Abstract
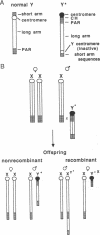
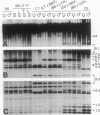
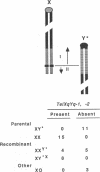
The pseudoautosomal (PA) region of the mammalian genome is the region of the X and Y chromosomes that shares extensive DNA sequence homology and is of special interest because it may play an essential role during male meiosis. We have identified three telomere-related restriction fragments from the PA region of the mouse genome, using an oligonucleotide probe composed of the mammalian telomere consensus sequence TTAGGG. PA assignment of two C57BL/6J-derived fragments was initially suggested by analysis of DNAs from progeny sired by C57BL/6J males carrying the rearranged Y chromosome, Y*: the hybridization intensity of both fragments was concordant with the sex-chromosome complement of the offspring. Further analysis indicated that both fragments were present in female and male F1, mice regardless of the sex of their C57BL/6J parent--a criterion for autosomal or PA linkage. Both fragments were closely linked to each other and located on the X chromosome distal to amelogenin (Amg)--in agreement with X or PA linkage. Confirmation of the PA derivation of these fragments was accomplished by following their segregation in a cross involving XY* males mated to DBA/2J females. A similar experiment identified a third PA-derived restriction fragment of LT/SvEi origin. Identification of PA-derived telomere-related restriction fragments will enable further genetic analysis of this region of the mouse genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar L. C., Arnaud D., Cambrou J., Guenet J. L., Avner P. R. Mapping of the mouse X chromosome using random genomic probes and an interspecific mouse cross. EMBO J. 1985 Dec 30;4(13B):3695–3700. doi: 10.1002/j.1460-2075.1985.tb04137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar L. C., Dandolo L., Hanauer A., Cook A. R., Arnaud D., Mandel J. L., Avner P. Conservation and reorganization of loci on the mammalian X chromosome: a molecular framework for the identification of homologous subchromosomal regions in man and mouse. Genomics. 1988 Apr;2(3):220–230. doi: 10.1016/0888-7543(88)90006-7. [DOI] [PubMed] [Google Scholar]
- Biddle F. G. Segregation distortion of X-linked marker genes in interspecific crosses between Mus musculus and M. spretus. Genome. 1987 Apr;29(2):389–392. doi: 10.1139/g87-067. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S. Genetic homology and crossing over in the X and Y chromosomes of Mammals. Hum Genet. 1982;61(2):85–90. doi: 10.1007/BF00274192. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Pollard C. E., Hawker S. G. Sex-reversed mice: XX and XO males. Cytogenetics. 1971;10(5):318–337. doi: 10.1159/000130151. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Rasberry C., Burtenshaw M. D., Evans E. P. Illegitimate pairing of the X and Y chromosomes in Sxr mice. Genet Res. 1990 Oct-Dec;56(2-3):121–128. doi: 10.1017/s0016672300035199. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Keitz B. T., Disteche C. M., Lau E. C., Snead M. L. Linkage of amelogenin (Amel) to the distal portion of the mouse X chromosome. Genomics. 1991 May;10(1):23–28. doi: 10.1016/0888-7543(91)90479-x. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Dautigny A., Mattei M. G., Morello D., Alliel P. M., Pham-Dinh D., Amar L., Arnaud D., Simon D., Mattei J. F., Guenet J. L. The structural gene coding for myelin-associated proteolipid protein is mutated in jimpy mice. 1986 Jun 26-Jul 2Nature. 321(6073):867–869. doi: 10.1038/321867a0. [DOI] [PubMed] [Google Scholar]
- Davisson M. T., Akeson E. C. An improved method for preparing G-banded chromosomes from mouse peripheral blood. Cytogenet Cell Genet. 1987;45(2):70–74. doi: 10.1159/000132432. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Hale D. W., Hunt P. A., Lee B. K., Tucker P. K., King T. R., Eppig J. T., Washburn L. L. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57(4):221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Washburn L. L. Assignment of genes to regions of mouse chromosomes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):946–950. doi: 10.1073/pnas.75.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. W., Yen C. H. DNA variants with telomere probe enable genetic mapping of ends of mouse chromosomes. Mamm Genome. 1991;1(2):118–122. doi: 10.1007/BF02443788. [DOI] [PubMed] [Google Scholar]
- Ellis N., Goodfellow P. N. The mammalian pseudoautosomal region. Trends Genet. 1989 Dec;5(12):406–410. doi: 10.1016/0168-9525(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Evans E. P., Burtenshaw M. D., Cattanach B. M. Meitoic crossing-over between the X and Y chromosomes of male mice carrying the sex-reversing (Sxr) factor. Nature. 1982 Dec 2;300(5891):443–445. doi: 10.1038/300443a0. [DOI] [PubMed] [Google Scholar]
- Hale D. W., Hunt P. A., Tucker P. K., Eicher E. M. Synapsis and obligate recombination between the sex chromosomes of male laboratory mice carrying the Y* rearrangement. Cytogenet Cell Genet. 1991;57(4):231–239. doi: 10.1159/000133153. [DOI] [PubMed] [Google Scholar]
- Harbers K., Soriano P., Müller U., Jaenisch R. High frequency of unequal recombination in pseudoautosomal region shown by proviral insertion in transgenic mouse. Nature. 1986 Dec 18;324(6098):682–685. doi: 10.1038/324682a0. [DOI] [PubMed] [Google Scholar]
- Keitges E. A., Schorderet D. F., Gartler S. M. Linkage of the steroid sulfatase gene to the sex-reversed mutation in the mouse. Genetics. 1987 Jul;116(3):465–468. doi: 10.1093/genetics/116.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitges E., Rivest M., Siniscalco M., Gartler S. M. X-linkage of steroid sulphatase in the mouse is evidence for a functional Y-linked allele. Nature. 1985 May 16;315(6016):226–227. doi: 10.1038/315226a0. [DOI] [PubMed] [Google Scholar]
- Kipling D., Cooke H. J. Hypervariable ultra-long telomeres in mice. Nature. 1990 Sep 27;347(6291):400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Lau E. C., Mohandas T. K., Shapiro L. J., Slavkin H. C., Snead M. L. Human and mouse amelogenin gene loci are on the sex chromosomes. Genomics. 1989 Feb;4(2):162–168. doi: 10.1016/0888-7543(89)90295-4. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Pearlman R. E. Telomere and centromere DNA are associated with the cores of meiotic prophase chromosomes. Chromosoma. 1990 Dec;100(1):8–14. doi: 10.1007/BF00337598. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Grant S. G., Stephenson D. A., Chapman V. M. Multilocus molecular mapping of the mouse X chromosome. Genomics. 1988 Oct;3(3):187–194. doi: 10.1016/0888-7543(88)90078-x. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Page D. C., Bieker K., Brown L. G., Hinton S., Leppert M., Lalouel J. M., Lathrop M., Nystrom-Lahti M., de la Chapelle A., White R. Linkage, physical mapping, and DNA sequence analysis of pseudoautosomal loci on the human X and Y chromosomes. Genomics. 1987 Nov;1(3):243–256. doi: 10.1016/0888-7543(87)90051-6. [DOI] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Physical mapping of the human pseudo-autosomal region; comparison with genetic linkage map. EMBO J. 1988 Aug;7(8):2369–2376. doi: 10.1002/j.1460-2075.1988.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Johnsson C., Vergnaud G., Cooke H. J., Weissenbach J. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature. 1986 Jan 23;319(6051):291–295. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- Snead M. L., Lau E. C., Zeichner-David M., Fincham A. G., Woo S. L., Slavkin H. C. DNA sequence for cloned cDNA for murine amelogenin reveal the amino acid sequence for enamel-specific protein. Biochem Biophys Res Commun. 1985 Jun 28;129(3):812–818. doi: 10.1016/0006-291x(85)91964-3. [DOI] [PubMed] [Google Scholar]
- Snead M. L., Zeichner-David M., Chandra T., Robson K. J., Woo S. L., Slavkin H. C. Construction and identification of mouse amelogenin cDNA clones. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7254–7258. doi: 10.1073/pnas.80.23.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Keitges E. A., Schorderet D. F., Harbers K., Gartler S. M., Jaenisch R. High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7218–7220. doi: 10.1073/pnas.84.20.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Levilliers J., Petit C., Rouyer F., Simmler M. C. Normal and abnormal interchanges between the human X and Y chromosomes. Development. 1987;101 (Suppl):67–74. [PubMed] [Google Scholar]