GTPase (Rho, Arf, and Rab) and heterotrimeric G protein families, are recruited to phagocytic membranes. Rab GTPases (>60 in mammalian cells) are deployed on membranes throughout cells where they engage pleiotropic effectors to bring about many functions.8 Previous proteomic and localization studies have identified many of the Rabs found on phagosomes during the uptake of different pathogens and particles, and roles for some of these Rabs in the aforementioned functions have been described (summarized in 9). For instance, there are well-known roles for endosomal Rabs in the trafficking and fusion of successive endosomes with the maturing phagosome, while coordinating actin polymerization for phagocytic cup formation involves Rab35, together with Arf6.10,11 The inactivation or knockdown of individual Rabs can halt phagosome formation or block maturation and pathogen killing, highlighting the essential nature of these GTPases in phagocytosis.9
The task of coordinating the complex, overlapping functions inherent in the early stages of phagocytosis requires a tightly linked, temporal sequence of Rabs and accessory proteins – a Rab cascade – to recruit sequential effectors. The Rab cascade model proposes that 2 or more Rabs in sequence can be coordinated by counter-currents of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which recruit and activate Rabs and then deactivate them, respectively.8,12 Thus the GTP-bound form of one Rab binds and recruits the GEF for the next Rab, and so on. Rabs can also be linked in cascades through their effectors or tethering factors.13 Examples of Rab cascades have been described in secretory, endocytic, and phagocytic pathways.12,14,15 Our focus in this study was to screen multiple Rabs to define a linked sequence of these GTPases required for formation and function of phagocytic cups and early phagosomes. The results presented here reveal a sequence of known and novel Rabs that form part of a putative cascade essential for the early stages of FcγR-mediated phagocytosis in macrophages.
Materials and methods
Reagents
Bacterial lipopolysaccharide (LPS), purified from Salmonella enterica serotype Minnesota Re 595, was purchased from Sigma Aldrich (St. Louis, MO, USA). For phagocytosis, human IgG (Invitrogen) was conjugated to 3-μm latex beads (Sigma Aldrich), and sheep red blood cells (Australian Ethical Biologicals, VIC, Australia) were conjugated with rabbit anti-sheep IgG (IgG-sRBCs, Sigma Aldrich). Alexa594-conjugated phalloidin and CellMask™ Orange was purchased from Invitrogen. All other chemicals and reagents were from Sigma Aldrich (Castle Hill, NSW, Australia).
Constructs
Mouse GFP-tagged Rab constructs were made in our laboratory or kindly provided by Mitsunori Fukuda (Tohoku University, Japan). Phosphoinositide probes (GFP-PLCδ1-PH, GFP-Akt-PH; kindly provided by Frederic Meunier, University of Queensland, Brisbane, Australia) were subcloned into a pmCherry-C1 vector (Clontech).
Cell culture, DNA and siRNA transfection
The mouse RAW 264.7 macrophage cell line was cultured in RPMI medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Thermo Trace) and 2 mM L-glutamine (Invitrogen) in humidified 5% CO2 at 37°C as previously described.16 For transient expression of cDNA constructs, cells at 50% confluency were transfected using Lipofectamine 2000 or Lipofectamine 3000 (Invitrogen) according to the manufacturer's instructions. Cells were typically used for experiments 18 h after transfection. Rab8a (MSS237261, MSS237262, MSS275712), Rab8b (MSS215069, MSS215070, MSS215071), Rab10 (MSS276679, MSS276680, MSS276681), Rab13 (MSS250415, MSS250416, MSS250417), Rab31 (MSS272451, MSS272452, MSS272453), and Rab35 (MSS233945, MSS233946, MSS233947) were silenced using the Stealth siRNA Primer Set from Life Technologies. Non-targeting siRNA was used as a control. Lipofectamine RNAiMAX was used to transfect RAW 264.7 cells with siRNA according to the manufacturer's instructions (Life Technologies).
Phagocytic assay
Human IgG-conjugated 3-μm latex beads were prepared according to the manufacturer's protocol (Sigma), centrifuged onto cells at 4°C, and incubated at 37°C to synchronize and initiate phagocytosis. Phagocytosis was allowed to proceed for a stipulated period and then stopped by adding 4% paraformaldehyde to fix the cells, and externally accessible beads were immunolabeled with goat anti-human IgG-Cy3. An image-based assay, previously described,17 was used to quantify beads at early stages of phagocytosis. Externally applied anti-IgG was used to differentiate beads that remained externally accessible from those that were fully internalized in closed phagosomes.
Immunofluorescence, fluorescence microscopy, and live cell imaging
Immunofluorescence staining was performed as previously described.18 Briefly, cells were fixed with 4% paraformaldehyde for 30 min and permeabilized using 0.1% Triton X-100 for 5 min, then incubated with primary and secondary antibodies. Coverslips were mounted in ProLong Gold reagent (Life Technologies). Epifluorescence images were taken with a 12-Mp differential contrast camera (DP71; Olympus) on an upright microscope (BX-51; Olympus) fitted for a 60X NA 1.35 oil objective using the associated DPController software (version 2.1; Olympus). For live cell experiments, RAW 264.7 macrophages were cultured on glass-bottom, 35-mm dishes (MatTek). Cells were maintained in CO2-independent medium in a 37°C incubation chamber before and during live cell imaging. Live and fixed cell imaging were also performed using a Personal DeltaVision Olympus IX71 inverted widefield deconvolution microscope equipped with Olympus U-apochromat 40X/1.35 oil DIC plan-apochromat 60X/1.42 oil DIC UPLS-apochromat 100X/1.40 Oil DIC and a 120 W xenon arc lamp. Images were captured using a Roper Coolsnap HQ2 monochrome camera. Imaging analysis of all other data was performed using ImageJ software (version 1.49; National Institutes of Health, Maryland, USA) and Adobe Photoshop CS6.
Real-time PCR
Total RNA was prepared using RNeasy mini-kits (Qiagen, Valencia, CA) and then reverse transcribed to cDNA using 1 μg of total RNA, Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA), and an oligo-dT primer. Gene expression was quantified by real-time PCR using SYBR Green PCR master mix (Applied Biosystems) according to previous studies,19 using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Real-time primers used for detecting Rab knockdown are as follows (forward/reverse sequences):
Rab8a (CGATCGCCTGAAGTGTGTCT/ACAATAGTGG-ATGCTCATTA)
Rab8b (CGGGAGCTCAGCTCTATCCT/CATATGCTCG-ATCTGATGTG)
Rab10 (CGGTGCAATCAAGTGTGTCT/CCAATAGTGG-TCGCTACGTC)
Rab13 (CGCGAGCTCACGCTAGCACT/GCATTAGTGG-AGTCGATCTA)
Rab35 (CGAGAGTTCACATATACACT/GCAATAGTGC-GTCTGATGTA)
HPRT (GGAGCAGTAGCACCTCCT/CATAACCTGGTT-CATCATCGC).
cDNA levels during the linear phase of amplification were normalized against the HPRT gene.
Statistical analysis
Statistics were calculated using Graphpad Prism version 7.0 (Graphpad Software, San Diego, CA). For all experiments in this study, Student's t-tests (Shapiro-Wilk test) were used unless stated otherwise. One-way ANOVA was used for post-test analysis of multiple comparisons by the Dunett's method *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Results
Defining and measuring early-stage phagocytosis and Rab recruitment
The phagocytosis of opsonized particles (or pathogens) occurs in a series of defined stages. Several parameters can be used to track these stages, including the transition of membrane PIs from PI(4,5)P2 in the plasma membrane and initial ruffles, to PI(3,4,5)P3 which gathers at the base of the phagocytic cup and is gradually enriched as the phagosome seals and internalizes,2,4 as depicted in Figure 1A.
Figure 1.
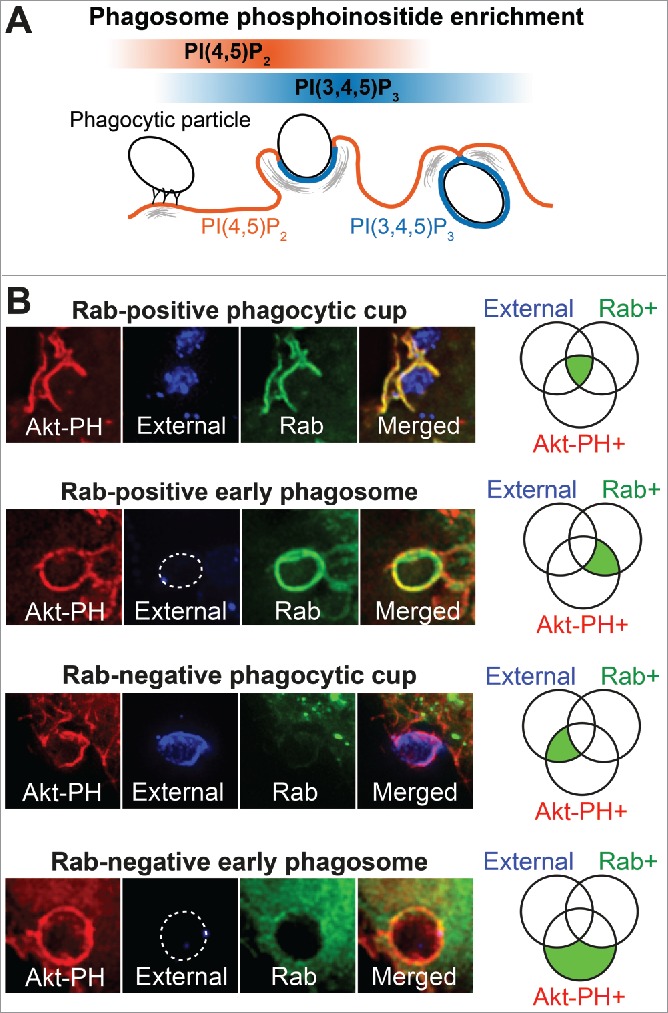
Defining and measuring early-stage phagocytosis and Rab recruitment (A) Schematic diagram of the progression of FcγR-mediated phagocytosis showing the classical enrichment of PI(4,5)P2 followed by PI(3,4,5)P3 on phagocytic cups and phagosomes. (B) Representative examples of the presence or absence of Rabs on phagocytic cups and phagosomes. Application of an external antibody against IgG-sRBC can distinguish the open phagocytic cup (blue signal in External) from the closed phagosome (loss of blue signal). These parameters correspond to a Venn diagram on the right where percentages of phagosome populations are quantified and classified into their corresponding areas in the Venn diagram (green shadings).
In order to identify the Rabs recruited to phagocytic cups and early phagosomes, we performed a colocalization screen by transiently co-expressing individual GFP-tagged Rabs with mCherry-Akt-PH (Akt-PH probe) as a marker for PI(3,4,5)P3 (and PI(3,4)P2). The Akt-PH probe typically labels phagocytic cups and early (newly sealed) phagosomes. Under the conditions used here and previously,20 coexpression of GFP-Rabs with lipid probes did not alter the membrane recruitment or localization of individual Rabs compared to cells expressing Rabs alone or with a general membrane lipid dye (Fig. S1). Cells were fixed for staining 10 mins after adding IgG-opsonized beads to initiate FcγR-mediated phagocytosis. To demark phagosome closure we applied an ‘external’ anti-IgG, which can only access the IgG-coated surface-attached or partially engulfed beads prior to cup closure.17 Representative images (Fig. 1B) show how the different staining patterns are interpreted to determine which Rabs are recruited to early phagocytic cups, before or after cup closure, and to eliminate those that are not recruited during this process. A total of 21 mammalian Rabs were thus expressed and screened by imaging of fixed, stained cells. Typically, these snapshot images of fixed cells capture and depict multiple beads per cell, which can be at different stages of internalization (See Figs. 2, 4 and 7).
Figure 2.
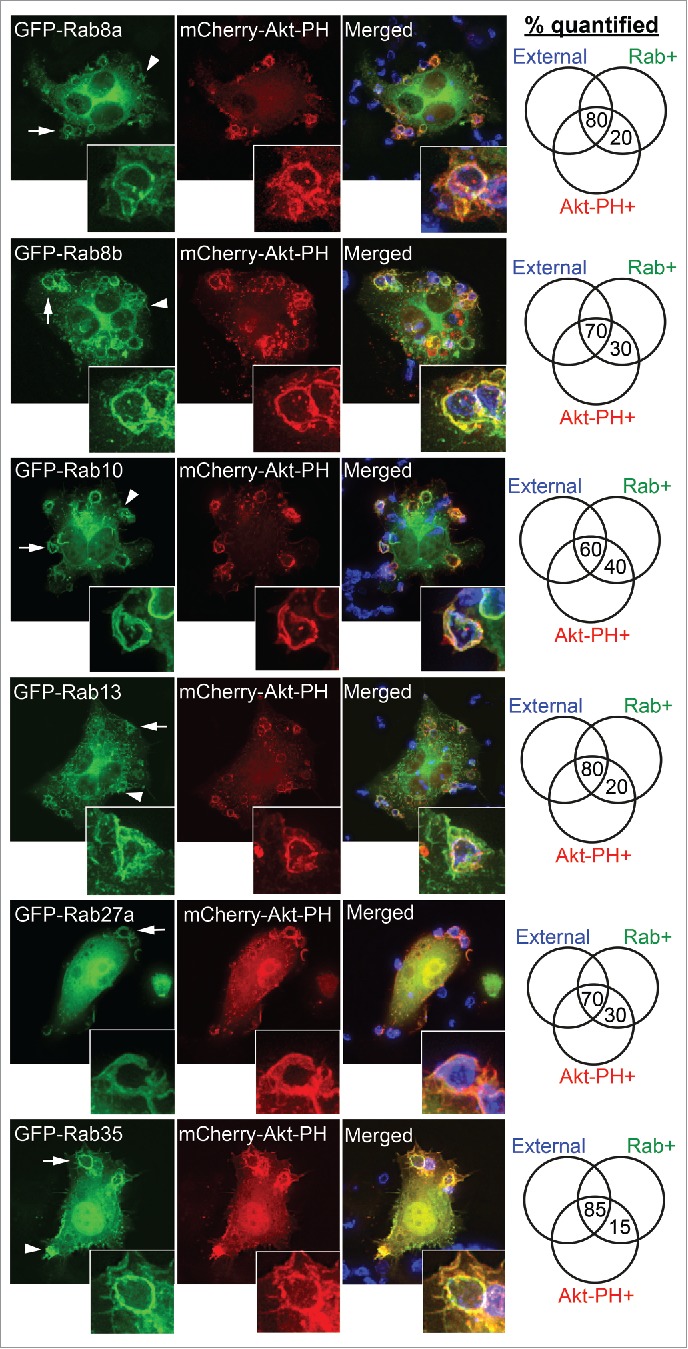
Rab GTPases recruited to PI(3,4,5)P3-enriched phagocytic cups prior to closure. RAW 264.7 macrophages were cotransfected to express GFP-tagged Rab8a, Rab8b, Rab10, Rab13, Rab27a, or Rab35 and mCherry-Akt-PH. Macrophages were exposed to IgG-opsonized sRBCs (IgG-sRBC) for 5 min and fixed. Exposed IgG-sRBC surfaces were probed with Alexa405 anti-human IgG. Representative images show examples of GFP-Rab recruitment to phagocytic cups depicted by enrichment of mCherry-Akt-PH and exposed IgG-sRBC. Insets correspond to arrows depiting representative phagocytic cups. Arrowheads depict recruitment of GFP-Rabs to membrane ruffle domains of the macrophage. Phagocytic cups and phagosomes counted per classification are expressed as percentages and recorded in the Venn diagram. At least 40 phagosomes from 5 or more different cells were quantified in each experiment.
Figure 4.
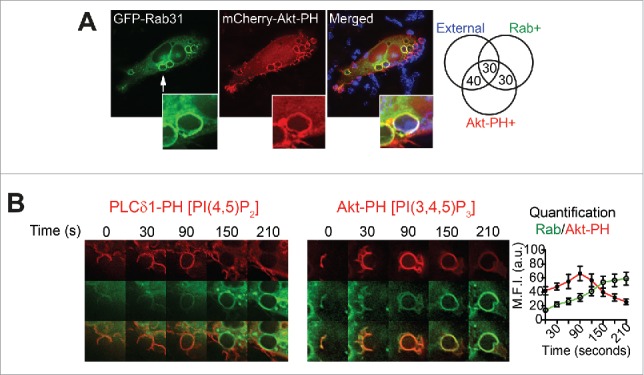
Rab31 is recruited upon phagocytic cup closure. (A) RAW 264.7 macrophages were cotransfected to express GFP-Rab31 and mCherry-Akt-PH, and macrophages were exposed to IgG-sRBC for 5 min and fixed. Exposed IgG-sRBC surfaces were probed with Alexa405 anti-human IgG. A representative image of a transfected macrophage is shown. Phagocytic cups and phagosomes counted per classification are expressed as percentages and recorded in the Venn diagram on the right. At least 40 phagosomes from 5 different cells were quantified. (B) Live cell imaging of a GFP-Rab31- and mCherry-Akt-PH cotransfected macrophage shows enrichment of both proteins on the phagosome. The quantification of maximum fluorescence intensities shows that Rab31 accumulates after the peak of PI(3,4,5)P3. Data are shown as mean ± SEM, n = 6 phagosomes from 3 different cells.
Figure 7.
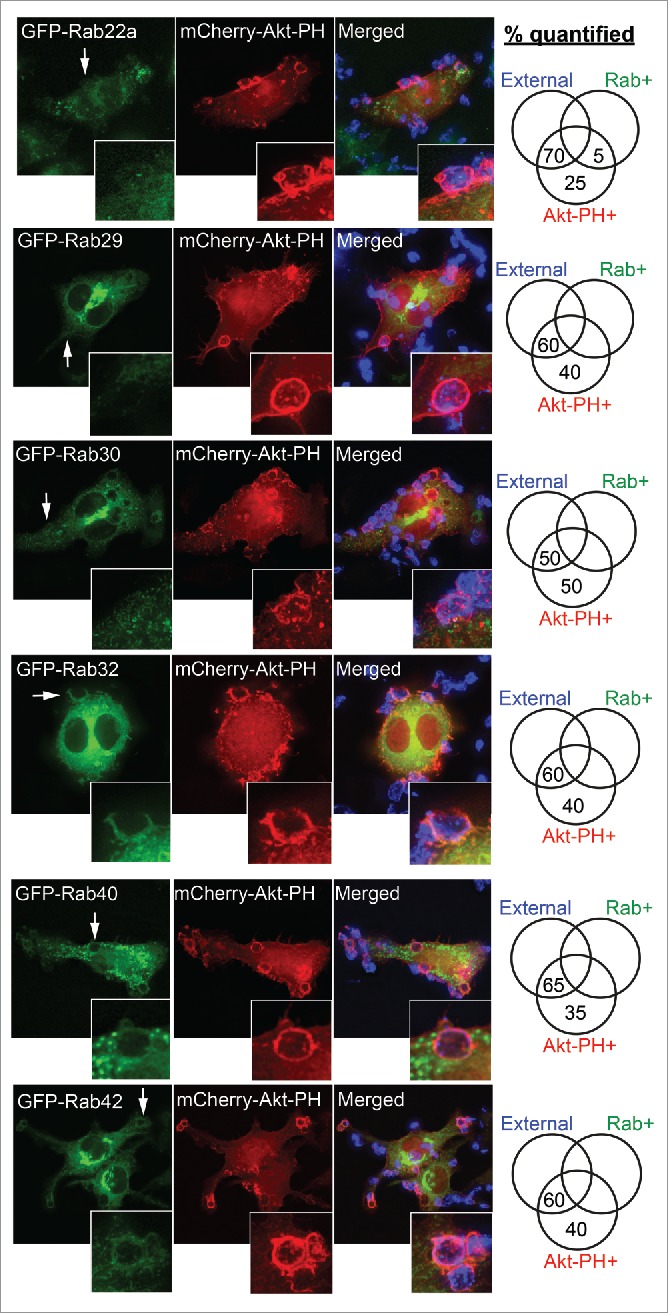
Rab GTPases not recruited during early stages of phagocytosis. RAW 264.7 macrophages were cotransfected to express GFP-tagged Rab22a, Rab29, Rab30, Rab32, Rab40, or Rab42 and mCherry-Akt-PH. Macrophages were exposed to IgG-sRBCs for 5 min and fixed with 4% paraformaldehyde to halt phagocytosis. Exposed IgG-sRBC surfaces were probed with Alexa405 anti-human IgG. Representative images of each Rab were chosen based on no observable GFP-Rab recruitment to phagocytic cups or phagosomes, detected by enrichment of mCherry-Akt-PH. Phagocytic cups and phagosomes counted per classification are expressed as percentages and recorded in the Venn diagrams on the right. At least 40 phagosomes from 5 different cells were quantified.
Rabs recruited to phagocytic cups prior to cup closure
The results represented in Figure 2 depict several GFP-Rabs (Rabs 8a, 8b, 10, 13, 27a, 35) that are first recruited to early-stage, ‘open’, PI(3,4,5)P3-enriched phagocytic cups, based on costaining the majority of phagocytic events with a GFP-Rab, an external antibody to detect beads, and Akt-PH. Triple-positive labeling (external, Akt-PH+, Rab+) was detected in 85% of GFP-Rab35 phagocytic cups, 80% of Rab13 and Rab8a cups, 70% of Rab8b and Rab27a cups, and 60% of Rab10 cups. For this group of Rabs, the remaining phagocytosed beads are visible in closed (no external labeling) structures that are still Akt-PH positive, consistent with retention of these Rabs on early phagosomes soon after closure. In addition, enrichment of these Rabs on phagocytic cups appears to coincide with PI(3,4,5)P3 accumulation, since separate labeling of Akt-PH and GFP was rarely seen.
Live cell imaging was then used to further dissect the temporal recruitment of selected Rabs during phagocytosis (same group as shown in Fig. 2) in cells coexpressing mCherry-PLCδ1-PH (PLC-PH probe for PI(4,5)P2) or Akt-PH (Fig. 3). Representative time-lapse images track GFP-Rab recruitment after synchronizing the events based on initial contact of IgG-sRBCs with the macrophage cell surface (at 0 sec). As diagrammed in Figure 1A, the PLC-PH probe initially labels phagocytic cups and the surrounding ruffles of the plasma membrane, but it was depleted entirely from the membrane of the sealed and internalized phagosome (Fig. 3A). In contrast, the Akt-PH probe is recruited to the open phagocytic cups and then retained on the sealed and internalized phagosomes, only beginning to disappear from them by the ∼ 4 min endpoint of these movies (Fig. 3B).
Figure 3.
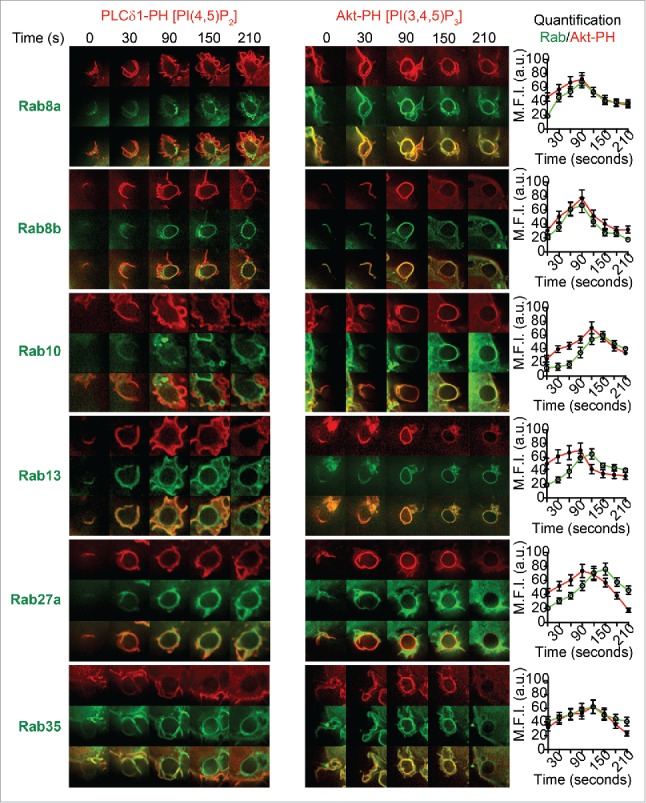
Spatiotemporal recruitment of Rabs during enrichment of PI(4,5)P2 and PI(3,4,5)3 at the phagocytic cups in live macrophages. RAW 264.7 macrophages were cotransfected to express GFP-tagged Rab8a, Rab8b, Rab10, Rab13, Rab27a, or Rab35 and either mCherry-PLC-PH (A) or mCherry-Akt-PH (B). Representative time lapse images are shown from 0 s to 210 s where 0 s represents the IgG-sRBC making contact with the cell surface. The quantification of maximum fluorescence intensities from GFP-Rabs and mCherry-Akt-PH are shown on the right, revealing the enrichment levels of these proteins throughout phagocytosis. Data are shown as mean ± SEM, n = 6 phagosomes from 3 different cells.
We particularly noted that Rabs 35 and 13 are recruited with similar timing to the earliest stages of phagocytic cup formation under the conditions in this study. Both are enriched on the PLC-PH-labeled ruffles that first begin to form the phagocytic cup (<30 s) and, at later time points (>150 s), these Rabs persist on the PLC-PH-negative inner leaflet of the internalized phagosome (Fig. 3A). A line scan of representative GFP-Rab13 localization shows its initial recruitment on PLC-PH-positive membranes followed by separation from this probe on membranes after cup closure. Though the peak fluorescence of both Rab35 and 13 coincided with Akt-PH on phagosomal membranes, they persisted after dissociation of PI(3,4,5)P3. (Fig. 3B) (Low levels of Rab35 can still be observed after PI(3,4,5)P3 dissociation).
A second subset of Rabs recruited prior to cup closure, Rabs 8a, 8b, and 10, also first appear on the PLC-PH-positive ruffles and phagocytic cups, but then persist on the early phagosomes after diminution of the PLC-PH labeling. A line scan of representative Rab8a labeling highlights, as an additional feature of this subgroup of Rabs, concentration on tubules and macropinosomes forming from and fusing with the remodeling phagosomes (Fig. 3A). Rabs 8a, 8b, and 10 are then enriched on Akt-PH positive early cups, with their enrichment on phagosomal membranes mirroring that of PI(3,4,5)P3 (Fig. 3B). Rab27a is present although less distinct on PLC-PH-negative phagosomes, and it persists mainly on membrane tubules of the Akt-PH-enriched phagosomes.
These observations confirm that this group of Rabs (Rabs 8a, 8b, 10, 13, 27a and 35) is recruited before cup closure, and among them we can distinguish at least 2 patterns of recruitment and localization. Typically these Rabs recruited before cup closure persist on the subsequent phagosome specifically during the duration of PI(3,4,5)P3 and PI(3,4)P2, based on Akt-PH labeling. By comparing multiple individual movies for each Rab, the sequence of phagosome recruitment we propose begins with Rab35, followed by Rab13, Rabs 8a, 8b, and 27a, and finally by Rab10.
Rab31 is recruited just prior to cup closure
We previously showed that Rab31 is recruited to early-stage phagosomes, where it participates in FcγR signaling.20 Here we examined its temporal recruitment in fixed and live cells for comparison with other Rabs under the same conditions. In fixed cells, GFP-Rab31 phagosomes and phagocytic cups are colabeled in roughly equal proportions with the Akt-PH probe and with external antibody, indicating that GFP-Rab31 is recruited just prior to cup closure (Fig. 4). However, many of the PI(3,4,5)3-enriched phagocytic cups are negative for GFP-Rab31, suggesting that Rab31 is recruited later than the other pre-closure Rabs. In live cells, Rab31 recruitment begins upon PI(4,5)P2 dissociation and it persists on early phagosomes during and after accumulation of PI(3,4,5)P3 (Fig. 4). This distribution shows that, while Rab31 begins to appear early on phagocytic cups, it peaks and persists later than the previous cascade of Rabs, defining yet another temporal point for recruitment.
Rabs recruited to early phagosomes after closure
Among those Rabs screened in this study, some are recruited only after cup closure to early phagosomes still enriched with PI(3,4,5)P3 (no external staining, but Rab+ and Akt-PH+) (Fig. 5). The Rabs in this category include canonical early endosomal Rab5a, Rab5b, Rab5c, and Rab14, the recycling endosome-associated Rab11a, as well as Rabs 20 and 33a. Rab33a does appear on a minor proportion of phagosomes that are still open to external antibody staining, suggesting it is recruited right around the time of phagosome sealing. All of these Rabs appear as punctate labeling around phagosomes in the fixed cells (Fig. 5), consistent with their presence on fusing (or departing) early and recycling endosomes that contribute to phagosome remodeling and subsequent maturation.
Figure 5.
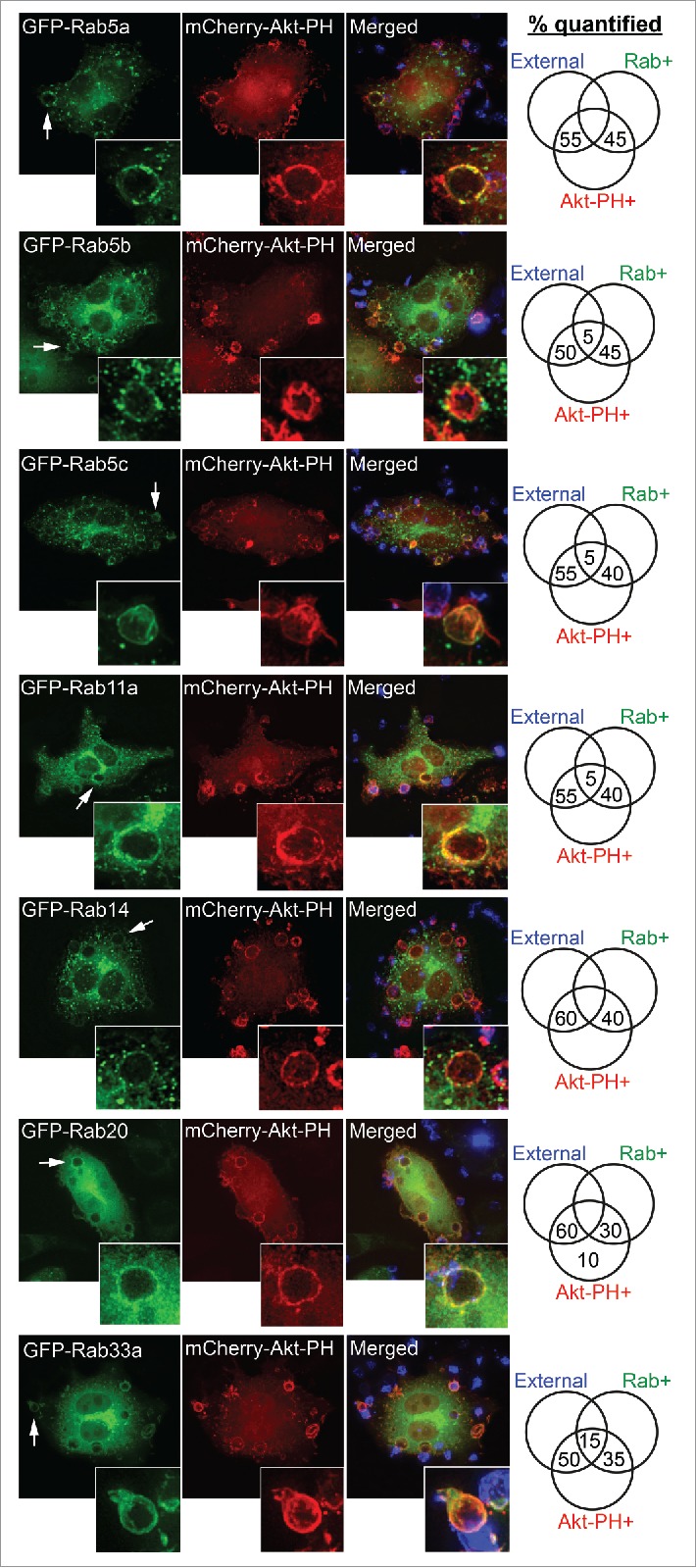
Rab GTPases recruited to PI(3,4,5)P3-enriched early phagosomes after closure. RAW 264.7 macrophages were cotransfected to express GFP-tagged Rab5a, Rab5b, Rab5c, Rab11a, Rab14, Rab20, or Rab33a and mCherry-Akt-PH. Macrophages were exposed to IgG-opsonized sRBCs (IgG-sRBC) for 5 min and fixed. Exposed IgG-sRBC surfaces were probed with Alexa405 anti-human IgG. Representative images show examples of GFP-Rab recruitment to phagocytic cups/phagosomes. Insets correspond to arrows indicating representative phagocytic cups/phagosomes. Phagocytic cups and phagosomes counted per classification are expressed as percentages and recorded in the Venn diagrams on the right. At least 40 phagosomes from 5 or more cells were quantified.
In live cells co-expressing the PI probes (Fig. 6), GFP-Rab5a, Rab5b, and Rab5c are apparent on the membrane of closed phagosomes after PI(4,5)P2 has converted to PI(3,4,5)P3, with a clear separation of the PLC-PH probe on the outer plasma membrane from the GFP-Rab5 labeling on the inner closed phagosome. GFP-Rab20 overlaps with some of the PLC-PH probe on the plasma membrane ruffles in live cells, but its labeling of the phagosomes is weaker than for the Rab5 isoforms. The Rab5 isoforms all accumulate on Akt-PH-labeled phagosomes and the intensity measurements quantify their continued presence and accumulation even after loss of PI(3,4,5)P3, consistent with their roles in ongoing maturation of PI3P-enriched phagosomes. Taken together, these results show that the endosomal Rabs form a cohort recruited generally after closure of the phagocytic cups.
Figure 6.
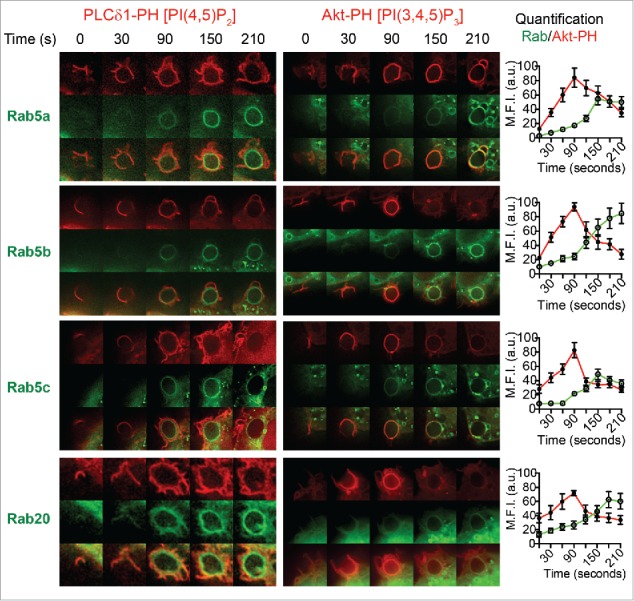
Spatiotemporal recruitment of Rabs after enrichment of PI(4,5)P2 and PI(3,4,5)3 on phagosomes in live macrophages. RAW 264.7 macrophages were cotransfected to express GFP-tagged Rab5a, Rab5b, Rab5c, or Rab20 and either mCherry-PLC-PH (left panels) or mCherry-Akt-PH (right panels). Representative time lapse images of each sample are shown from 0 s to 210 s where 0 s represents the first instance where the plasma membrane contacts with the IgG-sRBC, detected by enrichment of mCherry-PLC-PH/Akt-PH at the base of the phagocytic cup. Quantification of fluorescence intensities for GFP-Rab in relation to mCherryAkt-PH throughout phagocytosis is shown in the graphs on the right. Fluorescence is plotted using the mean fluorescence intensity around the circumference of the IgG-sRBC from the GFP and mCherry channels and data are shown as mean ± SEM, n = 6 phagosomes from 3 different cells.
Rabs not recruited during early stages of phagocytosis
The absence of labeling on PI(3,4,5)P3-enriched, Akt-PH-positive phagocytic cups or early phagosomes in fixed cells serves to define the GFP-Rabs that are not recruited during early stage phagocytosis (Fig. 7). This cohort includes Rabs 22a, 29, 30, 32, 40, and 42. Though Rab32 and Rab42 were observed on the phagosome, their enrichment on phagosomes is similar to their cytosolic distribution in cells. GFP-Rabs 29, 30, and 42 produce distinct labeling of perinuclear membranes, confirming that these Rabs can associate with membranes under the conditions used, but they are not localized on phagosomes. Rab40 is found on puncta throughout the cells, some of which abut the phagosomes, however Rab40 is absent from the phagosomal membranes themselves.
Rab35 and 13 are required for efficient phagocytosis
The Rabs recruited prior to phagocytic cup closure were selected for functional analysis, using siRNAs to target and deplete individual Rabs. siRNAs designed to target Rab8a, Rab8b, Rab10, Rab13, Rab31, and Rab35 depleted their respective mRNAs by > 80% (Fig. 8A). Control and knockdown macrophages transiently co-expressing PLC-PH and Akt-PH probes were imaged as shown in Figure 8B. Control cells show a patent transition of PI(4,5)P2 to PI(3,4,5)P3, closure of phagocytic cups (at ∼60 s), and depletion of PI(3,4,5)P3 (beginning at ∼150 s). Wortmannin, which inactivates Class I-PI3Ks, is known to block PI(3,4,5)P3 acquisition and phagocytic cup closure6,21 and here we treated cells with wortmannin as a positive control for disrupting phagocytosis and as a reference point for comparison with Rab knockdown cells. Classically we find in wortmannin-treated cells, that the PI(4,5)P2-labeled phagocytic cups form around the base of IgG-sRBCs, but instead of closing they continue to ‘cradle’ the particles in open phagocytic cups that do not accumulate PI(3,4,5)P3.
Figure 8.
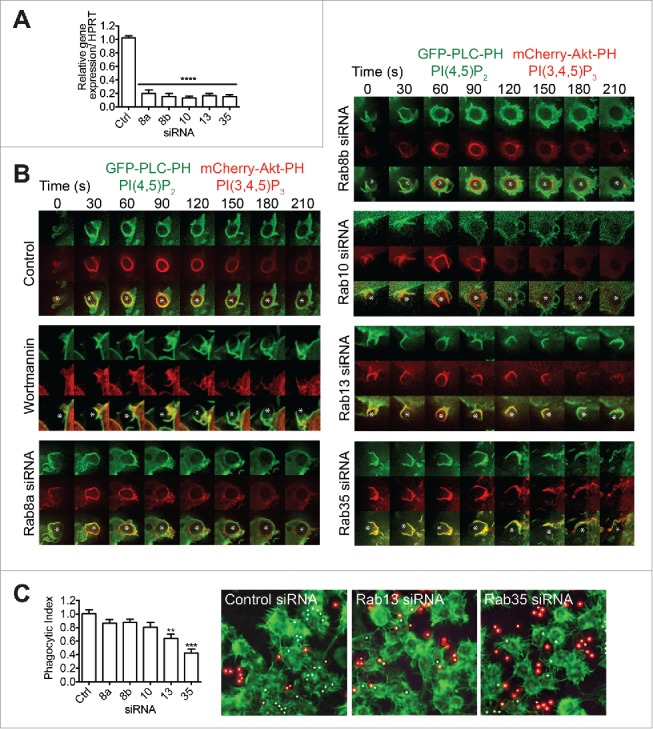
Depletion of early recruited Rabs differentially regulates FcγR-mediated phagocytosis. RAW 264.7 macrophages were treated with pooled siRNAs to target each of the following Rabs - Rab8a, Rab8b, Rab10, Rab13, or Rab35. Cells were siRNA treated for 48h. (A) Quantitative real-time PCR detection of Rab mRNA showing ˜80% depletion of Rab8a, Rab8b, Rab10, Rab13, Rab31, and Rab35 in respective samples. Graph values represent mean ± SEM, n = 3. (B) Cells were cotransfected to express both GFP-PLC-PH and mCherry-Akt-PH. Phagocytosis of IgG-sRBCs (asterisk) was imaged in live cells and representative time-lapse (0–210 s) images are shown. Control cells and cells pre-treated with wortmannin demonstrate normal and disrupted progression of phagocytosis. Cells treated with siRNAs to deplete specific Rabs and then examined in the same regime are then shown. (C) siRNA-treated macrophages were allowed to phagocytose IgG-beads for 15 min then fixed. A phagocytosis assay was used to determine a phagocytic index (proportion of fully internalized beads in each test cell line relative to control). Graph shows mean ± SEM. At least 200 phagosomes from 4 fields of view and 3 individual experiments were quantified. Representative images are shown for knockdown of Rab13 and Rab35. Cells are labeled with phalloidin488 (green); red indicates the externally exposed beads stained with anti-IgG and white only beads are fully internalized
In cells depleted of Rab8a, Rab8b, or Rab10, phagocytosis proceeds, with a transition from PI(4,5)P2 to PI(3,4,5)P3 and sealing of the phagosome, although this is delayed after depletion of Rab8b or Rab10, but not Rab 8a (Fig. 8B). We thus conclude that Rabs 8a, 8b, and 10 are not essential for membrane closure. In contrast, depletion of Rab13 or Rab35 dramatically perturbs the progression of phagocytosis of IgG-sRBCs; in these cells, phagocytic cups can form, but do not progress to closure. Unlike wortmannin-treated cells, PI(3,4,5)P3 accumulates in the aborted cups, although this is more pronounced after loss of Rab35 than in Rab13 depletion. This suggests that loss of Rab35 or 13 does not directly inhibit PI3K action, but that another facet of cup closure is perturbed in the absence of either of these 2 Rabs.
We next quantified early-stage phagocytosis using an image-based assay to quantify surface attached and fully internalized IgG opsonized beads in cells after knockdown of individual Rabs17 (Fig. 8C). Compared to control cells, knockdown of Rab8a, Rab8b, or Rab10 did not significantly reduce the phagocytic index. However depletion of Rab13 or Rab35 did significantly reduce the amount of phagocytosis - by ∼35% and 60%, respectively. Representative images of Rab13- or 35-depleted cells demonstrate these effects (Fig. 8C). Thus the combined results from fixed and live cell imaging show that Rab13 and Rab35 are both required for efficient cup closure and the normal progression of phagosome internalization. This replicates the known role(s) of Rab35 and its requirement for successful phagocytosis (discussed below) and it reveals Rab13 as a new and essential mediator of phagocytosis in macrophages. Taken together, these results reveal that the sequence of Rabs recruited to phagocytic cups includes Rabs with different roles, pinpointing Rabs13 and 35 as the only ones with roles in closure and phagosome formation.
Discussion
Phagocytosis is a multi-stage process that, in its early stages, is designed to support multiple host cell responses. Rab GTPases are required at successive stages of phagocytosis in order to recruit a sequence of effectors to bring about sequential changes in the composition and trafficking of membranes, cytoskeletal rearrangements, and receptor signaling. By carefully comparing the localization of multiple Rabs in fixed and live cells we have defined their sequential recruitment for the early stages of phagocytosis, providing a basis to further investigate their coordinated functions. Of 21 Rabs screened, we show that Rabs 35, 13, 8a, 8b, 27a, and 10 are recruited early, before phagocytic cup closure, and then retained on PI(3,4,5)P3-positive, early-stage phagosomes soon after closure (Fig. 9). This cohort of Rabs thus defines the focus for recruiting effectors required to initiate phagocytosis through actin or membrane transformations, and for regulating receptor signaling to activate macrophages. We propose that this sequence of Rabs forms the basis for a regulatory Rab cascade and future studies can now focus on identifying the accessory proteins or effectors that link adjacent Rabs in this sequence. While the results here are based on the expression of exogenous GFP-Rabs, we believe our findings faithfully reflect the behavior of endogenous Rabs, supported by our previous work showing identical spatiotemporal recruitment of tagged and endogenous Rab31, for instance, on phagosomes.20 Similarly, the localization we report for PI probes reflects the known PI transitions that occur during phagocytosis3 and fact that GFP-Rabs show consistent localization with and without coexpressed tags20 (see Fig. S1) suggests that the PI probes as used here do not alter Rab membrane recruitment.
Figure 9.
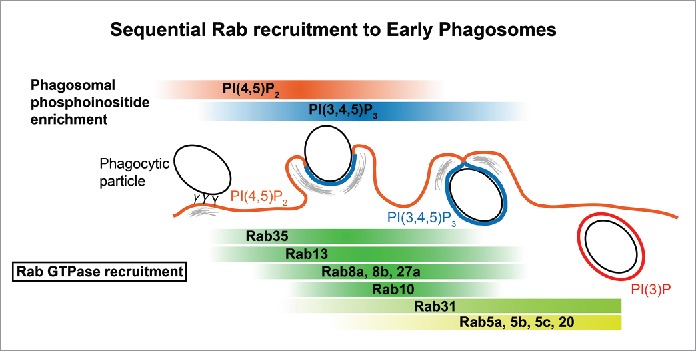
Summary of sequential Rab recruitment and enrichment during early stages of phagocytosis. A schematic diagram summarizing the relationships between Rab and phosphoinositide enrichment on membranes throughout phagocytosis. Rab35 and Rab13 are present on the PI(4,5)P2-enriched plasma membrane and are rapidly recruited to phagocytic cups, overlapping with enrichment of PI(3,4,5)P3, while Rab13 persists after cup closure. Rab8a, Rab8b, and Rab27a are locally enriched on the nascent phagocytic cup, mirroring the association and dissociation of PI(3,4,5)P3 on the phagosomal membrane. This is followed by Rab10 in concert with PI(3,4,5)P3 enrichment and dissociation. Rab31 is recruited just prior to cup closure and it persists during phagosome maturation. Rab5a, Rab5b, Rab5c, and Rab20 are distinctly enriched on the phagosome after dissociation of PI(3,4,5)P3 and all persist during maturation.
Our results for the temporal recruitment of individual Rabs also coincide with their reported functions. More generally, Rab35 is implicated in peripheral endocytic recycling steps in cellular processes such as cytokinesis, cell migration, and neurite outgrowth, and it is a known regulator of phagocytosis.22-24 Cell surface-associated Rab35 is known to modulate actin and membrane dynamics and cell shape via regulating the activity and recruitment of many actin and membrane remodelling proteins including the GTPases Arf6, Rac1, and Cdc42, and the phosphatase, OCRL.10,24,25 Rab35 is one of the earliest Rabs recruited to PI(3,4,5)P3-rich phagocytic cups.10,11 In a knockdown screen of Rab GTPases in Drosophila, depletion of Rab35 produced the only marked reduction in hemocyte phagocytosis of labeled E.coli (by ∼50%) (noting that Rab13 was not reported in that screen).10 This reflects the similar impairment of phagocytosis recorded by us after Rab35 depletion in mouse macrophages and by Egami et al11 in macrophages expressing either the dominant-negative or constitutively active mutants of Rab35. Our live imaging also showed that phagocytic cups cannot fully form after Rab35 depletion and this requirement for Rab35 might derive from its roles in actin remodeling through Rac1 and Cdc4210,11 and/or its recruitment of ACAP2 (an Arf6 GTPase-activating protein [GAP]) during focal exocytosis for pseudopod extension.11 Interestingly, Rab35 and the scaffolding effector MICAL-L1 also serve to recruit Rab13 and Rab8a to membranes,26 in keeping with the sequence of Rab recruitment our results show for early phagocytosis.
The role of Rab13 has not previously been reported in either macrophages or phagocytosis. Evidence in other cell types implicates Rab13 in post-Golgi trafficking, cell migration, and actin reorganisation at the plasma membrane.27-29 Specifically, Rab13 recruits vesicles containing the RhoA GTPase to the leading edge of migrating cells via its effector MICAL-L2,28,29 and it has been implicated in cancer metastasis.27 It is thus tempting to speculate that Rab13 could similarly have a role in actin dynamics for phagocytosis, given that its depletion severely compromised phagocytic cup closure without blocking PI transitions in our experiments - effects that were similar to those we observed after Rab35 depletion. Thus Rab13 is revealed as a new regulator of FcγR-phagocytosis in macrophages.
Our imaging shows the closely related Rabs 8a, 8b, and 10 recruited in a temporally overlapping window to ruffles preceding phagocytic cup closure. Our depletion experiments further show that these Rabs are not directly required for phagocytic closure and the literature supports them each having alternative functions in receptor signaling or membrane trafficking. In LPS-activated macrophages, membrane ruffle-associated Rab8a regulates TLR4 signaling via the recruitment of the p110γ subunit of PI3K as an effector.30 Depletion of Rab8a or PI3Kγ attenuates TLR4-mediated Akt and mTORC activation, and enhances the pro-inflammatory cytokine response.30 Delivery of TLR4 to the surface and its cytokine output are also regulated by Rab10 in macrophages.
Rabs 35, 13, 8a, 8b, 27a, and 10 thus emerge as putative Rabs for a cascade that regulates early phagocytosis, by recruiting sequential effectors to coordinate receptor signaling and phagocytic cup closure. The aforementioned studies and others provide further evidence to support interplay between this series of Rabs and to flesh-out other molecules that could contribute to a cascade in early phagocytosis. Indeed, Rab35 and Rab10 in different systems both have the Arf6 GAP, ACAP, as effectors,11 and the Rabs 35, 13 and 8a share associations with the effector MICAL-L1.26 Rabs 8a and 8b have both redundant and non-redundant roles in different cells, and some roles overlapping with Rab10, as revealed by the phenotypes of single and double Rab8 knockouts in mice.31 Further studies are needed to assign relevant GTPase activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) to this early phagocytosis Rab cascade. A previous study outlines a Rab cascade consisting of Rab20, the Rab5 GEF, Rabex5 and Rab5a recruited at a later stage to interferon-stimulated phagosomes.15 Thus a series of Rab cascades is likely to operate throughout the phagocytic process.
Rab5a, Rab5b, Rab5c, Rab11a, and Rab14 were identified on phagosomes, but not on early phagocytic cups enriched with PI(3,4,5)P3. Rab5 is well established as the classic Rab that regulates the early endocytic pathway, and as such, it is crucial for endosomal and phagosomal maturation.32 Accordingly, our results show that Rab5a, Rab5b, and Rab5c persist on phagosomes after dissociation of PI(3,4,5)P3, consistent with a known role in the recruitment of effector VPS34, which regulates the formation of PI(3)P on internalized phagosomes.32-34 Rab14 is recruited for phagosome maturation during uptake of Candida albicans and Mycobacterium tuberculosis where its recruitment to phagosomes favors pathogen survival and escape.35,36 Rab11a localizes to early phagosomes to function in recycling membranes to and from the phagosome.37-39 Several other Rabs previously implicated in phagocytosis were not observed on the early PI(3,4,5)P3-positive phagosome by our work. These included Rab22a, Rab29, Rab30, Rab32, Rab39, Rab40, and Rab42. While some of these Rabs are not recruited at all for macrophage phagocytosis, others may act at later stages of phagosome remodeling and progression to a phagolysosome. The subversion of phagolysosome formation by M. tuberculosis includes inhibiting the function of Rab22a,40,41 while Rab39, Rab20, and Rab32 were also implicated in the later stages of phagocytosis, in processes such as phagosomal acidification and delivery of cathepsin D.42 Finally, some Rabs are involved at multiple stages of phagocytosis by recruiting different effectors. A recent structural study43 shows that the recycling endosome-localized Rab11a can simultaneously bind to the effectors FIP3 and Rabin8, the GEF for Rab8a, indicating the potential for roles and interactions between pre- and post-closure Rabs in phagocytosis. Rab27a was identified here on early phagocytic cups where one of its purported functions is to prolong the F-actin assembled around the phagocytic cup44 that normally depolymerizes commensurate with closure and internalization. However, Rab27a also functions on the later phagolysosome in dendritic cells, to control phagosomal pH and NADPH.45 Similarly, Rab11a has been attributed with delivering cargo to early and late phagosomes, namely TLR4 and the NADPH oxidase complex, respectively.37,39
In this study, we have systematized information regarding the sequential recruitment of Rab GTPases to FcγR-mediated phagocytic cups and early phagosomes. We thereby provide new information on the necessity of Rabs 35 and 13 for phagocytic cup closure. Overall the data will add to our understanding of critical regulators and steps in phagocytosis and receptor signaling that can be targeted to fight infection or perhaps to manipulate the removal of cell debris or protein aggregates in chronic disease.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge M. Fukuda for generously providing Rab constructs and we thank T. Khromykh and J. Venturato for valuable technical assistance.
Funding
This work was supported by grant (APP606788) and fellowship (APP1003021) funding to JLS from the National Health and Medical Research Council of Australia. Imaging was performed in the ACRF-funded Cancer Biology Imaging Facility at the Institute for Molecular Bioscience
References
- [1].Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol (2013); 14:986-95; PMID:24048120; http://dx.doi.org/ 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol (2008); 9:639-49; PMID:18612320; http://dx.doi.org/ 10.1038/nrm2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bohdanowicz M, Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev (2013); 93:69-106; PMID:23303906; http://dx.doi.org/ 10.1152/physrev.00002.2012 [DOI] [PubMed] [Google Scholar]
- [4].Freeman SA, Grinstein S. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev (2014); 262:193-215; PMID:25319336; http://dx.doi.org/ 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- [5].Amer AO, Swanson MS. A phagosome of one's own: a microbial guide to life in the macrophage. Curr Opin Microbiol (2002); 5:56-61; PMID:11834370; http://dx.doi.org/ 10.1016/S1369-5274(02)00286-2 [DOI] [PubMed] [Google Scholar]
- [6].Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol (1996); 135:1249-60; PMID:8947549; http://dx.doi.org/ 10.1083/jcb.135.5.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schink KO, Raiborg C, Stenmark H. Phosphatidylinositol 3-phosphate, a lipid that regulates membrane dynamics, protein sorting and cell signalling. Bioessays (2013); 35:900-12; PMID:23881848 [DOI] [PubMed] [Google Scholar]
- [8].Hutagalung AH, Novick PJ. Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol Rev (2011); 91:119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gutierrez MG. Functional role(s) of phagosomal Rab GTPases. Small GTPases (2013); 4:148-58; PMID:24088602; http://dx.doi.org/ 10.4161/sgtp.25604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shim J, Lee S-M, Lee MS, Yoon J, Kweon H-S, Kim Y-J. Rab35 Mediates Transport of Cdc42 and Rac1 to the Plasma Membrane during Phagocytosis. Mol Cell Biol (2010); 30:1421-33; PMID:20065041; http://dx.doi.org/ 10.1128/MCB.01463-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during Fc gamma R-mediated phagocytosis. J Cell Sci (2011); 124:3557-67; PMID:22045739; http://dx.doi.org/ 10.1242/jcs.083881 [DOI] [PubMed] [Google Scholar]
- [12].Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci U S A (2009); 106:14408-13; PMID:19666511; http://dx.doi.org/ 10.1073/pnas.0906536106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Markgraf DF, Peplowska K, Ungermann C. Rab cascades and tethering factors in the endomembrane system. FEBS Lett (2007); 581:2125-30; PMID:17316615; http://dx.doi.org/ 10.1016/j.febslet.2007.01.090 [DOI] [PubMed] [Google Scholar]
- [14].Rana M, Lachmann J, Ungermann C. Identification of a Rab GTPase-activating protein cascade that controls recycling of the Rab5 GTPase Vps21 from the vacuole. Mol Biol Cell (2015); 26:2535-49; PMID:25971802; http://dx.doi.org/ 10.1091/mbc.E15-02-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pei G, Repnik U, Griffiths G, Gutierrez MG. Identification of an immune-regulated phagosomal Rab cascade in macrophages. J Cell Sci (2014); 127:2071-82; PMID:24569883; http://dx.doi.org/ 10.1242/jcs.144923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shurety W, Merino-Trigo A, Brown D, Hume DA, Stow JL. Localization and post-Golgi trafficking of tumor necrosis factor-α in macrophages. J Interferon Cytokine Res (2000); 20:427-38; PMID:10805378; http://dx.doi.org/ 10.1089/107999000312379 [DOI] [PubMed] [Google Scholar]
- [17].Yeo JC, Wall AA, Stow JL, Hamilton NA. High-throughput quantification of early stages of phagocytosis. Biotechniques (2013); 55:115-24; PMID:24003943 [DOI] [PubMed] [Google Scholar]
- [18].Pagan JK, Wylie FG, Joseph S, Widberg C, Bryant NJ, James DE, Stow JL. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr Biol (2003); 13:156-60; PMID:12546791; http://dx.doi.org/ 10.1016/S0960-9822(03)00006-X [DOI] [PubMed] [Google Scholar]
- [19].Schroder K, Irvine KM, Taylor MS, Bokil NJ, Cao KAL, Masterman KA, Labzin LI, Semple CA, Kapetanovic R, Fairbairn L, et al.. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc Natl Acad Sci U S A (2012); 109:E944-E53; PMID:22451944; http://dx.doi.org/ 10.1073/pnas.1110156109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yeo JC, Wall AA, Luo L, Stow JL. Rab31 and APPL2 enhance FcγR-mediated phagocytosis through PI3K/Akt signaling in macrophages. Mol Biol Cell (2015); 26:952-65; PMID:25568335; http://dx.doi.org/ 10.1091/mbc.E14-10-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cox D, Tseng C-C, Bjekic G, Greenberg S. A Requirement for Phosphatidylinositol 3-Kinase in Pseudopod Extension. J Biol Chem (1999); 274:1240-7; PMID:9880492; http://dx.doi.org/ 10.1074/jbc.274.3.1240 [DOI] [PubMed] [Google Scholar]
- [22].Allaire PD, Sadr MS, Chaineau M, Sadr ES, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci (2013); 126:722-31; PMID:23264734; http://dx.doi.org/ 10.1242/jcs.112375 [DOI] [PubMed] [Google Scholar]
- [23].Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathwav essential for the terminal steps of cytokinesis. Curr Biol (2006); 16:1719-25; PMID:16950109; http://dx.doi.org/ 10.1016/j.cub.2006.07.020 [DOI] [PubMed] [Google Scholar]
- [24].Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-β 2 (ACAP2) during neurite outgrowth. J Cell Sci (2012); 125:2235-43; PMID:22344257; http://dx.doi.org/ 10.1242/jcs.098657 [DOI] [PubMed] [Google Scholar]
- [25].Dambournet D, Machicoane M, Chesneau L, Sachse M, Rocancourt M, El Marjou A, Formstecher E, Salomon R, Goud B, Echard A. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol (2011); 13:981-U245; PMID:21706022; http://dx.doi.org/ 10.1038/ncb2279 [DOI] [PubMed] [Google Scholar]
- [26].Kobayashi H, Etoh K, Ohbayashi N, Fukuda M. Rab35 promotes the recruitment of Rab8, Rab13 and Rab36 to recycling endosomes through MICAL-L1 during neurite outgrowth. Biol Open (2014); 3:803-14; PMID:25086062; http://dx.doi.org/ 10.1242/bio.20148771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ioannou MS, Bell ES, Girard M, Chaineau M, Hamlin JNR, Daubaras M, Monast A, Park M, Hodgson L, McPherson PS. DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior. J Cell Biol (2015); 208:629-48; PMID:25713415; http://dx.doi.org/ 10.1083/jcb.201407068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu CS, Agrawal S, Vasanji A, Drazba J, Sarkaria S, Xie J, Welch CM, Liu ML, Anand-Apte B, Horowitz A. Rab13-dependent Trafficking of RhoA Is Required for Directional Migration and Angiogenesis. J Biol Chem (2011); 286:23511-20; PMID:21543326; http://dx.doi.org/ 10.1074/jbc.M111.245209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sakane A, Honda K, Sasaki T. Rab13 Regulates Neurite Outgrowth in PC12 Cells through Its Effector Protein, JRAB/MICAL-L2. Mol Cell Biol (2010); 30:1077-87; PMID:20008558; http://dx.doi.org/ 10.1128/MCB.01067-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luo L, Wall AA, Yeo JC, Condon ND, Norwood SJ, Schoenwaelder S, Chen KW, Jackson S, Jenkins BJ, Hartland EL, et al.. Rab8a interacts directly with PI3Kγ to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun (2014); 5; 4407. [DOI] [PubMed] [Google Scholar]
- [31].Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, et al.. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci (2014); 127:422-31; PMID:24213529; http://dx.doi.org/ 10.1242/jcs.136903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol (2001); 154:631-44; PMID:11489920; http://dx.doi.org/ 10.1083/jcb.200106049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, et al.. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol (2005); 170:607-18; PMID:16103228; http://dx.doi.org/ 10.1083/jcb.200505128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature (1999); 397:621-5; PMID:10050856; http://dx.doi.org/ 10.1038/17618 [DOI] [PubMed] [Google Scholar]
- [35].Okai B, Lyall N, Gow NAR, Bain JM, Erwig LP. Rab14 Regulates Maturation of Macrophage Phagosomes Containing the Fungal Pathogen Candida albicans and Outcome of the Host-Pathogen Interaction. Infect Immun (2015); 83:1523-35; PMID:25644001; http://dx.doi.org/ 10.1128/IAI.02917-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kyei GB, Vergne I, Chua J, Roberts E, Harris J, Junutula JR, Deretic V. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J (2006); 25:5250-9; PMID:17082769; http://dx.doi.org/ 10.1038/sj.emboj.7601407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas Ø, Nilsen NJ, Stenmark H, Latz E, Lien E, et al.. The Rab11a GTPase Controls Toll-like Receptor 4-Induced Activation of Interferon Regulatory Factor-3 on Phagosomes. Immunity (2010); 33:583-96; PMID:20933442; http://dx.doi.org/ 10.1016/j.immuni.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38.].Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci U S A (2000); 97:680-5; PMID:10639139; http://dx.doi.org/ 10.1073/pnas.97.2.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Casbon A-J, Allen L-AH, Dunn KW, Dinauer MC. Macrophage NADPH Oxidase Flavocytochrome b Localizes to the Plasma Membrane and Rab11-Positive Recycling Endosomes. J Immunol (2009); 182:2325-39; http://dx.doi.org/ 10.4049/jimmunol.0803476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Egami Y, Araki N. Rab20 Regulates Phagosome Maturation in RAW264 Macrophages during Fc Gamma Receptor-Mediated Phagocytosis. Plos One (2012); 7:e35663; PMID:22545127; http://dx.doi.org/ 10.1371/journal.pone.0035663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roberts EA, Chua J, Kyei GB, Deretic V. Higher order Rab programming in phagolysosome biogenesis. J Cell Biol (2006); 174:923-9; PMID:16982798; http://dx.doi.org/ 10.1083/jcb.200603026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Seto S, Tsujimura K, Koide Y. Rab GTPases Regulating Phagosome Maturation Are Differentially Recruited to Mycobacterial Phagosomes. Traffic (2011); 12:407-20; PMID:21255211; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01165.x [DOI] [PubMed] [Google Scholar]
- [43].Vetter M, Stehle R, Basquin C, Lorentzen E. Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat Struct Mol Biol (2015); 22:695-702; PMID:26258637; http://dx.doi.org/ 10.1038/nsmb.3065 [DOI] [PubMed] [Google Scholar]
- [44].Yokoyama K, Kaji H, He JS, Tanaka C, Hazama R, Kamigaki T, Ku YS, Tohyama K, Tohyama Y. Rab27a Negatively Regulates Phagocytosis by Prolongation of the Actin-coating Stage around Phagosomes. J Biol Chem (2011); 286:5375-82; PMID:21169636; http://dx.doi.org/ 10.1074/jbc.M110.171702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM-C, Pascolo S, Gougerot-Pocidalo M-A, Raposo G, Seabra MC, et al.. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol (2007); 9:367-U29; PMID:17351642; http://dx.doi.org/ 10.1038/ncb1552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


