Abstract
An 80 year old lady with a history of metastatic sigmoid carcinoma presented with expressive dysphasia and unsteady gait 4 days after commencement of adjuvant capecitabine chemotherapy. MRI demonstrated restricted diffusion and T2/FLAIR hyperintensity involving the course of the bilateral corticospinal tracts, the corpus callosum and the middle cerebellar peduncles. Discontinuation of chemotherapy lead to symptom resolution in 2 days; repeat MRI at 2 months demonstrated reversal of the diffusion changes and improvement of the previous T2W/FLAIR hyperintensity. This report describes the first case of capecitabine induced leukoencephalopathy causing restricted diffusion along the corticospinal tracts, which should be differentiated from other entities that involve the corticospinal tracts (i.e. amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS), hypoglycemic coma, etc.)
Keywords: Capecitabine induced leukoencephalopathy, corticospinal tracts, capecitabine toxicity, drug-induced leukoencephalopathy, corpus callosum
CASE REPORT
An 80 year old lady presented with a 3 day duration of transient expressive dysphasia, non-vertiginous giddiness, and unsteady tandem gait. Her past medical history was significant for carcinoma of the sigmoid colon with bladder and pulmonary metastases, for which she underwent anterior resection of the rectum as well as resection of the bladder tumor. Adjuvant capecitabine chemotherapy at a dose of 1000mg twice daily was commenced 4 days prior to presentation.
CT brain performed upon presentation was unremarkable except for a small chronic lacunar infarct in the right lentiform nucleus (Fig. 2). MRI performed 2 days later demonstrated symmetric areas of diffusion restriction and mild T2/FLAIR hyperintensity in the bilateral corticospinal tracts, the splenium of the corpus callosum, and in the middle cerebellar peduncles (Fig 1 and 2). No signal changes on other performed sequences (i.e. T1, T1 post contrast) were seen. Although there was no known association of capecitabine leukoencephalopathy with corticospinal tract involvement in the documented literature, this provisional diagnosis was made in account of the close temporal relationship between commencement of chemotherapy and the patient’s symptoms. Capecitabine chemotherapy was thus discontinued. The patient’s neurological symptoms subsequently resolved 2 days later, and a repeat MRI performed 2 months after admission demonstrated complete resolution of diffusion changes, and improvement of the previous T2W/FLAIR hyperintensity (Fig. 3). The patient subsequently declined alternative adjuvant chemotherapeutic agents and opted for best supportive medical care in her treatment plan. The patient was still alive 11 months after the episode of Capecitabine-induced leukoencephalopathy, although she had underwent resection of a metachronous recurrence of the rectal carcinoma 9 months after cessation of Capecitabine.
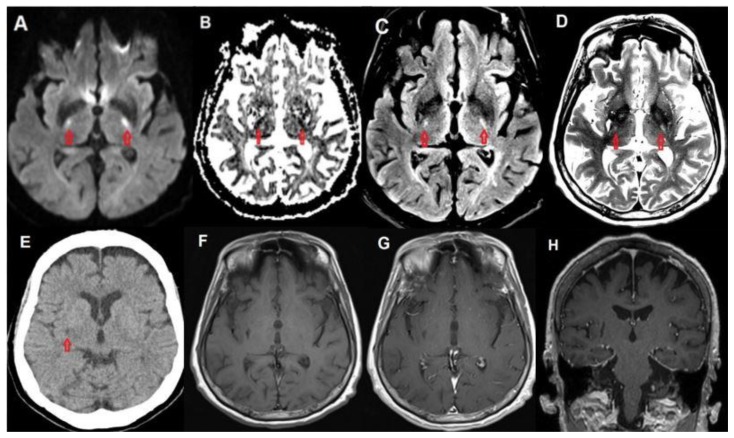
Figure 2.
80 year old female with capecitabine-induced leukoencephalopathy.
FINDINGS: Restricted diffusion (A, B), as well as FLAIR (C) and T2 (D) hyperintensity is shown in the bilateral corticospinal tracts at the level of the posterior limb of the internal capsules. Initial CT (E) is unremarkable except for a small chronic lacunar infarct in the right lentiform nucleus. Pre contrast (F) and post contrast axial (G) and coronal (H) T1 weighted images demonstrate no corresponding signal abnormality.
TECHNIQUE: CT. mAs: 2146 kV: 120 Slice Thickness: 3mm TECHNIQUE: MRI. Magnet Strength: 3.0 Telsa. T1 - Plane: Axial. TE: 8.4ms TR 500ms FOV: 210mm × 210mm Matrix: 265 × 256 Slice Thickness: 4mm. T2 - Plane: Axial. TE: 92ms TR: 2830ms FOV: 210mm × 210mm Matrix: 384 × 384 Slice Thickness: 4mm. FLAIR - Plane: Axial. TE: 132ms TR: 6900ms FOV: 210mm × 210mm Matrix: 256 × 256 Slice Thickness: 4mm. T1 Contrast Axial - TE: 8.4ms TR 500ms FOV: 210mm × 210mm Matrix: 265 × 256 Slice Thickness: 4mm. T1 Contrast Coronal - TE: 2.5ms TR: 1700ms FOV: 187 × 230 Matrix: 412 × 512 Slice Thickness: 0.9mm. Contrast: IV Magnevist 10mls.
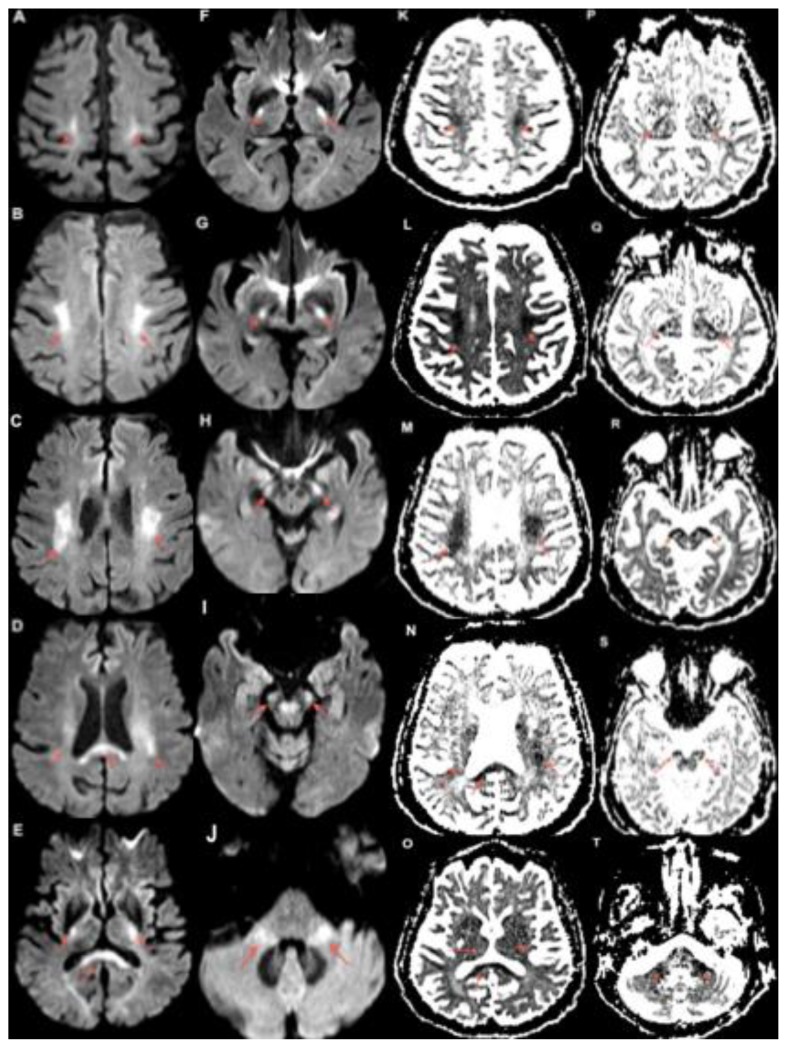
Figure 1.
80 year old female with capecitabine-induced leukoencephalopathy.
FINDINGS: Diffusion weighted MRI imaging demonstrates restricted diffusion along the course of the bilateral corticospinal tracts. The bilateral centrum semiovale (A, B, K, L), corona radiata (C, D, M, N), posterior limbs of the internal capsules (E, F, G, P, Q, R) and cerebral peduncles (H, I, R, S) are involved. There is also involvement of the corpus callosum (D, E, N, O) and middle cerebellar peduncles (J, T) seen.
TECHNIQUE: MRI. Magnet Strength: 3.0 Telsa. Plane: Axial. DWI b=1000 s/mm2 TE: 101ms TR: 4700ms FOV: 230mm × 230mm. Matrix: 192 × 192 Slice Thickness: 4.00mm
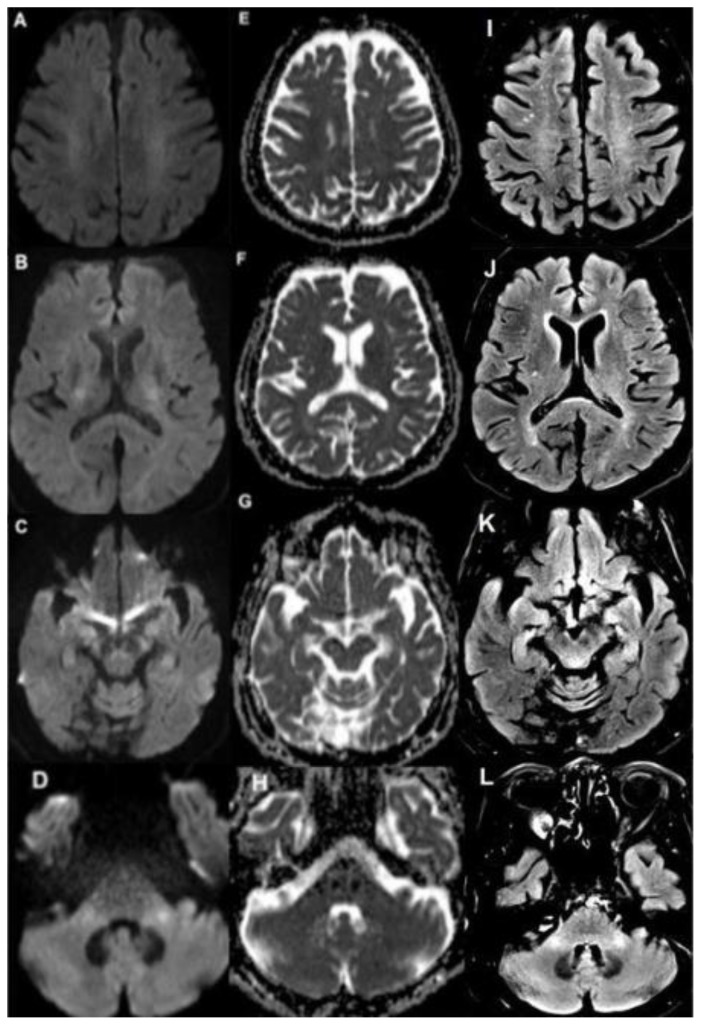
Figure 3.
80 year old female with capecitabine-induced leukoencephalopathy.
FINDINGS: Follow up MRI demonstrates resolution of restricted diffusion and improvement of the previous FLAIR hyperintensity along the course of the bilateral corticospinal tracts involving the bilateral corona radiata (A, E, I), posterior limb of the internal capsules and corpus callosum (B, F, J), cerebral peduncles (C, G, K), as well as the middle cerebellar peduncles (D, H, L). TECHNIQUE: MRI. Magnet Strength: 3.0 Telsa. DWI - Plane: Axial. DWI b=1000 s/mm2 TE: 101ms TR: 4500ms FOV: 230mm × 230mm. Matrix: 163 × 192 Slice Thickness: 4.00mm. FLAIR - Plane: Axial. TE: 132ms TR: 6800ms FOV: 220mm × 220mm Matrix: 256 × 256 Slice Thickness: 4.00mm.
DISCUSSION
Etiology & Demographics
Demographics
Capecitabine is an oral fluoropyrimidine chemotherapeutic agent used primarily in the treatment of colorectal and breast cancer. Capecitabine-induced leukoencephalopathy was first described by Niemann et al in 2004 [1]. There are to date 12 cases of this disease with associated MRI findings of leukoencephalopathy reported [1–8]. The age of patients affected by this condition ranges from 40–82 years. This entity appears to be more common in females, with a female to male ratio of 5:1. Capecitabine was administered for colorectal (6 cases [4–8]), breast (5 cases [1–3]) and pancreatic (1 case [2]) malignancy respectively.
Etiology
Capecitabine is converted in a three-step cascade to its active product, 5-fluorouracil (5-FU), an antineoplastic agent that principally acts to inhibit thymidine synthesis and DNA replication. Although the exact mechanism of capecitabine neurotoxicity is not well understood, it is known that an intermediate metabolite of capecitabine, 5′-deoxy-5-fluorouridine (5′-DFUR), is able to cross the blood brain barrier to enter the CSF [9], and that thymidine phosphorylase, the last enzyme in the three-step cascade of capecitabine conversion to 5-FU, is found preferentially in the white matter tracts as compared to grey matter [10]. A separate murine study [11] of 5-fluorouracil (5-FU), has also been shown to cause acute as well as delayed damage to the myelinated tracts of the central nervous system. These findings when taken together have been postulated by Lyros et al [6] to explain the propensity of white matter tract involvement in this disease entity.
Clinical and imaging findings
There is a wide spectrum of symptoms reported in the clinical presentation of capecitabine-induced leukoencephalopathy, ranging from milder symptoms such as fatigue [2,7] and nausea [2] to more severe symptoms such as dysphasia [2,3,5,7] seizures [1,6] and drowsiness [4]. The onset of symptoms has been reported to occur as soon as 3–7 days after drug initiation [1–5], although other cases of symptom onset after 1–2 months have also been reported [6,8].
Radiological findings in this condition typically include signal changes in the cerebral white matter seen on MRI, specifically restricted diffusion and/or increased signal intensity on T2/FLAIR sequences. There appears to be a strong propensity for involvement of the corpus callosum in this condition [2,4,5], which is also seen in our case. The areas of white matter involvement previously described include the subcortical regions [4,6], the periventricular regions [2,8], as well as the posterior parietal and anterior thalamic regions [3]. However, this is the first case to document involvement of the course of the bilateral corticospinal tracts, warranting consideration of this condition among other entities that also involve the corticospinal tracts.
Treatment and Prognosis
Capecitabine-induced leukoencephalopathy is treated by the cessation of capecitabine chemotherapy. Excellent recovery upon cessation of capecitabine with no or minimal residual deficit has been reported to occur within several days in all reported cases, with one case report [5] reporting symptom resolution within a day of chemotherapy cessation. Interval follow up imaging that documented near complete resolution of the earlier detected imaging abnormalities was performed in at least 4 cases [1,4,6], including our current case.
Differential Diagnoses
This report provides the first reported case of capecitabine-induced leukoencephalopathy involving the course of the bilateral corticospinal tracts. It is worth reiterating that the provisional diagnosis in this case was premised upon the close temporal relationship between chemotherapy commencement and the patient’s symptoms, despite corticospinal tract involvement in capecitabine-induced leukoencephalopathy not being described in the existing literature. Thus in the appropriate clinical context, this disease entity should also warrant consideration in the radiological differential diagnosis of leukoencephalopathy involving the bilateral corticospinal tracts, which would include amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS), hypoglycaemic coma, X-linked adrenoleukodystrophy (ALD), Wilson disease as well as Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) (Table 3).
Table 3.
Differential diagnosis of leukoencephalopathy involving the corticospinal tracts.
| Diagnosis | MRI findings | Clinical issues |
|---|---|---|
| Capecitabine induced leukoencephalopathy | - Restricted diffusion and/or increased T2W/FLAIR signal in the cerebral white matter - Involvement of the course of the bilateral corticospinal tracts, as well as of the corpus callosum, has been described |
Excellent recovery upon cessation of Capecitabine with no or minimal residual deficit |
| Amyotrophic lateral sclerosis (ALS) | - Bilateral hyperintensities along the corticospinal tracts extending from the corona radiata to the brainstem seen on T2WI and FLAIR, reduced diffusion on DWI. | - Mixed upper and lower motor neuron signs - Mean age of onset: 62 years |
| Primary lateral sclerosis (PLS) | - Bilateral hyperintensities along corticospinal tracts on T2WI and reduced diffusion on DWI | - Upper motor neuron without lower motor neuron signs |
| X-linked Adrenoleukodystrophy (XAD) | - Symmetrical, confluent demyelination in the peritrigonal region T1W: reduced signal of involved white matter, enhancement present T2W/FLAIR: Increased signal of involved white matter Bilateral corticospinal tract involvement described |
- Spectrum of phenotypes: childhood cerebral forms, adrenomyeloneuropathy, isolated adrenal insufficiency |
| Hypoglycaemic coma | - Parietal/occipital lobe, amygdala, basal ganglia involvement T1W: Gyral swelling, sulcal effacement T2W: Parieto-occipital infarcts, tends to spare cerebral white matter DWI: reduced diffusion along bilateral corticospinal tracts |
- Altered mental state - Associated with hypoglycaemic state from Diabetes medications without adequate glucose intake or glucose utilization |
| Wilson disease | T1W: Reduced in basal ganglia T2W: Bilateral symmetrical hyperintensity in putamina, thalami, caudate nuclei, globus pallidi Bilateral corticospinal tract involvement described |
- Inherited copper metabolism disorder - Autosomal recessive - Hepatic, neurological and psychiatric manifestations |
| Adult onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) | White matter lesions that are hyperintense on T2W/FLAIR and hypointense on T1W are seen. Bilateral corticospinal tract involvement has been described. | - Autosomal dominant - Range: early adulthood to 8th decade of life - Supportive management |
Amyotrophic lateral sclerosis is a progressive degenerative motor neuron disease that classically presents as a combination of upper and lower motor neuron signs and symptoms. Bilateral hyperintensities and reduced diffusion along the corticospinal tracts extending from the corona radiata to the brainstem are seen on T2WI/FLAIR and DWI MRI sequences respectively. ALS has an annual incidence of 1–3 cases per 100,000/year and a prevalence of 3–7 cases per 100,000 [12–14]. Although the etiology of this disease is as yet unknown, ALS is associated with increased age (mean age of onset − 62 years [15]) and a positive family history, with sporadic ALS accounting for 90 percent of cases and familial forms the remaining 10 percent [16]. The median survival of ALS from the time of diagnosis is 30–37 months [17–18]. The treatment of ALS is primarily symptomatic, with Riluzole the only known agent with a modest effect on slowing disease progression [19].
Primary lateral sclerosis is a progressive degenerative motor neuron disease that unlike ALS is associated with upper motor neuron signs and symptoms without lower motor neuron involvement. The imaging findings on MRI of PLS are similar to that of ALS, with bilateral hyperintensities and reduced diffusion along the corticospinal tracts seen on T2WI and DWI sequences respectively [20]. The etiology is this disease is yet unknown. The true incidence of PLS is not known, with a prevalence of 2 per million inferred from a study by Pringle at al [21]. The mean age of disease onset is 55 years [22]. PLS portends a better prognosis than ALS, with a median survival of 20 years [22]. The treatment of PLS is symptomatic.
Hypoglycaemic coma is a severe presentation of the neuroglycopenic symptoms of hypoglycemia. The parietal and occipital lobes, amygdala, and basal ganglia appear to be involved, with gyral swelling and sulcal effacement seen on T1W sequences and parieto-occipital infarcts that appear to spare the cerebral white matter seen on T2W sequences. Diffusion abnormalities involving the corticospinal tracts have also been reported [23,24]. Insulin and oral hypoglycaemic therapy for the treatment of diabetes mellitus are the most common causes of hypoglycemia, with rarer causes including beta-cell tumors. Hypoglycemic coma is treated by the immediate administration of glucagon as well as IV glucose infusion. Although the prevalence of hypoglycaemic coma is not well characterized, the incidence of severe hypoglycemia, defined as symptoms incapacitating enough to require the necessary administration of carbohydrate or glucagon to the affected patient by another person [25], is approximately 1.15 to 0.35 events per patient per year for type 1 and type 2 diabetes respectively [26].
X-linked adrenoleukodystrophy is a disorder of peroxisome oxidation that results in the accumulation of very long chain fatty acids in tissues. ALD comprises of a spectrum of differing phenotypes. These include childhood cerebral forms that present as learning disabilities and behavior problems with progressive neurological deterioration; adrenomyeloneuropathy, which presents in adults as spinal cord dysfunction with associated cerebellar symptoms and adrenal insufficiency; as well as isolated adrenal insufficiency. Symmetrical, confluent demyelination changes in the white matter of the peritrigonal region have been described on MRI, with reduced signal on T1W, increased signal on T2W, reduced diffusion on DWI and contrast enhancement seen. Patterns of corticospinal tract involvement have also been described in the adult population, which is associated with and slower progression of disease [27]. The disease is X-linked in inheritance. Disease frequency in the United States is estimated at 1 in 42,000 in hemizygotes and 1 in 16,800 in hemizygotes plus heterozygotes respectively [28]. Treatment strategies for ALD would include corticosteroid replacement therapy for adrenal insufficiency, as well as the use of dietary therapy and hematopoietic stem cell transplantation in selected cases.
Wilson disease is caused by an autosomal recessive genetic abnormality of cellular copper transport. The impaired excretion of copper leads to its accumulation within organs such as the liver and brain, resulting in hepatic manifestations such as hepatitis and cirrhosis, neurologic manifestations such as dysarthria, gait abnormalities and dystonia, as well as psychiatric manifestations such as depression. Kayser-Fleischer rings may be seen in the cornea. On MRI, bilateral symmetrical hyperintensities in the putamina, thalami, caudate nuclei, globus pallidi have been reported on T2W sequences, with basal ganglia hypodensity on T1W sequences. A study of patterns of white matter abnormalities in Wilson disease has described involvement of the bilateral corticospinal tracts [29]. Normal radiological findings may also be seen on MRI in pre-symptomatic patients. The prevalence of the disease is approximately 1 case per 30,000 in most populations [30]. The disease tends to present between 5–40 years of age, although cases in patients younger than 3 years and in adults older than 70 years have been reported [31]. Treatment in based on disease control by copper chelation, with liver transplantation a consideration in patients with acute liver failure.
Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia is an autosomal dominant disease characterized by executive dysfunction, memory decline, personality changes, motor impairment and seizures. On MRI, white matter lesions which are hyperintense on T2W/FLAIR and hypointense on T1W are seen, with involvement of the corticospinal tracts being previously described [32]. The mean age of onset is usually in the fourth decade, but ranges from early adulthood to the 8th decade of life [32]. The course of the disease may range from 2 to more than 30 years, with a mean of 8 years [32]. The prevalence of ALSP has not yet been reported. No specific therapy is currently available for ALSP aside from supportive management [33].
TEACHING POINT
Capecitabine, an oral chemotherapeutic agent, uncommonly causes leukoencephalopathy that presents as diffusion/T2W/FLAIR abnormalities of the cerebral white matter in varied patterns, including that of the bilateral corticospinal tracts. This disease entity should thus be considered in the differential diagnosis of leukoencephalopathy involving the corticospinal tracts.
Table 1.
Summary table of Capecitabine induced leukoencephalopathy.
| Etiology | Not known, injury to cells in the cerebral white matter |
| Gender Ratio | Female > Male (Ratio of 5:1 in 12 cases) |
| Age predilection | Adult population (Range: 40–82 years) |
| Risk factors | Capecitabine |
| Treatment | Cessation of Capecitabine chemotherapy |
| Prognosis | Excellent recovery upon cessation of Capecitabine with no or minimal residual deficit |
| Findings on imaging | Restricted diffusion and/or increased T2W/FLAIR signal in the cerebral white matter |
Table 2.
Reported case characteristics of Capecitabine induced leukoencephalopathy.
| Age/Sex | Tumor | Symptom Onset after Capecitabine started | Symptom Resolution after Capecitabine stopped | MRI Findings (i.e. regions of increased signal on T2W/FLAIR, and/or restricted diffusion on DWI) | Symptom | Source |
|---|---|---|---|---|---|---|
| 80F | Sigmoid | 4 days | Within days, exact timing not stated | DWI: Corticospinal tracts; splenium of corpus callosum; middle cerebellar peduncles T2/FLAIR: cerebral white matter |
Expressive aphasia; unsteady gait | Current case |
| 61F | Breast | 7 days, previously had 6 cycles 1 year ago | 2 days | T2: Subcortical white matter changes in the bilateral cerebral hemispheres and basal ganglia. | Epileptic symptoms | Niemann1 |
| 52F | Breast | 6 days | 5 days | DWI/FLAIR/T2: Brachium pontis; splenium of corpus callosum, deep white matter | Vertigo; dysarthria; glove stocking numbness | Videnovic2 |
| 40F | Breast | 7 days | 3 days | DWI/FLAIR/T2: Splenium of corpus callosum; deep white matter | Word finding difficulty; pseudobulbar affect, glove and stocking numbness | Videnovic2 |
| 74F | Pancreas | 7 days | Within days, exact timing not stated | DWI/T2: deep periventricular areas without enhancement | Confusion, unsteady, word finding, right pronator drift, dysmetria | Videnovic2 |
| 44F | Breast | 3 days | Within days, exact timing not stated | FLAIR/T2: increased signal in splenium of corpus callosum, no enhancement | Fatigue, nausea, slurred speech, no signs | Videnovic2 |
| 41F | Breast | 7 days | 3 days | DWI: Bilateral posterior parietal white matter and anterior thalamus | Dysarthria | Baehring3 |
| 62M | Colon | 3 days | 9 days | DWI: subcortical white matter of cerebral hemispheres, corpus callosum | Dizziness and drowsiness | Endo4 |
| 63F | Colon | 5 days | 1 day | DWI: Corpus callosum and Corona radiata | Dysarthria, dysphagia | Shimoyama5 |
| 69M | Rectal | 75 days | Yes, timing not stated | FLAIR, T2, DWI: Subcortical white matter; ADC: corresponding increased signal | Recurrent generalized seizures | Lyros6 |
| 52F | Colorectal | 1st cycle | 6 days | T2: Deep white matter | Weakness, slurred speech, trismus, gait abnormalities, ocular changes | Couch7 |
| 82F | Colorectal | 42–63 days | Within days, exact timing not stated | Not detailed, reported as mild and diffuse hyperintensity of the white matter in periventricular regions of the bilateral frontal and parietal lobes | Dizziness, unsteadiness, confusion, leg weakness | Fantini8 |
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Matthew Ng, an oncologist from the National Cancer Centre, Singapore and Dr. John Ng, a neurologist from the National Neuroscience Institute, Singapore, for their contributions to the clinical assessment of this case.
ABBREVIATIONS
- 5-FU
5-Fluorouracil
- 5′-DFUR
5′-deoxy-5-fluorouridine
- ALD
X-linked adrenoleukodystrophy
- ALS
Amyotrophic Lateral Sclerosis
- ALSP
Adult-onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia
- CSF
Cerebrospinal Fluid
- CT
Computed Tomography
- DWI
Diffusion Weighted Imaging
- ETL
Echo Train Length
- FLAIR
Fluid-Attenuated Inversion Recovery
- FOV
Field of View (MRI)
- MRI
Magnetic Resonance Imaging
- PLS
Primary Lateral Sclerosis
- TE
Echo Time (MRI)
- TR
Repetition Time (MRI)
REFERENCES
- 1.Niemann B, Rochlitz C, Herrmann R, Pless M. Toxic encephalopathy induced by capecitabine. Oncology. 2004;66:331–335. doi: 10.1159/000078335. [DOI] [PubMed] [Google Scholar]
- 2.Videnovic A, Semenov I, Chua-Adajar R, et al. Capecitabine-induced multifocal leukoencephalopathy: a report of five cases. Neurology. 2005 Dec 13;65(11):1792–4. doi: 10.1212/01.wnl.0000187313.83515.7e. [DOI] [PubMed] [Google Scholar]
- 3.Baehring JM, Fulbright RK. Delayed leukoencephalopathy with stroke-like presentation in chemotherapy recipients. J Neurol Neurosurg Psychiatry. 2008 May;79(5):535–9. doi: 10.1136/jnnp.2007.123737. [DOI] [PubMed] [Google Scholar]
- 4.Endo A, Yoshida Y, Nakashima R, Takahashi N, Tanabe K. Capecitabine induces both cardiomyopathy and multifocal cerebral leukoencephalopathy. Int Heart J. 2013;54(6):417–20. doi: 10.1536/ihj.54.417. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama R, Ban T, Miyake K, et al. Early diagnosis of capecitabine-induced acute leukoencephalopathy by using diffusion-weighted MRI. Gan To Kagaku Ryoho. 2014 Oct;41(10):1251–3. [PubMed] [Google Scholar]
- 6.Lyros E, Walter S, Keller I, Papanagiotou P, Fassbender K. Subacute reversible toxic encephalopathy related to treatment with capecitabine: a case report with literature review and discussion of pathophysiology. Neurotoxicology. 2014 May;42:8–11. doi: 10.1016/j.neuro.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Couch L, Groteluschen DL, Stewart JA, Mulkerin L. Capecitabine-related neurotoxicity presenting as trismus. Clin Colorect Cancer. 2003;3:121–123. doi: 10.3816/CCC.2003.n.019. [DOI] [PubMed] [Google Scholar]
- 8.Fantini M, Gianni L, Tassinari D, et al. Toxic encephalopathy in elderly patients during treatment with capecitabine: literature review and a case report. J Oncol Pharm Pract. 2011 Sep;17(3):288–91. doi: 10.1177/1078155210374891. [DOI] [PubMed] [Google Scholar]
- 9.Heier MS, Heintz R, Fossa SD. Passage of 5′-dFUrd and its metabolites 5-FU and 5-FUH2 to CSF in a clinical phase 1 study. Acta Neurol Scand. 1986;74:240–4. doi: 10.1111/j.1600-0404.1986.tb07862.x. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa H, Yamada M, Fukushima M, Ikenaka K. Intrathecal 5-fluoro-2′-deoxyuridine (FdUrd) for the treatment of solid tumor neoplastic meningitis: an in vivo study. Cancer Chemother Pharmacol. 1999;43(3):247–56. doi: 10.1007/s002800050891. [DOI] [PubMed] [Google Scholar]
- 11.Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191(1–2):3. doi: 10.1016/s0022-510x(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 13.Logroscino G, Traynor BJ, Hardiman O, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385. doi: 10.1136/jnnp.2009.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan H, Rechtman L, Wagner L, Kaye WE. Amyotrophic lateral sclerosis surveillance in Baltimore and Philadelphia. Muscle Nerve. 2015 Jun;51(6):815–21. doi: 10.1002/mus.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–30. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(6):623. doi: 10.1136/jnnp.2010.224501. [DOI] [PubMed] [Google Scholar]
- 17.Chio A, Mora G, Leone M, et al. Early symptom progression rate is related to ALS outcome: a prospective population based study. Neurology. doi: 10.1212/wnl.59.1.99. 20025999–103.103. [DOI] [PubMed] [Google Scholar]
- 18.Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci. doi: 10.1016/0022-510x(93)90245-t. 199311848–55.55. [DOI] [PubMed] [Google Scholar]
- 19.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu JT, Shang XL. Magnetic resonance imaging and diffusion tensor imaging in primary lateral sclerosis. Neurol India. 2011;59:767–8. doi: 10.4103/0028-3886.86560. [DOI] [PubMed] [Google Scholar]
- 21.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992 Apr;115( Pt 2):495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- 22.Tartaglia MC, Rowe A, Findlater K, Orange JB, Grace G, Strong MJ. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007 Feb;64(2):232–6. doi: 10.1001/archneur.64.2.232. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Sato T, Hasegawa K, Ishizaki R, Saiki M. Reversible hyperintensity lesion on diffusion-weighted MRI in hypoglycemic coma. Neurology. 2004;63:392–93. doi: 10.1212/01.wnl.0000130181.05016.68. [DOI] [PubMed] [Google Scholar]
- 24.Lo L, Tan CHA, Umapathi T, Lim CC. Diffusion-Weighted MR Imaging in Early Diagnosis and Prognosis of Hypoglycemia. AJNR June. 2006;27:1222–1224. [PMC free article] [PubMed] [Google Scholar]
- 25.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845. doi: 10.1210/jc.2012-4127. [DOI] [PubMed] [Google Scholar]
- 26.Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 27.Loes DJ, Fatemi A, Melhem ER, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. 2003 Aug 12;61(3):369–74. doi: 10.1212/01.wnl.0000079050.91337.83. [DOI] [PubMed] [Google Scholar]
- 28.Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49(4):512. [PubMed] [Google Scholar]
- 29.van Wassenaer-van Hall HN, van den Heuvel AG, Jansen GH, Hoogenraad TU, Mali WP. Cranial MR in Wilson disease: abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am J Neuroradiol. 1995 Nov-Dec;16(10):2021–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010 Oct;24(5):531–9. doi: 10.1016/j.bpg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Roberts EA, Schilsky ML American Association for Study of Liver Diseases (AASLD). American Association for Study of Liver Diseases (AASLD) Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008 Jun;47(6):2089–111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 32.Sundal C, Van Gerpen JA, Nicholson AM, et al. MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology. 2012 Aug 7;79(6):566–74. doi: 10.1212/WNL.0b013e318263575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundal C, Wszolek Z. Adult-Onset Leukoencephalopathy with Axonal Spheroids and Pigmented Glia. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2012. Aug 30, [Updated 2014 Dec 18] 1993–2015. [Google Scholar]