Abstract
Objectives
To review published cost-effectiveness analyses (CEA) assessing bortezomib (BTZ) for multiple myeloma (MM) and explore possible bias affecting the cost-effectiveness of BTZ.
Methods
Literature was searched for published CEAs assessing BTZ or BTZ-containing regimens for MM from 2003 to 2015. The reported incremental cost-effectiveness ratios (ICER) were adjusted by 2014 country-specific gross domestic product per capita (GDPPC) to compare the cost-effectiveness threshold of the World Health Organization (3 GDPPC per gained quality-adjusted life year [QALY]).
Results
A total of 17 published CEAs were included in this review. When compared to non-BTZ treatments, BTZ-containing regimens were cost-effective for induction treatment prior to stem cell transplantation (SCT) in Canada, Poland, and Germany (ICER per QALY: 0.9299–2.254 GDPPC). BTZ/melphalan/prednisolone (VMP) was cost-effective for previously untreated and SCT-ineligible MM patients when compared to melphalan plus prednisolone (MP), melphalan/prednisone/lenalidomide with lenalidomide maintenance, and cyclophosphamide/thalidomide/dexamethasone (CTD) (ICER per QALY: dominant to 2.374 GDPPC) in Canada, UK, and USA. BTZ was cost-effective for relapsed/refractory MM when compared to best supportive care (ICER per life year: 0.9317–1.8210 GDPPC) in the UK and the USA, thalidomide in USA (0.5178 GDPPC/LY), and dexamethasone (DEX) in four Nordic countries (€54,451–€81,560/QALY). However, the cost-effectiveness for VMP versus MP plus thalidomide (MPT) and continuous lenalidomide (LEN) plus low-dose DEX (RD) for previously untreated and SCT-ineligible MM patients and BTZ versus LEN/DEX for relapsed/refractory MM patients could be unreliable because of the bias associated with model design and the indirect comparisons of treatment effects.
Conclusion
Published CEAs suggested that BTZ or BTZ-containing regimens were cost-effective when compared to most non-BTZ treatments for MM. However, the conflicting cost-effectiveness for VMP versus MPT for previously untreated and SCT-ineligible MM and BTZ versus LEN/DEX for relapsed/refractory MM needs more robust evidence for further clarification.
Keywords: cost-effectiveness, bortezomib, multiple myeloma, systematic review
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm characterized by proliferation of neoplastic plasma cells that impair hematopoiesis and activate osteoclastic bone resorption and also secrete a monoclonal paraprotein (M-protein) in serum and/or urine.1 MM is ranked as the second most common hematological malignancy (12%–15% of all cases).2 The 5-year survival rate of MM has substantially improved since the launch of bortezomib (BTZ),3 a breakthrough in the treatment of MM. Two open-label, Phase II trials (SUMMIT4 and CREST5) established the treatment efficacy of BTZ for overall survival (OS) in heavily pretreated patients with MM. The Phase III APEX trial proved the superiority of BTZ over high-dose dexamethasone (DEX) for any relapsed MM.6 The VISTA trial, a large Phase III trial including 682 patients, observed significantly extended median OS (13.3 months) associated with BTZ/melphalan/prednisone (VMP) when compared to melphalan/prednisone (MP) (56.4 versus 43.1 months).7 Another Phase III trial, the IFM 2005-01 trial, reported that BTZ plus DEX was superior to vincristine/doxorubicin/DEX regarding postinduction overall response rate (78.5% versus 62.8%) and median progression-free survival (PFS) (36.0 versus 29.7 months) when used for the treatment of MM as induction treatment prior to stem cell transplantation (SCT).8 Thus, BTZ has been approved and recommended in all treatment settings for MM.
Even though BTZ treatments have been proven to be effective in randomized clinical trials (RCTs), the cost- effectiveness of BTZ is often required to support reimbursement decision-making in many countries where there are public health systems or third payers for health care. When compared to clinical efficacies that are usually associated with strong internal validity,9 the cost-effectiveness could vary substantially because of heterogeneity and bias associated with study design, data sources, evidence synthesis methods, and country settings.10 To guide the interpretation of the published cost-effectiveness of BTZ for MM patients, we conducted this systematic review to summarize the published cost-effectiveness analyses (CEA) assessing BTZ or BTZ-containing regimens for MM and to explore the potential bias affecting the cost-effectiveness of BTZ or BTZ-containing regimens.
Study methods
This study was designed as a systematic review of published CEAs assessing BTZ or BTZ-containing regimens for MM by treatment settings.
Literature search
A literature search was conducted in the electronic bibliographic databases, including MEDLINE, EMBASE, and Web of Science. The literature search strategy was developed by combining the keywords for disease (“myeloma”, “multiple myeloma”, “myelomatosis”, “Kahler’s disease”, and “MM”), treatment (“bortezomib” and “Velcade”), and cost-effectiveness (“cost-effectiveness”, “CEA”, “cost- utility”, “CUA”, “cost-benefit”, “CBA”, “cost-minimization”, “CMA”, “incremental cost-effectiveness ratio”, “ICER”, “incremental cost-utility ratio”, and “ICUR”). The literature search time was defined from May 2003 to September 2015, the first market authorization of BTZ for MM. Additionally, conference proceedings and presentations from MM-related medical conferences (ASH annual meeting, Congress of EHA, and ASCO annual meeting) and health economics conferences (ISPOR, HTAi, and SMDM) in 2013 and 2014 were searched to include any eligible CEAs that had not been fully published prior to our literature search.
Identifying qualified CEA
The identified references from literature search were pooled together to delete duplicated records. Two reviewers independently screened the cleaned references for relevance to the cost-effectiveness of BTZ for MM by reading titles and abstracts. The full publications of the relevant references were retrieved and reviewed for further assessment according to the following inclusion and exclusion criteria. The inclusion criteria were 1) original CEA assessing the cost-effectiveness of BTZ or BTZ-containing regimens for MM; 2) containing sufficient information to assess the cost-effectiveness of BTZ or BTZ-containing regimens for MM; 3) using life years (LY) and/or quality-adjusted life years (QALY) as health benefit measurements in CEA; and 4) published in English. The exclusion criteria were: 1) studies in patients with hematological malignancies other than MM; 2) the study only assessing the treatment effects or costs associated with BTZ or BTZ-containing regimens for MM; and 3) reviews, letters, or commentaries citing the cost-effectiveness of BTZ or BTZ-containing regimens for MM.
Data extraction
A data extraction form was developed in Microsoft Excel according to ISPOR health economic evaluation publication guideline and clinical practices guidelines for MM. Two reviewers independently reviewed the publications of the included studies to extract study characteristics (publication type, publication year, country setting, study patients, and treatment strategies assessed in CEA), the design of the CEA (time horizon, health benefit measurement, cost perspective, currency, currency year, and annual discounting rate for health benefits and costs), CEA model information (model design, model structure, health states, model variables, and model assumption), data sources and evidence synthesis methods for model variables, and the results of base case analysis and sensitivity analysis, such as one-way sensitivity analysis and probabilistic sensitivity analysis (PSA).
Data analysis
The included CEAs were summarized by treatment settings, including induction treatment prior to SCT, treatment for previously untreated and SCT-ineligible MM patients, and treatment for patients with refractory/relapsed MM. A narrative review approach was used to describe the study characteristics, CEA design, CEA model characteristics, data sources, and evidence synthesis methods used to estimate model variables. The reported base case incremental cost-effectiveness ratios (ICER) in the included CEAs were adjusted to the 2014 currency value according to the country-specific annual inflation rates in the past and further divided by 2014 country-specific gross domestic product per capita (GDPPC) to compare with the cost-effectiveness threshold defined by the World Health Organization (WHO) (3 GDPPC per gained QALY).11 The reported key factors affecting the cost-effectiveness of BTZ treatments in one-way sensitivity analysis and the results of the PSA were summarized to confirm the robustness of base case analysis. The key model assumptions in the included CEAs were also reviewed for their impacts on the cost-effectiveness of BTZ treatments for MM. Finally, potential bias associated with study design, model structure, data sources, and evidence synthesis methods in the included CEAs were explored to guide the interpretation of the reported cost-effectiveness of BTZ.
Results
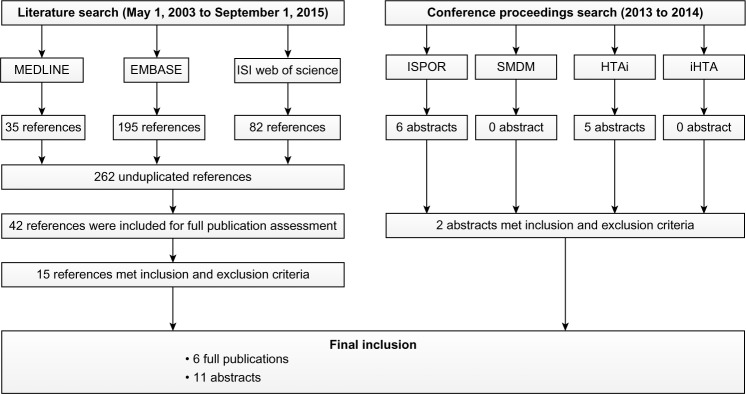
Our literature searches of MEDLINE, EMBASE, and Web of Sciences identified 312 references. After the deletion of the duplicate references, 262 references were included for further assessment of the relevance of the cost-effectiveness of BTZ or BTZ-containing regimens for MM by reading their titles and abstracts. The full publications of 42 relevant references were retrieved for final eligibility assessment using the defined inclusion and exclusion criteria. After the exclusion of 27 references (6 abstracts which were fully published, 4 CEAs without assessing BTZ or BTZ-containing regimens for MM, 4 studies not qualified for CEA, 4 studies without sufficient information for cost-effectiveness assessment, 2 CEAs without using LY or QALY as health benefit measurement, 2 reviews, 1 letter to editor, 1 published in Italian, 1 assessing BTZ for follicular lymphoma, 1 assessing budget impact, and 1 without control strategy for the calculation of ICER), 15 references met both inclusion and exclusion criteria, and so their full publications were reviewed for data extraction. An additional search of the conferences proceedings and presentations identified eleven relevant abstracts. Of these eleven abstracts, only two abstracts were qualified for inclusion. Thus, this systematic review included 17 CEAs, including 3 CEAs (Kouroukis et al,12 Mucha et al,13 and Van Beurden-Tan et al14) assessing BTZ-containing regimens as induction treatment prior to SCT, 6 CEAs (Rickert et al,15 Garrison et al,16 Oster et al,17 Picot et al,18 Yoong et al,19 and Cavenagh et al20) assessing VMP for previously untreated and SCT-ineligible MM, and 8 CEAs (Bagust et al,21 Mehta et al,22 Felix et al,23 Fragoulakis et al,24 Hornberger et al,25 Jiang et al,26 Liwing et al,27 and Moller et al28) assessing BTZ for relapsed/refractory MM. The literature search process is illustrated in Figure 1, and the excluded references from the final assessment of study eligibility are summarized in Table S1. The study characteristics, data sources of model variables, and the base case ICER adjusted by 2014 country-specific GDPPC of the included CEAs are summarized in Tables 1–3, respectively.
Figure 1.
Flow chart to illustrate the literature search process for identification of articles eligible for the cost-effectiveness analyses assessing bortezomib or bortezomib-contained regimens for multiple myeloma.
Table 1.
Summary of study characteristics of the included cost-effectiveness analyses assessing bortezomib or bortezomib-contained regimens for multiple myeloma
| Included study | Publication type | Country | Study design | Model design | Model cycle length | Time horizon | Cost perspective | Annual discount rate for health benefits (%) | Annual discount rate for costs (%) | Model health state |
|---|---|---|---|---|---|---|---|---|---|---|
| Kouroukis et al12 | Abstract | Canada | Cost-utility | Markov | Not reported | 50 years | Public health system | 5 | 5 | Progress free Progressive disease Death |
| Mucha et al13 | Abstract | Poland | Cost-utility | Markov | Not reported | Lifetime | National Health Foundation | 4 | 5 | First-line Second-line Third-line Subsequent line Death |
| Van Beurden-Tan et al14 | Abstract | Germany | Cost-effectiveness/utility | Markov | 1 month | Lifetime | Third payer | 3 | 3 | First-line Second-line Third-line Fourth-line Death |
| Rickert et al15 | Abstract | Sweden | Cost-utility | Partitioned survival model | Not reported | 30 years | Public health system | Not reported | Not reported | Progress free Progressive disease Death |
| Garrison et al16 | Full publication | USA | Cost-effectiveness/utility | Markov | 1 month | 20 years | Third payer | 3 | 3 | Stable disease Minor response Partial response Complete response Treatment free Progressive disease Second-line Death |
| Oster et al17 | Abstract | USA | Cost-effectiveness/utility | Partitioned survival model | Not reported | Lifetime | Third payer | 3 | 3 | Progress free Progressive disease Death |
| Picot et al18 | Full publication | UK | Cost-utility | Partitioned survival model | 6 weeks | 30 years | National health system | 4 | 4 | Treatment Posttreatment Progressive disease |
| Yoong et al19 | Abstract | Canada | Cost-utility | Not reported | Not reported | 10 years | Public health system | Not reported | Not reported | Not reported |
| Cavenagh et al20 | Abstract | USA | Cost-effectiveness/utility | Partitioned survival model | Not reported | Lifetime | US payer | 3 | 3 | Not reported |
| Bagust et al21 | Abstract | UK | Cost-utility | Partitioned survival model | Not reported | Lifetime | National health system | 0 | 0 | Not reported |
| Mehta et al22 | Full publication | USA | Cost-utility | Decision making tree | Not reported | Lifetime | Third payer | 0 | 0 | Not reported |
| Felix et al23 | Abstract | Portugal | Cost-effectiveness/utility | Markov | Not reported | Lifetime | Public health system | 5 | 5 | Not reported |
| Fragoulakis et al24 | Full publication | Greece | Cost-effectiveness/utility | Discrete event simulation | Not reported | Lifetime | Public health system | 4 | 4 | Partial response Complete response Stable disease Progressive disease |
| Hornberger et al25 | Full publication | Sweden | Cost-effectiveness/utility | Partitioned survival model | 3 weeks | 10 years | Public health system | 3 | 3 | Progress free Progressive diseas Death |
| Jiang et al26 | Abstract | UK | Cost-effectiveness/utility | Partitioned survival model | Not reported | Lifetime | National health system | 4 | 4 | Progressive free Progressive disease Death |
| Liwing et al27 | Abstract | Nordic four countries (Finland, Norway, Sweden, Denmark) | Cost-effectiveness/utility | Partitioned survival model | Not reported | Lifetime | Public health system | Not reported | Not reported | Progress free Progressive disease Death |
| Moller et al28 | Full publication | Norway | Cost-effectiveness/utility | Discrete event simulation | Not reported | 2 years | Public health system | 4 | 4 | Partial response Complete response Stable disease Progressive disease |
Table 2.
Summary of the data sources of treatment effects, costs, and utility in the included cost-effectiveness analyses assessing bortezomib or bortezomib-contained regimens for multiple myeloma
| Treatment setting | Comparison | Included studies | Data source for treatment effects | Data sources for costs | Data source for utility |
|---|---|---|---|---|---|
| Induction treatment prior to SCT | BTZ treatment versus non-BTZ treatment | Kouroukis et al12 | IFM2005-01 trial | Medical costs for chemotherapy, maintenance therapy, SCT, palliative care, and adverse events | Van Agthoven et al42 |
| VD versus CTD | Mucha et al13 | IFM2005-01 trial | Medical costs for chemotherapy, maintenance therapy, SCT, palliative care, and adverse events | Van Agthoven et al42 | |
| VD versus VAD | Mucha et al13 | IFM2005-01 trial | Medical costs for chemotherapy, maintenance therapy, SCT, palliative care, and adverse events | Van Agthoven et al42 | |
| VTD versus CTD | Mucha et al13 | PETHEMA trial | Medical costs for chemotherapy, maintenance therapy, SCT, palliative care, and adverse events | Van Agthoven et al42 | |
| VTD versus TD | Mucha et al13 | PETHEMA trial | Medical costs for chemotherapy, maintenance therapy, SCT, palliative care, and adverse events | Van Agthoven et al42 | |
| Van Beurden-Tan et al14 | Medical costs for treatment, SCT, and adverse events | Not reported | |||
| Previously untreated MM but ineligible for SCT | VMP versus MP | Rickert et al15 | VISTA trial | Medical costs for treatment, adverse events, disease relapse, and palliative care | Not reported |
| Garrison et al16 | VISTA trial | Medical costs for treatments, adverse events, and second-line treatment | VISTA trial | ||
| Oster et al17 | Palumbo et al,46 San Miguel et al,32 Facon et al,34 Hulin et al,33 Palumbo et al,35 any VISTA trial | Medical costs for treatment and adverse events | Not reported | ||
| Yoong et al19 | VISTA trial | Medical costs for treatment and adverse events, maintenance therapy, and second-line treatment | Not reported | ||
| Picot et al18 | VISTA trial and Palumbo et al,46 San Miguel et al,32 Facon et al,34 Hulin et al,33 and Palumbo et al35 | Medical costs for treatment and adverse events, maintenance therapy, and second-line treatment | Study mapping EORTC QLQ-C30 to EQ-5D43 | ||
| VMP versus MPT | Rickert et al15 | VISTA trial and Palumbo et al,46 San Miguel et al,32 Facon et al,34 Hulin et al,33 and Palumbo et al35 | Medical costs for treatment, adverse events, relapse, and palliative care | Not reported | |
| Garrison et al16 | VISTA trial and IFM99-06 trial | Medical costs for treatment, adverse events, and second-line treatment | VISTA trial | ||
| Yoong et al19 | VISTA trial | Medical costs for treatment, adverse events, and second-line treatment | Not reported | ||
| Picot et al18 | VISTA trial and Palumbo et al,46 San Miguel et al,32 Facon et al,34 Hulin et al,33 and Palumbo et al35 | Medical costs for treatment, adverse events, and second-line treatment | Study mapping EORTC QLQ-C30 to EQ-5D43 | ||
| VMP versus MPR-R | Garrison et al16 | VISTA trial and MM-015 trial | Medical costs for treatment, adverse events, and second-line treatment | VISTA trial | |
| Oster et al17 | Palumbo et al,46 San Miguel et al,32 Facon et al,34 Hulin et al,33 and Palumbo et al,35 MM-015, and VISTA trial | Medical costs for treatment, adverse events | Not reported | ||
| VMP versus RD | Cavenagh et al20 | FIRST trial and VISTA trial | Medical costs for drugs, administration, medical care, second- and third-line antimyeloma regimens, and management of toxicity | Not reported | |
| Relapsed/refractory MM | BTZ versus BSC | Bagust et al21 | SUMMIT1 trial | Medical costs for treatment and adverse events | Not reported |
| Mehta et al22 | SUMMIT1 trial | Medical costs for treatment and adverse events | Not reported | ||
| BTZ versus THD | Mehta et al22 | SUMMIT1 trial | Medical costs for treatment and adverse events | Not reported | |
| BTZ versus DEX | Hornberger et al25 | APEX trial, MM-009 trial, and MM-10 trial | Medical costs for treatment and adverse events | Van Agthoven et al42 | |
| Liwing et al27 | APEX trial, MM-009 trial, and MM-10 trial | Medical costs for treatment, adverse events, and palliative care | Not reported | ||
| BTZ versus LEN/DEX | Felix et al23 | APEX trial, MM-009 trial, and MM-10 trial | Not reported | Not reported | |
| Fragoulakis et al24 | APEX trial, MM-009 trial, and MM-10 trial | Medical costs for treatment, adverse events, and palliative care | Van Agthoven et al42 | ||
| Hornberger et al25 | APEX trial, MM-009 trial, and MM-10 trial | Medical costs for treatment, adverse events, and palliative care | Van Agthoven et al42 | ||
| Jiang et al26 | APEX trial, MM-009 trial, and MM-10 trial | Not reported | Not reported | ||
| Liwing et al27 | APEX trial, MM-009 trial, and MM-10 trial | Not reported | Not reported | ||
| Moller et al28 | APEX trial, MM-009 trial, and MM-10 trial | Medical costs for treatment, adverse events, and palliative care | Van Agthoven et al42 |
Abbreviations: BSC, best supportive care; BTZ, bortezomib; CTD, cyclophosphamide/thalidomide/dexamethasone; DEX, dexamethasone; LEN, lenalidomide; MM, multiple myeloma; MP, melphalan/prednisone; MPR-R, melphalan/prednisone/lenalidomide with lenalidomide maintenance; MPT, melphalan/prednisone/thalidomide; RD, lenalidomide plus low-dose dexamethasone; SCT, stem cell transplantation; THD, thalidomide; TD, thalidomide/dexamethasone; VD, bortezomib/dexamethasone; VAD, vincristine/adriamycin/dexamethasone; VMP, bortezomib/melphalan/prednisone; VTD, bortezomib/thalidomide/dexamethasone.
Table 3.
Summary of the published base case cost-effectiveness studies of bortezomib or bortezomib-contained regimens for multiple myeloma (ICER was adjusted by 2014 country-specific GDPPC)
| Treatment setting | Comparison | Included studies | Country | Adjusted ICER by 2014 GDPPC |
|---|---|---|---|---|
| Induction treatment prior to SCT | BTZ-containing regimen versus non-BTZ treatment | Kouroukis et al12 | Canada | 2.2544/QALY |
| VD versus CTD | Mucha et al13 | Poland | 1.2584/QALY | |
| VD versus VAD | Mucha et al13 | Poland | 1.0083/QALY | |
| VTD versus CTD | Mucha et al13 | Poland | 0.5588/QALY | |
| VTD versus TD | Mucha et al13 | Poland | 0.9864/QALY | |
| Van Beurden-Tan et al14 | Germany | 0.9299/QALY | ||
| Previously untreated MM but ineligible for SCT | VMP versus MP | Yoong et al19 | Canada | 1.1060/QALY |
| Garrison Jr et al16 | USA | 1.1622/QALY | ||
| Picot et al18 | UK | 1.2070/QALY | ||
| Rickert et al15 | Sweden | 2.3744/QALY | ||
| Oster et al17 | USA | 2.0059/QALY | ||
| VMP versus MPT | Rickert et al15 | Sweden | 2.0248/QALY | |
| Garrison Jr et al16 | USA | Dominant | ||
| Yoong et al19 | Canada | 0.7323/QALY | ||
| Picot et al18 | UK | Dominated | ||
| VMP versus MPR-R | Garrison Jr et al16 | USA | Dominant | |
| Oster et al17 | USA | 1.3979/QALY (MPR-R versus VMP) | ||
| VMP versus CTD | Picot et al18 | UK | 1.1911/QALY | |
| VMP versus RD | Cavenagh et al20 | USA | 1.5775/QALY (RD versus VMP) | |
| Relapsed/refractory MM | BTZ versus BSC | Bagust et al21 | UK | 0.9317–1.8210/LY |
| Mehta et al22 | USA | 1.0933/LY | ||
| Mehta et al22 | USA | 1.2004/LY | ||
| BTZ versus THD | Mehta et al22 | USA | 0.5178/QALY | |
| BTZ versus DEX | Hornberger et al25 | Sweden | 3.2062/QALY | |
| Liwing et al27 | Nordic countries | €54451–€81560/QALY | ||
| BTZ versus LEN/DEX | Felix et al23 | Portugal | 2.5532–3.0187/QALY (LEN/DEX versus BTZ) | |
| Fragoulakis et al24 | Greece | 2.0259/QALY (LEN/DEX versus BTZ) | ||
| Hornberger et al25 | Sweden | Dominant | ||
| Jiang et al26 | UK | Dominant | ||
| Liwing et al27 | Nordic countries | Dominant | ||
| Moller et al28 | Norway | 0.5205/QALY (LEN/DEX versus BTZ) |
Abbreviations: BSC, best supportive care; BTZ, bortezomib; CTD, cyclophosphamide/thalidomide/dexamethasone; DEX, dexamethasone; GDPPC, gross domestic product per capita; ICER, incremental cost-effectiveness ratio; LEN, lenalidomide; MM, multiple myeloma; MP: melphalan/prednisone; MPR-R, melphalan/prednisone/lenalidomide with lenalidomide maintenance; MPT, melphalan/prednisone/thalidomide; RD, lenalidomide plus low-dose dexamethasone; SCT, stem cell transplantation; THD, thalidomide; TD, thalidomide/dexamethasone; VD, bortezomib/dexamethasone; VAD, vincristine/adriamycin/dexamethasone; VMP, bortezomib/melphalan/prednisone; VTD, bortezomib/thalidomide/dexamethasone.
BTZ-containing regimens as induction treatment prior to SCT
Three included CEAs12–14 assessing the cost-effectiveness of BTZ-containing regimens as induction treatment prior to SCT in Canada, Poland, and Germany were published in abstracts and had similar study characteristics. The base case ICER per gained QALY for BTZ-containing regimens versus non-BTZ treatments in the Canadian CEA based on IFM 2005-01 phase III trial29 was 2.2544 GDPPC. The Polish CEA13 compared bortezomib/dexamethasone (VD) versus vincristine/adriamycin/dexamethasone (VAD) and cyclophosphamide/thalidomide/DEX (CTD) likely using an indirect comparison method to estimate treatment efficacies associated with the two treatments from two trials.29,30 The reported base case ICERs per gained QALY for VD versus VAD and CTD were 1.0083 and 1.2584 GDPPC, respectively. Two CEAs compared BTZ/thalidomide/DEX (VTD) versus CTD and thalidomide/DEX (TD) in Poland and Germany, respectively.13,14 The base case ICERs per gained QALY for VTD versus CTD and TD were 0.5588 and 0.9864 GDPPC, respectively in Poland. However, the base case ICER per gained QALY for VTD versus TD in Germany (0.9299 GDPPC) was only half that of the ICER in Poland. The key factors affecting the cost-effectiveness of VTD included the induction cost and transplant percentage. The cost-effectiveness proportion for VTD versus TD was 57.1% in Germany at the cost-effectiveness threshold of €35,000 per gained QALY.
VMP for previously untreated and SCT-ineligible MM
Five included CEAs assessed the cost-effectiveness of BTZ-containing regimens for previously untreated MM patients who were ineligible for SCT due to old age. These five CEAs compared VMP versus melphalan plus prednisone (MP),15–17,19 MP plus thalidomide (MPT),15,16,18,19 lenalidomide (LEN) maintenance (MPR-R),16,17 and continuous LEN plus low-dose DEX (RD).20
VMP versus MP
Five included CEAs compared VMP versus MP for previously untreated and SCT-ineligible MM patients in USA, Sweden, UK, and Canada. Three of these CEAs used a partitioned survival model that decomposed survival by disease progression status. One CEA used a Markov model with seven health states for simulation. One included CEA did not report the model design. The VISTA trial31 was the data source for the treatment effects of VMP. However, the methods comparing VMP versus MP for survival outcomes in these CEAs were not exactly the same. Three included CEAs15,16,19 directly used the VISTA trial to estimate hazard ratio associated with VMP versus MP for PFS (0.48) and OS (0.695). However, the other two studies17,18 estimated the baseline hazard rate associated with MP for PFS and OS from five RCTs (VISTA trial,32 IFM 01/01 trial,33 IFM 99/06,34 the GIMEMA trial,35 and MMIX02 trial36) and projected higher hazard ratio associated with VMP versus MP for PFS (0.578). The reported base case ICER per gained QALY for VMP versus MP in these five studies ranged from 1.1060 to 2.3744 GDPPC. Four of the included CEAs reported that the survival differences between VMP and MP had the strongest impact on the cost-effectiveness of VMP. The reported cost-effective proportion for VMP versus MP in PSA was 20.4% at the cost-effectiveness threshold of US$50,000 per QALY, but it increased to 100% at the cost-effectiveness threshold of US$100,000 per QALY.
VMP versus MPT
Four included CEAs compared VMP versus MPT for previously untreated and SCT-ineligible MM patients in Sweden,15 USA,16 UK,18 and Canada.19 Due to the lack of trials directly comparing VMP versus MPT for previously untreated MM, indirect comparison methods were used in these four studies to estimate the impact of VMP versus MPT on survival in the CEAs. Even though the indirect comparisons used the same data sources (VISTA trial for VMP and published RCTs comparing MPT versus MP for MPT), the indirect comparisons generated conflicting results for OS associated with the two treatments. For example, a mixed treatment comparison meta-analysis in one CEA16 estimated longer OS associated with VMP (61 versus 50.2 months), whereas another CEA18 projected slightly shorter OS associated with VMP (6.64 versus 6.66 years) according to the extracted survival probabilities from Kaplan–Meier plots in the five RCTs. Thus, the two CEAs reported conflicting data on cost-effectiveness of VMP by reporting dominance of VMP over MPT and dominance of VMP by MPT. The other two included CEAs15,19 reported that the base case ICERs per gained QALY for VMP versus MPT were 2.0248 and 0.7323 GDPPC, respectively. The reported key factors affecting the cost-effectiveness of VMP relative to MPT included drug costs of MPT and the survival differences between VMP and MPT. The PSA in the CEA18 reporting the dominance of MPT over VMP projected that the probability of dominance of MPT over VMP was 95% at the cost-effectiveness thresholds of GBP 20,000 and 30,000 per QALY.
VMP versus MPR-R
Two included CEAs compared VMP versus MPR-R for the previously untreated and SCT-ineligible MM patients in USA.16,17 One included CEA16 used a Markov model with seven health states, and the other CEA17 used a partitioned survival model decomposing survival by the onset of progressive disease to simulate lifetime health benefits and costs. The data sources for the survival outcomes associated with VMP and MPR-R were based on the VISTA trial (for VMP) and the MM-015 trial (for MPR-R).37 The MM-015 trial showed significantly longer PFS but comparable OS for MPR-R versus MP. However, the VISTA trial showed longer PFS and OS associated with VMP when compared to MP. Thus, VMP was expected to generate more survival benefits than MPR-R and become dominant over MPR-R because of lower treatment costs. However, the other included CEA17 comparing VMP versus MPR-R estimated longer OS associated with MPR-R through an indirect comparison assuming the same postprogression survival associated with the two treatments to adjust “crossover” effects associated with patients receiving MP in the VISTA trial. The reported base case ICER per gained QALY for MPR-R versus VMP in this CEA was 1.3979 GDPPC, instead of the previously reported dominance of VMP when compared with MPR-R. The identified key model variables affecting the cost-effectiveness included the drug costs of MPR-R and the survival differences between MPR-R and VMP. No PSA was performed in these two CEAs.
VMP versus CTD
One included CEA used a partitioned survival model comparing VMP versus CTD for health benefits and costs over a time frame of 30 years in patients with newly diagnosed MM who were ineligible for SCT in the UK.18 The VISTA trial was used to estimate the hazard ratio for VMP versus MP for OS and PFS, and the MMIX trial was used to estimate the hazard ratios for CTD versus MP for OS and PFS outcomes. Indirect comparisons of PFS and OS were conducted to estimate the survival differences between VMP and CTD. The reported base case ICER per gained QALY for VMP versus CTD was 1.1911 GDPPC.
VMP versus continuous RD
One included CEA compared continuous RD versus fixed duration VMP in previously untreated but SCT-ineligible patients. Due to the lack of clinical trials directly comparing continuous RD versus VMP for PFS and OS, this CEA assumed that VMP had the same treatment efficacy as MPT regarding PFS and OS. Thus, the superior treatment efficacy associated with continuous RD relative to MPT in the FIRST trial38 was applied to the comparisons for continuous RD versus VMP. Thus, this CEA projected more health benefits and medical costs associated with continuous RD relative to VMP during the patient’s lifetime, and the estimated ICER was 1.5775 GDPPC per QALY.
BTZ for relapsed/refractory MM
Eight included CEAs compared BTZ versus best supportive care (BSC), THD, DEX, and LEN/DEX for relapsed/refractory MM.
BTZ versus BSC
Two included CEAs compared BTZ versus BSC for relapsed/refractory MM in UK and the USA.21,22 These two CEAs took life year as health benefits measurement in CEA. The UK CEA used partitioned survival model for simulation. The USA CEA constructed a decision analytic model to simulate life expectancy and lifetime medical costs associated with BTZ and BSC.22 Both CEAs used the SUMMIT trial,39 a multicenter, open-label, nonrandomized Phase II trial, assessing BTZ in 202 patients with relapsed/refractory MM, as the data source for treatment effects. Both CEAs projected longer life years and more medical costs associated with BTZ when compared to BSC. In the UK, the base case ICER per gained life year for BTZ versus BSC ranged from 0.9317 to 1.8210 GDPPC. In the United States, the base case ICER per gained life years for BTZ versus BSC ranged from 1.0933 to 1.2004 GDPPC.
BTZ versus THD
One included CEA compared BTZ versus THD using a modified Delphi technique to survey OS and health resources utilization associated with the two treatments.22 Based on this approach, BTZ was associated with longer OS and higher medical costs than THD in patients without the previous use of THD. The estimated base case ICER per gained life year for BTZ versus THD was 0.5178 GDPPC.
BTZ versus DEX
Two included CEAs compared BTZ versus DEX for relapsed/refractory MM from the perspective of the public health systems in Sweden25 and four Nordic countries, including Finland, Norway, Sweden, and Denmark.27 Both studies used partitioned survival model with three health states (PFS, PPS, and death) to simulate health benefits and medical costs. Additionally, the two CEAs used the same trial, the APEX trial,39 as the data source for the survival benefits associated with BTZ. The survival benefits of DEX were based on two trials (the MM-00940 and MM-01041 trials) comparing LEN/DEX versus DEX to avoid the bias associated with “crossover” effects in the APEX trial, in which patients in the DEX arm were switched to the BTZ arm after disease progression. The base case ICERs per gained LY and QALY for BTZ versus DEX were 1.8603 and 3.2062 GDPPC, respectively, in Sweden. The estimated base case ICER per gained LY ranged from €42,145 to €62,748, and the estimated base case ICER per gained QALY ranged from €54,451 to €81,560 in the four Nordic countries (we were unable to use GDPPC for adjustment because of the lack of ICER for each country). Of these four Nordic countries, Denmark was associated with the highest ICER per gained LY and QALY. The identified key model variables affecting the cost-effectiveness of BTZ in these two studies included utility and treatment costs of BTZ. The 95% credible interval of ICER per gained QALY for BTZ versus DEX in Sweden ranged from 1.4453 to 2.7021 GDPPC.
BTZ versus LEN/DEX
Six included CEAs (three in full publication24,25,28 and three in abstract23,26,27) compared BTZ versus LEN/DEX for relapsed/refractory MM in Portugal,23 Greece,24 Sweden,25 UK,26 Nordic countries,27 and Norway.28 Of these included six CEAs, three CEAs used partitioned survival model,25–27 two CEAs used a discrete event simulation model,24,28 and one CEA used Markov model to simulate health benefits and costs.23 The survival outcomes of BTZ were based on the APEX trial, which directly compared BTZ versus high-dose DEX for relapsed/refractory MM. The survival outcomes of LEN/DEX were based on the MM-009 and MM-010 trials, which used an identical study design for comparing LEN/DEX versus DEX. Indirect comparison methods were used to compare BTZ versus LEN/DEX for survival difference. Of these six CEAs, three CEAs using partitioned survival model reported the dominance of BTZ over LEN/DEX.25–27 However, two CEAs using DES model reported longer survival and higher costs associated with LEN/DEX.24,28 The base case ICERs per gained QALY for LEN/DEX versus BTZ in these two studies were 0.5205 GDPPC (CEA31) in Norway and 2.0259 GDPPC (CEA20) in Greece. The one CEA using Markov model also reported more health benefits and higher costs associated with LEN/DEX (base case ICER per gained QALY: 2.5532–3.0187 GDPPC). The identified main drivers for the cost-effectiveness of BTZ versus LEN/DEX included the costs of LEN/DEX and survival differences between BTZ and LEN/DEX. The proportion of cost-effectiveness for LEN/DEX versus BTZ was reported over 95% at the cost-effectiveness threshold of €60,000 per QALY in Greece.
Discussion
This systematic review summarized 17 published CEAs assessing BTZ or BTZ-containing regimens in the treatment settings from previously untreated MM to relapsed/refractory MM. According to the cost-effectiveness threshold defined by the WHO (3 GDPPC per gained QALY), BTZ-containing regimens including VD and VTD appeared cost-effective when compared to non-BTZ treatments for previously untreated MM prior to SCT; VMP was cost-effective when compared to MP and CTD for previously untreated and SCT-ineligible MM patients; and BTZ was cost-effective when compared to BSC, THD, and DEX for relapsed/refractory MM. However, our review also found that indirect comparisons, model assumptions, and model structure might introduce bias in the CEA comparing VMP versus MPT, MPR-R, and continuous RD for previously untreated but SCT-ineligible MM and the cost-effectiveness analysis comparing BTZ versus LEN/DEX for relapsed/refractory MM. The one-way sensitivity analyses conducted in the included CEAs had a common finding indicating that the survival difference was the driving factor for the cost-effectiveness of BTZ or BTZ-based regimens for MM. Thus, the cost-effectiveness of BTZ or BTZ-containing regimens was more reliable and consistent if the survival outcomes were based on the direct head-to-head comparisons of RCTs.
The included CEAs assessing VD and VTD for previously untreated MM prior to SCT treatment used the same data sources for treatment efficacy, health resources utilization, and quality of life.42,43. Even though the reported cost-effectiveness was adjusted by country-specific GDPPC, the cost-effectiveness for the same comparison, such as VTD versus TD, was more attractive in Germany when compared to that for Poland. Thus, the country setting seems to have strong confounding effects on cost-effectiveness even after the adjustment of GDPPC. Our finding could be explained by the relatively small variance associated with treatment costs of patented drugs that are likely to be more affordable in countries with a higher income. Thus, caution is needed when interpreting the high-income countries-based cost-effectiveness for low-income countries. Additionally, interpreting the reported cost-effectiveness of VD and VTD as induction treatment in these three included CEAs should take into account the uncertainty associated with subsequent treatment patterns, which could significantly affect life expectancy, QALY, and health resources utilization after induction treatment.
In our review, apart from the observed impact of country setting on the cost-effectiveness of BTZ-containing regimens as induction treatment prior to SCT for previously untreated MM, indirect comparison of survival outcomes between treatment strategies due to the lack of direct head-to-head comparison trials was another significant bias affecting the cost-effectiveness of BTZ or BTZ-containing regimens. For example, the reported cost-effectiveness of VMP relative to MPT for previously untreated and SCT-ineligible MM patients was conflicting because of opposite survival outcomes estimated by the indirect comparison between VMP and MPT. Of the four included CEAs using indirect comparison methods to estimate the survival differences between VMP and MPT, three reported longer survival associated with VMP and one reported longer survival associated with MPT. After careful review of the indirect comparison methods in these four CEAs, the method used to estimate survival from survival curves, potential confounding effects associated with patient baseline characteristics from different trials could not be adjusted. Because the survival difference between VMP and MPT was likely to be small and very sensitive to confounding effects, any slight confounding effect associated with patient baseline characteristics could completely change the dominance of survival and cause the change of cost-effectiveness dominance. Because the other three CEAs estimated longer survival and better cost-effectiveness associated with VMP using different indirect comparison methods, VMP might be more appropriate than MPT for previously untreated MM in patients who were ineligible for SCT from the cost-effectiveness perspective. However, future direct evidence comparing VMP versus MPT in this setting is still needed for confirmation. Additionally, the potential differences in treatment efficacy between VMP and MPT could have a profound impact on the included CEAs comparing continuous RD versus VMP as this study assumed the same treatment efficacy for VMP versus MPT, the control used to estimate relative treatment efficacy associated with continuous RD. Thus, the reported cost-effectiveness for continuous RD versus VMP could be overestimated. Finally, an indirect comparison method used for the CEA comparing VMP versus MPR-R was also controversial, as the survival advantage associated with VMP over MPR-R has not been fully established.
The included six CEAs assessing BTZ for relapsed/refractory MM suggested that CEA model design could introduce bias affecting cost-effectiveness. For example, the six CEAs used the same data sources for survival outcomes associated with BTZ and LEN/DEX but used different model designs for CEA. The CEAs using Markov model or partitioned survival model projected longer survival associated with BTZ. However, the two CEAs based on the same discrete event model projected longer survival associated with LEN/DEX. Because discrete event model was designed to predict survival outcomes using established relationship between patient baseline characteristics and treatment response,44 the model assumed that the APEX trial comparing BTZ versus DEX had comparable patient baseline characteristics as the MM-009 and MM-010 trials comparing LEN/DEX versus DEX. This assumption could introduce bias if the relationship between patient baseline characteristics and treatment response derived from MM-009 and MM-010 trials was not validated in the APEX trial. This might explain why the discrete event model without the adjustment of patient baseline characteristics projected longer OS associated with LEN/DEX (4.14 versus 3.14 years), while the other indirect comparison methods suggested lower mortality risk associated with BTZ when compared to LEN/DEX (0.59 versus 0.71). Thus, future studies directly comparing BTZ versus LEN/DEX are still needed to clarify the survival difference between the two treatments and confirm the cost-effectiveness of BTZ versus LEN/DEX for relapsed/refractory MM.
Because the included CEAs used highly similar methods to estimate health resources utilization, which usually took into account treatment cost, subsequent treatment, serious adverse events, and palliative care, the health resources utilization was unlikely to be the significant source of the discrepancies associated with cost-effectiveness in this review. However, the main source of quality of life in these included cost-effectiveness analyses is a cost-utility analysis assessing chemotherapy for MM.42 Thus, these CEAs were unlikely to adjust for quality of life by treatment efficacy and treatment toxicity associated with assessed treatments. Thus, future studies assessing the quality of life in MM patients receiving varied treatments are needed to further improve the robustness of the included CEAs in this review. The data sources of treatment effects in these included CEAs were all based on RCTs, which usually had poorer generalizability because highly selected patients were included for analysis.45 Thus, the generalizability of the summarized cost-effectiveness of BTZ or BTZ-containing regimens in our review could also be limited, and future cost-effectiveness based on real-world treatment effects, quality of life, and health resource utilization is needed to support more robust reimbursement decision making. Additionally, the RCTs seldom captured the impact of BTZ or BTZ-containing treatments on MM-related complication due to short follow-up time, and so the reported cost-effectiveness of BTZ or BTZ-containing regimens could be underestimated. Even though our literature search strategies were developed to identify any cost-effectiveness analysis comparing BTZ or BTZ-containing regimens versus any non-BTZ treatments, the published CEAs were unlikely to cover all possible treatments for MM. For example, we did not find any published CEAs comparing BTZ or BTZ-containing regimens versus cyclophosphamide or bendamustine-based regimens, the conventional regimens used to treat refractory/relapsed MM. Thus, future CEAs comparing BTZ or BTZ-containing regimens versus all existing non-BTZ regimens are needed for comprehensive assessment of BTZ or BTZ-containing regimens for MM. Another major limitation of our review is the lack of quality assessment of the data used in the included cost-effectiveness analysis. Even though almost all included CEAs clearly indicated the data sources of treatment efficacy, the data sources for direct medical costs and quality of life were usually not well described. Thus, our review was unable to assess the quality of data for costs and utility and explore the potential bias associated with these data in the cost-effectiveness analysis. This systematic review also included CEAs published in abstracts that usually contain insufficient information for quality assessment. Thus, caution is needed when interpreting the cost-effectiveness of BTZ or BTZ-containing regimens from abstracts. Finally, the administration practices for BTZ treatment has been evolving, and the current practices might differ from the practices applied in the included cost-effectiveness analysis. For example, BTZ administration is now changing from being an intravenous to a subcutaneous injection, which could reduce neuropathic toxicity and improve treatment convenience. The treatment schedule for less intensively treated patients could change from a twice weekly schedule to a weekly schedule. Thus, the published cost-effectiveness of BTZ should be interpreted after taking into account the potential impact of health benefits and costs associated with current practices for BTZ treatment.
In summary, the systematic review of published CEAs suggested that BTZ and BTZ-containing regimens appeared cost-effective treatment strategies for MM in most circumstances according to the cost-effectiveness threshold defined by WHO. However, this systematic review also observed conflicting cost-effectiveness for the comparisons of VMP versus MPT for previously untreated and SCT-ineligible MM patients and BTZ versus LEN/DEX for relapsed/refractory MM due to the bias associated with indirect comparison and model structure. Thus, the conflicting cost-effectiveness of VMP relative to MPT and BTZ relative to LEN/DEX needs further head-to-head comparison studies for clarification. Finally, this systematic review found that the impact of a country setting on the cost-effectiveness could be substantial. Because most of the included CEAs were based in high-income countries, caution is needed when interpreting the published cost-effectiveness of BTZ or BTZ-containing regimens for middle or low-income countries.
Supplementary materials
Table S1.
Summary of excluded references and their exclusion reasons
| Excluded references | Exclusion reason |
|---|---|
| Mehta et al1 | Full manuscript has been published |
| Fragoulakis et al2 | Full manuscript has been published |
| Wang et al3 | Full manuscript has been published |
| Wang et al4 | Full manuscript has been published |
| Wang et al5 | Full manuscript has been published |
| Wang et al6 | Full manuscript has been published |
| Popat et al7 | BTZ was not studied |
| Schey et al8 | BTZ was not studied |
| Tuffaha et al9 | BTZ was not studied |
| Gaultney et al10 | BTZ was not studied |
| Gaultney et al11 | Not CEA (cohort study for clinical outcomes) |
| Hornberger et al12 | Not CEA (cost study) |
| Teitelbaum et al13 | Not CEA (cost study) |
| Vitale et al14 | Not CEA (cost study) |
| Gaultney et al15 | Insufficient information |
| Gibbons et al16 | Insufficient information |
| Blommestein et al17 | Insufficient information |
| Durie et al18 | Outcome measures for health benefits were not LY and/or QALY |
| Gooding et al19 | Outcome measures for health benefits were not LY and/or QALY |
| Schey and Higginson20 | Review |
| Haycox and Tolley21 | Review |
| Cecchi et al22 | Letter |
| Lucioni et al23 | Non-English |
| Vandekerckhove et al24 | Not MM patients |
| Shustik et al25 | Budget impact analysis |
| Blommestein et al26 | Insufficient information |
| Gooding et al27 | Control group was lacking for the calculation of ICER |
Abbreviations: BTZ, bortezomib; CEA, cost-effectiveness analyses; LY, life year; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio; MM, multiple myeloma.
References
- 1.Mehta J, Duff S, Gupta S. Bortezomib is cost-effective for the treatment of relapsed and refractory multiple myeloma. Blood. 2003;102(11):500A–501A. [Google Scholar]
- 2.Fragoulakis V, Kourlaba G, Maniadakis N. Economic evaluation of lenalidomide in the management of previously treated multiple myeloma (PTMM) patients in Greece. Value Health. 2012;15(4):A104–A105. [Google Scholar]
- 3.Wang ST, Huang H, Ba-Mancini A, et al. The cost-effectiveness of bortezomib plus melphalan and prednisone versus lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment for the initial treatment of multiple myeloma in the United States. Blood. 2010;116(21):A62. doi: 10.1634/theoncologist.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ST, Huang H, Ba-mancini A, et al. The cost-effectiveness of bortezomib plus melphalan and prednisone versus lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment for the initial treatment of multiple myeloma in the United States. Value Health. 2011;14(3):A62. [Google Scholar]
- 5.Wang ST, Huang H, Shi H, et al. The cost-effectiveness of bortezomib for the initial treatment of multiple myeloma in the United States. Blood. 2009;114(22):561–562. [Google Scholar]
- 6.Wang ST, Huang H, Shi H, et al. Modeling the cost-effectiveness of bortezomib for the initial treatment of multiple myeloma in the United States. Value Health. 2010;13(3):A210. [Google Scholar]
- 7.Popat R, Dickson J, Khan I, et al. An alternate day dosing strategy for lenalidomide in multiple myeloma improves cost-effectiveness whilst maintaining efficacy. Blood. 2011;118(21):1797. [Google Scholar]
- 8.Schey S, Stern S, Dhanasiri S, et al. Cost-effectiveness of lenalidomide in multiple myeloma patients with 1 prior therapy in England and Wales. Blood. 2011;118(21):1789–1790. [Google Scholar]
- 9.Tuffaha HW, Hussein AA, Abdel-Rahman FA. Comparative cost utility analysis of plerixafor plus GCSF versus cyclophosphamide plus GCSF as salvage mobilization regimens in multiple myeloma patients. Biol Blood Marrow Transplant. 2012;18(2):S248. [Google Scholar]
- 10.Gaultney JG, Redekop WK, Sonneveld P, et al. Early-stage economic evaluation of stratified medicine in multiple myeloma. Value Health. 2013;16(7):A417. [Google Scholar]
- 11.Gaultney J, Francenken M, Van Gils CW, et al. Outcomes research of bortezomib indicated for multiple myeloma in the context of the Dutch reimbursement policy for expensive medicines: threats to the internal validity of the incremental effectiveness estimate. Value Health. 2009;12(7):A259. [Google Scholar]
- 12.Hornberger J, Rickert J, Dhawan R, et al. Comparisons of regimen costs of bortezomib and lenalidomide for treatment of advanced, relapsed multiple myeloma. Haematologica. 2008;93:477. [Google Scholar]
- 13.Teitelbaum A, Ba-Mancini A, Huang H, et al. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data. Oncologist. 2013;18(1):37–45. doi: 10.1634/theoncologist.2012-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitale V, Pinto Neto JV, Asano E. Total treatment costs analysis between subcutaneous and intravenous bortezomib under Brazilian private health care system perspective. Value Health. 2014;17(3):A77. [Google Scholar]
- 15.Gaultney J, Franken M, Huijgens PC, et al. Real-world cost-effectiveness of bortezomib in relapsed or refractory multiple myeloma in The Netherlands. Value Health. 2010;13(7):A467. [Google Scholar]
- 16.Gibbons CJ, Yong K, Roberts G. Pegylated liposomal doxorubicin in combination with bortezomib for the treatment of relapsed multiple myeloma-a cost-effectiveness study for Scotland. Value Health. 2008;11(6):A467. [Google Scholar]
- 17.Blommestein HM, Verelst SG, De Groot S, et al. One line does not make a picture: Real-world cost-effectiveness of multiple myeloma treatments using a full disease model. Blood. 2013;122(21):2930. [Google Scholar]
- 18.Durie BG, Binder G, Pashos CL, et al. Cost-effectiveness of treatments (TX) for newly-diagnosed multiple myeloma patients (NDMM PTS) Clin Lymphoma Myeloma Leuk. 2013;13:S216. [Google Scholar]
- 19.Gooding S, Lau IJ, Sheikh M, et al. Double refractory myeloma: analysis of clinical outcomes and medical-resource utilisation in a single centre. Blood. 2013;122(21):1727. [Google Scholar]
- 20.Schey S, Higginson I. Cost-effectiveness of lenalidomide in multiple myeloma. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):229–238. doi: 10.1586/erp.10.19. [DOI] [PubMed] [Google Scholar]
- 21.Haycox A, Tolley K. Bortezomib economic evaluation limited. Hospital Pharma. 2005;12(1):35–36. [Google Scholar]
- 22.Cecchi M, Caccese E, Messori A, et al. Cost-effectiveness of bortezomib in multiple myeloma. Pharma World Sci. 2007;29(5):485–486. [Google Scholar]
- 23.Lucioni C, Cavo M, Mazzi S, et al. Economic evaluation of two therapeutic sequences in the treatment of relapsed/refractory multiple myeloma. Pharmaco Econ. 2013;15(1):1–8. [Google Scholar]
- 24.Vandekerckhove S, Lamotte M, Iannazzo S, et al. Cost utility analysis of bortezomib in a biomarker positive subgroup of relapsed and refractory folicular lymphoma. Value Health. 2012;15(7):A427. [Google Scholar]
- 25.Shustik J, Tay J, Hollmann S, et al. A Canadian cost impact analysis comparing maintenance therapy with bortezomib versus lenalidomide in multiple myeloma patients ineligible for stem cell transplant. Value Health. 2014;17(3):A76. [Google Scholar]
- 26.Blommestein HM, Verelst SG, de Groot S, et al. A cost-effectiveness analysis of real-world treatment for elderly patients with multiple myeloma using a full disease model. Eur J Haematol. 2016;96(2):198–208. doi: 10.1111/ejh.12571. [DOI] [PubMed] [Google Scholar]
- 27.Gooding S, Lau IJ, Sheikh M, et al. Double relapsed and/or refractory multiple myeloma: clinical outcomes and real world healthcare costs. PLoS One. 2015;10(9):e0136207. doi: 10.1371/journal.pone.0136207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
We want to thank the authors of the included CEAs who helped make our review comprehensive and meaningful for future clinical and reimbursement decision-making for patients with multiple myeloma. Dr Wendong Chen formulated the research idea and developed the study design for literature search, data extraction, and data analysis. Yi Chen and Fen Du conducted the literature search and assessed the studies for inclusion eligibility. Yi Chen, Fen Du, and Huan Zhan conducted the data extraction and data analysis. Dr Yicheng Yang participated in the discussion on study design and critically reviewed the manuscript. Dr Wendong Chen developed the manuscript. All authors read the manuscript and have approved the submission of this developed manuscript. The abstract of this paper was presented at the ISPOR 18th Annual European Congress as a poster presentation in Milan, Italy. The poster’s abstract was published in “Poster Abstracts” in Value in Health, 2015;18(7):A456.
Footnotes
Disclosure
Dr Wendong Chen is the founder of Normin Health, a Canadian organization receiving industry funds for health economics and outcomes research. Dr Yicheng Yang is the employee of Xian Janssen. Yi Chen, Fen Du, and Huan Zhan are the employees of the representative office of Normin Health in Changsha, People’s Republic of China. The authors report no other conflicts of interest in this work.
References
- 1.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Pulte D, Redaniel MT, Brenner H, Jansen L, Jeffreys M. Recent improvement in survival of patients with multiple myeloma: variation by ethnicity. Leuk Lymphoma. 2014;55(5):1083–1089. doi: 10.3109/10428194.2013.827188. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Barlogie B, Berenson J, et al. A Phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 5.Jagannath S, Barlogie B, Berenson J, et al. A Phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 7.San Miguel JF, Schlag R, Khuageva NK, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2012;31:448–455. doi: 10.1200/JCO.2012.41.6180. [DOI] [PubMed] [Google Scholar]
- 8.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply? Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 10.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cost effectiveness and strategic planning (WHO-CHOICE) [Accessed March 6, 2016]. Available from: http://www.who.int/choice/costs/CER_thresholds/en/
- 12.Kouroukis T, White D, Kruse M, Lawrence D, Trambitas C, Cheung MC. Cost-utility of bortezomib in induction treatment prior to autologous stem-cell transplantation (ASCT) in previously untreated multiple myeloma patients in Canada. Blood. 2013;122(21):1735. [Google Scholar]
- 13.Mucha J, Walczak J, Tronczynski K, Skrzekowska-Baran I. Bortezomib-based regimens used as induction in newly diagnosed multiple myeloma (ndMM) patients eligible for stem cell transplantation (SCT) the cost-utility analysis. Value Health. 2014;17(3):A229. [Google Scholar]
- 14.Van Beurden-Tan C, Rosinol L, Diels J, et al. Cost-effectiveness of induction treatment with bortezomib added to thalidomide and dexamethasone in newly diagnosed multiple myeloma patients eligible for autologous stem cell transplantation in Germany. Value Health. 2013;16(7):A409–A410. [Google Scholar]
- 15.Rickert JB, Hornberger J, Liwing J, Aschan J, Gjonnes L, Dhawan R. Bortezomib is cost-effective for first-line treatment of multiple myeloma in Sweden. Value Health. 2010;13(3):A40. [Google Scholar]
- 16.Garrison LP, Jr, Wang ST, Huang H, et al. The cost-effectiveness of initial treatment of multiple myeloma in the US with bortezomib plus melphalan and prednisone versus thalidomide plus melphalan and prednisone or lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment. Oncologist. 2013;18(1):27–36. doi: 10.1634/theoncologist.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oster G, Berger A, Bornheimer R, Binder G, Nagarwala Y. Cost-effectiveness of lenalidomide and bortezomib in patients with previously untreated multiple myeloma (MM) Blood. 2013;122(21):5604. [Google Scholar]
- 18.Picot J, Cooper K, Bryant J, Clegg AJ. The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technol Assess. 2011;15(41):1–204. doi: 10.3310/hta15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoong K, Attard C, Jivraj F, Shustik C, Reece D. Cost effectiveness analysis of bortezomib in previously untreated multiple myeloma patients in Canada. Value Health. 2009;12(7):A272. [Google Scholar]
- 20.Cavenagh JD, Belch AR, Hulin C, et al. Cost-effectiveness in newly diagnosed multiple myeloma (NDMM): lenalidomide plus low-dose dexamethasone (RD) versus bortezomib plus melphalan and prednisone (VMP) Haematologica. 2014;99:379. [Google Scholar]
- 21.Bagust A, Haycox A, Mujica-Mota R, Dhawan R, Dubois D. Cost-effectiveness of bortezomib (VELCADE) for relapsed and refractory multiple myeloma. Value Health. 2004;7(6):670. [Google Scholar]
- 22.Mehta J, Duff SB, Gupta S. Cost effectiveness of bortezomib in the treatment of advanced multiple myeloma. Manag Care Interface. 2004;17(9):52–61. [PubMed] [Google Scholar]
- 23.Felix J, Almeida J, Vandewalle B. A comprehensive cost-effectiveness analysis of lenalidomide for multiple myeloma patients who have received at least one prior therapy. Value Health. 2012;15(4):A218. doi: 10.1016/j.jval.2014.08.111. [DOI] [PubMed] [Google Scholar]
- 24.Fragoulakis V, Kastritis E, Psaltopoulou T, Maniadakis N. Economic evaluation of therapies for patients suffering from relapsed-refractory multiple myeloma in Greece. Cancer Manag Res. 2013;5(1):37–48. doi: 10.2147/CMAR.S43373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornberger J, Rickert J, Dhawan R, Liwing J, Aschan J, Lothgren M. The cost-effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85(6):484–491. doi: 10.1111/j.1600-0609.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Spencer M, Gauthier A, Pacou M. A cost-effectiveness analysis for second-line treatment of relapsed/refractory (RR) multiple myeloma (MM) in the United Kingdom. Value Health. 2011;14(7):A452. [Google Scholar]
- 27.Liwing J, Gjonnes L, Sandberg I, et al. The cost-effectiveness of bortezomib for relapsed/refractory multiple myeloma – a nordic comparison. Value Health. 2009;12(7):A273. [Google Scholar]
- 28.Moller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14(6):690–697. doi: 10.3111/13696998.2011.611841. [DOI] [PubMed] [Google Scholar]
- 29.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 Phase III trial. J Clin Oncol. 2010;28(30):4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 30.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosiñol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized Phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 32.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 33.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:4848–4857. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 34.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 36.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–1238. doi: 10.1182/blood-2011-02-338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MM-015 Investigators Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 38.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 39.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 40.Multiple Myeloma (009) Study Investigators Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 41.Multiple Myeloma (010) Study Investigators Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 42.van Agthoven M, Segeren CM, Buijt I, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised Phase III study. Eur J Cancer. 2004;40(8):1159–1169. doi: 10.1016/j.ejca.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Kontodimopoulos NL, Aletras VH, Paliouras D, et al. Mapping the cancer-specific EORTC QLQ-C30 to the preference-based EQ-5D, SF-6D, and 15D instruments. Value Health. 2009;12(8):1151–1157. doi: 10.1111/j.1524-4733.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 44.Pooch UW, Wall JA. Discrete Event Simulation: A Practical Approach. Vol. 4. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- 45.Stuart EA, Bradshaw CP, Leaf PJ. Assessing the generalizability of randomized trial results to target populations. Prev Sci. 2015;16(3):475–485. doi: 10.1007/s11121-014-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Summary of excluded references and their exclusion reasons
| Excluded references | Exclusion reason |
|---|---|
| Mehta et al1 | Full manuscript has been published |
| Fragoulakis et al2 | Full manuscript has been published |
| Wang et al3 | Full manuscript has been published |
| Wang et al4 | Full manuscript has been published |
| Wang et al5 | Full manuscript has been published |
| Wang et al6 | Full manuscript has been published |
| Popat et al7 | BTZ was not studied |
| Schey et al8 | BTZ was not studied |
| Tuffaha et al9 | BTZ was not studied |
| Gaultney et al10 | BTZ was not studied |
| Gaultney et al11 | Not CEA (cohort study for clinical outcomes) |
| Hornberger et al12 | Not CEA (cost study) |
| Teitelbaum et al13 | Not CEA (cost study) |
| Vitale et al14 | Not CEA (cost study) |
| Gaultney et al15 | Insufficient information |
| Gibbons et al16 | Insufficient information |
| Blommestein et al17 | Insufficient information |
| Durie et al18 | Outcome measures for health benefits were not LY and/or QALY |
| Gooding et al19 | Outcome measures for health benefits were not LY and/or QALY |
| Schey and Higginson20 | Review |
| Haycox and Tolley21 | Review |
| Cecchi et al22 | Letter |
| Lucioni et al23 | Non-English |
| Vandekerckhove et al24 | Not MM patients |
| Shustik et al25 | Budget impact analysis |
| Blommestein et al26 | Insufficient information |
| Gooding et al27 | Control group was lacking for the calculation of ICER |
Abbreviations: BTZ, bortezomib; CEA, cost-effectiveness analyses; LY, life year; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio; MM, multiple myeloma.