Abstract
Giant encephalocele is an uncommon congenital anomaly with very few published reports available in the English literature. Tetralogy of Fallot associated with situs inversus is also infrequently reported. To our knowledge there are no published reports of an association between giant encephalocele and Tetralogy of Fallot. The additional finding of situs inversus results in a rare pathologic triad, not heretofore described.
Keywords: Giant encephalocele, Situs inversus, Tetralogy of Fallot, Occipital encephalocele, Lipomyelomeningocele
CASE REPORT
A female patient was born to a 26-year-old G2P1 mother at 35 weeks gestational age. Outside institution prenatal ultrasound was notable for encephalocele, micrognathia, dextrocardia, and polyhydraminos. Fetal magnetic resonance imaging (MRI) demonstrated a giant occipital encephalocele with dysmorphic brain and lumbosacral dysraphism [Figure 1]. Of note, the patient’s older sibling was full term at birth with normal development.
Figure 1.
A female fetus at 31 weeks gestation with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. Noncontrast T2-weighted sequence from fetal MRI in sagittal plane with respect to the baby reveals a giant occipital encephalocele (arrows).
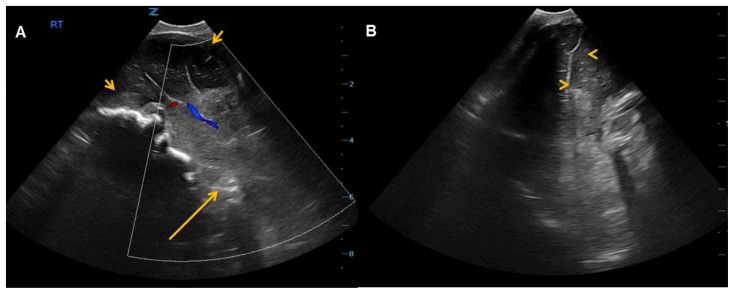
Ultrasound (US) of the head on the second day of life confirmed a giant occipital encephalocele with dysmorphic brain [Figure 2]. Closure of encephalocele was attempted on the third day of life with debridement and superficial skin reconstruction. Cranial vault reconstruction was postponed until further growth of the calvarium. On the fourth day of life, the patient experienced bradycardia with an episode of desaturation suspicious for seizure, and phenobarbital treatment was initiated. On the fifth day of life, the patient developed hypernatremia up to 150 mmol/L with a suspected etiology of central diabetes insipidus and was maintained on careful fluid balance.
Figure 2.
2-day-old female with triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. (A) Sagittal and (B) coronal ultrasound shows frontal lobes within the skull (short arrows) with the remainder of the brain located outside the cranial vault in the encephalocele (long arrow). The sagittal image is obtained near the midline, with the brain in similar configuration to the sagittal T1-weighted MRI brain image in Figure 3. Only the anterior interhemispheric fissure (arrowheads) can be identified on the coronal images.
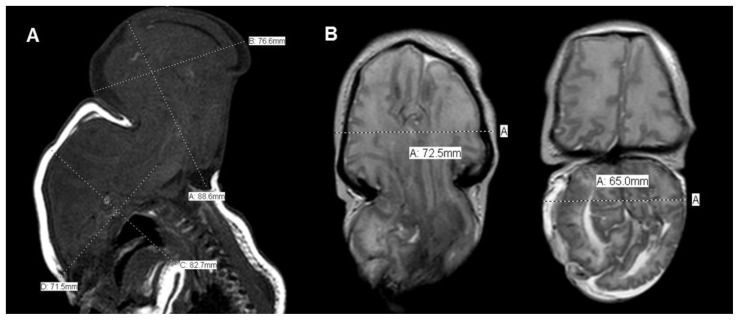
Ophthalmologic evaluation showed optic nerve hypoplasia. Brain MRI revealed occipital encephalocele containing brainstem, cerebellum, occipital lobes and a significant proportion of the temporal and the parietal lobes. The encephalocele measured approximately 8.9 × 7.7 × 6.5 cm (maximum CC × AP × TV), and approximately 3.5 cm at its neck. In comparison, the head measured approximately 8.3 × 7.2 × 7.2 cm (maximum CC × AP × TV). The herniated contents were dysmorphic [Figure 3]. MRI of the spine revealed lipomyelomeningocele with tethered cord and sacral dysgenesis [Figures 4 and 5]. Evoked potential study was notable for no visual response and minimal auditory response in one ear.
Figure 3.
18-day-old female with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. (A) Sagittal T1-weighted and (B) axial T2-weighted noncontrast MRI of the brain shows giant occipital encephalocele, measuring approximately 8.9 × 7.7 × 6.5 cm (maximum CC × AP × TV), and approximately 3.5 cm at its neck. In comparison, the head measured approximately 8.3 × 7.2 × 7.2 cm (maximum CC × AP × TV). The encephalocele contains a large proportion of the brainstem, cerebellum, occipital lobes, temporal lobes and parietal lobes. The herniated contents are dysmorphic.
Figure 4.
18-day-old female with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. (A) Sagittal T2-weighted noncontrast MRI of the normal upper spinal cord. (B) Sagittal T1- and (C) T2-weighted noncontrast MRI of lower spine demonstrates a lipomyelomeningocele at L4 through S1 levels with tethered cord (long arrows). Small syrinx within distal cord at L2–3 to L4 levels (short arrow).
Figure 5.
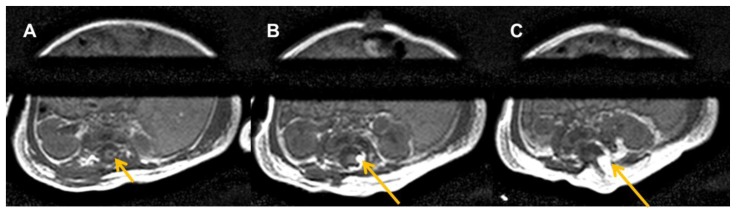
18-day-old female with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. Axial T1-weighted noncontrast MRI of the lumbar spine at three progressively caudal lumbar levels (A, B, C) shows syrinx (short arrow) and lipomyelomeningocele (long arrows) with sacral dysgenesis.
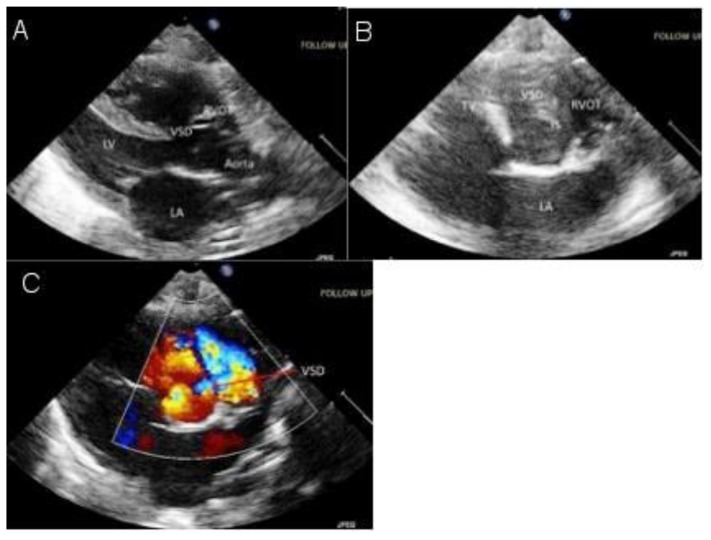
Cardiovascular assessment with echocardiography revealed situs inversus totalis with dextrocardia and mirror image segmental cardiac anatomy with Tetralogy of Fallot [Figure 6]. There was no significant right ventricular outflow tract obstruction, and therefore the infant had normal oxygen saturation. The localizer image from the MR of the lumbar spine partially showed findings consistent with situs inversus totalis [Figure 7]. Abdominal ultrasound confirmed situs inversus totalis, with otherwise normal abdominal organs.
Figure 6.
A newborn female with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus totalis. Echocardiography (A) parasternal grayscale and (B, C) short axis grayscale and color Doppler images demonstrate a widely patent ventricular septal defect and overriding aorta.
LA - left atrium; LV - left ventricle; RVOT - right ventricular outflow tract; TV - tricuspid valve; VSD - ventricular sepal defect.
Figure 7.
A newborn female with a triad of neural tube defect, Tetralogy of Fallot, and situs inversus. Coronal T2-weighted images from noncontrast MRI of the entire spine showing situs inversus totalis. (A) The larger T2 hypointense liver is on the patient’s left, while the smaller spleen is on the patient’s right, with the medially adjacent rounded T2 hyperintense stomach. Small bowel is largely located in the right side of the abdomen. (B) The cardiac apex is partially visualized (arrow) and is on the right, consistent with dextrocardia.
DISCUSSION
Encephalocele and myelomeningocele are two of the most common neural tube defects. Giant encephalocele is truly a rare finding, with only a few published reports available in the English literature [1]. Furthermore, Tetralogy of Fallot with situs inversus is infrequently reported [2]. Only a single case with mild similarity has been previously reported, wherein a patient with occipital encephalocele and tectocerebellar dysraphia had associated abdominal situs inversus and conotruncal anomaly of double outlet right ventricle and pulmonary stenosis [3]. We report an extremely rare case of a newborn female with giant occipital encephalocele and lipomyelomeningocele, conotruncal anomaly of Tetralogy of Fallot, and situs inversus totalis. There are no previous reports of such association and to the best of our knowledge this triad has never been described. In this report, we highlight potential surgical management of both complex congenital heart disease and severe neural tube defects as applicable to this newborn. In addition, we discuss the possibility of a yet undiscovered genetic association amongst the described pathologies, especially as several known genetic syndromes include the finding of occipital encephalocele. However, none of the known syndromes are specifically associated with the rarer entity of giant encephalocele.
Etiology & Demographics
Neural tube defects result from developmental failure between 25 and 27 days after conception. In the United States, occipital encephalocele is the most common form of congenital encephalocele with incidence between 1 in 3000 to 1 in 10,000 live births. Isolated encephaloceles are sporadic. The incidence of Tetralogy of Fallot is approximately 3 of every 10,000 live births, and accounts for 7–10% of all severe congenital cardiac malformations [4]. Situs inversus occurs in isolation in 1 of every 10,000 live births. Tetralogy of Fallot associated with dextrocardia and situs inversus is only rarely reported [2]. The first case of Tetralogy of Fallot with situs inversus was published in 1952 in which a 2½-year-old female who presented with prolonged cough, failure to thrive, marked cyanosis, and clubbing in the presence of unrepaired Tetralogy of Fallot [4]. A review of the medical literature since the year 2000 yielded only one reported case of Tetralogy of Fallot and dextrocardia with abdominal situs solitus, detected on ultrasound in a fetus of 26 weeks gestation [5]. In 147 adults with Tetralogy of Fallot, two patients had dextrocardia [6]. The reported prevalence of the three anomalies together (Tetralogy of Fallot, situs inversus, and dextrocardia) is low [2]. A study of 63 patients with dextrocardia yielded one patient with Tetralogy of Fallot and situs inversus [7].
Neural tube defects occur because of a defect in the neurulation process. Many chromosomal abnormalities are associated with neural tube defects, such as: monosomy X; numerous trisomies including 13, 18, and 21, as well as lesser known 5, 7, 8, 11 mosaicism, 14, 15, 16, and 20 mosaicism; and tetraploidy. Neural tube defects may be linked to aneuploidy [8]. Tetralogy of Fallot has been associated with chromosome 22q11.2 deletion [9]. Studies have suggested linkage of situs inversus and other left-right axis anomalies to chromosome 6p [10]. Therefore there may be yet an unidentified genetic association of the rare clinical triad of encephalocele, Tetralogy of Fallot, and situs inversus.
Aside from chromosomal abnormalities, NTD arise from environmental factors. The risk factors for Tetralogy of Fallot include maternal age over 40 years old, viral infection, alcoholism, and poor nutrition during pregnancy; presence of Down’s syndrome or DiGeorge syndrome; and a parent with Tetralogy of Fallot. Non-genetic causes of situs inversus include gene-environmental interaction, monozygotic twinning, and parental cocaine use.
Clinical & Imaging findings
The three most common neural tube defects are anencephaly, myelomeningocele, and encephalocele. Anencephaly is complete absence of a majority of the brain and is incompatible with life. Myelomeningocele is characterized by a defect in the vertebral column and skin with exposure of meninges and spinal cord. An encephalocele is herniation of the brain, cerebrospinal fluid (CSF), and meninges through a defect in the skull that is “closed” or covered with skin. There are several types of encephalocele, including sincipital (fronto-ethmoidal), basal (trans-sphenoidal, spheno-ethmoidal, trans-ethmoidal, and spheno-orbital), and occipital. Anterior cranial fossa encephalocele has equal gender ratio, while 70% of occipital encephaloceles occur in females [11]. Giant encephalocele is distinguished by head size smaller than the encephalocele [12,13].
MR findings of encephalocele include T1 and T2-weighted sequences that demonstrate characteristic signal of brain tissue and CSF. MR venography characterizes the dural venous anatomy in relation to the encephalocele. Computed tomography (CT) delineates the bone margins, demonstrates the bone defect, and the brain tissue. US delineates the encephalocele and will identify encephalocele in the fetus.
Occipital encephalocele in association with other congenital abnormalities can be part of a genetic syndrome. Meckel-Gruber syndrome includes occipital encephalocele, microcephaly, microphthalmia, cleft lip and palate, polycystic kidneys, ambiguous genitalia, polydactyly, and other malformations. Von Voss-Cherstvoy syndrome involves occipital encephalocele, upper limb phocomelia, urogenital abnormalities, and thrombocytopenia. Walker-Warburg syndrome is an autosomal recessive congenital muscular dystrophy with lissencephaly, occipital encephalocele, cerebellar malformations, and retinal dysplasia. Knobloch syndrome is characterized by extreme myopia, retinal detachment, cataracts as well as occipital encephalocele and aplasia [14].
Lipomyelomeningocele is a form of closed spinal dysraphism, a rare but complex neurological disorder that may present with neurological deterioration secondary to an inherent tethered spinal cord [15]. It usually presents as a subcutaneous fatty mass just above the intergluteal cleft. However, some lipomyelomeningoceles may occur at other locations along the spinal canal [16].
Tetralogy of Fallot is a congenital conotruncal malformation of anterior malalignment of the infundibular septum resulting in a large ventricular septal defect (VSD), varying degree of right ventricular outflow tract obstruction, overriding aorta, and right ventricular hypertrophy. Situs inversus is a congenital condition in which all major organs are mirrored from their normal position. Tetralogy of Fallot associated with dextrocardia and situs inversus is only rarely reported [2].
Imaging of Tetralogy of Fallot demonstrates conotruncal malformation of anterior malalignment of the cardiac infundibular septum resulting in a large VSD, varying degree of right ventricular outflow tract obstruction, overriding aorta, and right ventricular hypertrophy. MR in steady state free procession (SSFP) demonstrates the right ventricular hypertrophy and cine images may demonstrate the interventricular shunt. The ejection fraction of both ventricles may be calculated. MR arteriography depicts the anatomy of the major vessels. Phase imaging permits calculation of the flow in the aortic valve and the degree of stenosis. CT arteriography depicts the anatomy of the major vessels and collaterals. Ultrasound may delineate the anatomy in fetus and color Doppler may demonstrate the direction of the interventricular shunt. Flow across the valve may be demonstrated.
In situs inversus totalis all major organs are mirrored from their normal position. This abnormal anatomical configuration can be easily delineated on CT and on MR, however US has a limited field of view and can only effectively be utilized in the abdomen to evaluate single solid organs.
Treatment & Prognosis
Surgical management of newborns with encephalocele varies and depends upon the amount of neural tissue in the sac, state of CSF pathway, neurological status of the patient and presence or absence of associated congenital anomalies elsewhere in body [17]. Presence of a large occipital sac poses difficulties in positioning for intubation. MRI is the imaging modality of choice for assessing the contents of the sac and its relationship to the neighboring cerebral structures and for surgical planning. Visual evoked response is used in cases of giant occipital encephalocele to determine whether the sac contains functional tissue from visual cortex [18]. In case of nonfunctional gliotic tissue, it can be transected. In case of normal neural tissue, attempts are made to preserve the tissue. Expansion cranioplasty can be performed by surrounding the extracranial structures with tantalum mesh and using daily digital pressure to expand the calvarium [19]. Ventricular volume reduction technique is a two-step process with initial dural closure which leads to increased intracranial pressure and hydrocephalus, followed by subsequent drainage with transposition of neural tissue into the intracranial space [20]. The tentorium can also be incised to create infratentorial space to preserve herniated neural tissues [21].
Myelomeningocele is characterized by the extrusion of the spinal cord into a sac filled with CSF. Surgical repair of myelomeningocele is necessary to close the abnormal opening, to decrease the risk of infection and to protect the integrity of the spinal column and the tissue inside. Myelomeningocele should be treated surgically as soon after birth as possible, irrespective of whether the myelomeningocele has ruptured. The goals of surgery are to protect the neural elements, to remove excess skin tissue, and to obtain a watertight dural closure to prevent infection without exacerbating neurological deficits. Postnatal disabilities could in part be related to the spinal damage and to the cerebral repercussion of the leak of CSF from the defect. Several experimental studies in animals have demonstrated that a surgical repair of the lesion at middle gestation reduced the postnatal disabilities. These results were confirmed in humans by the Management of Myelomeningocele (MOM) Trial. However, the prenatal surgical repair is associated with some maternal and fetal morbidity [22].
The management of Tetralogy of Fallot during the neonatal period depends on the degree of cyanosis due to severe right ventricular outflow obstruction at multiple levels: subvalvular, valvular and supravalvular. As this patient did not have significant right ventricular outflow tract obstruction, cardiovascular intervention during the neonatal period was unnecessary. Given that the patient continued to have normal oxygen saturations, the goal was to maintain increased blood flow to the lungs if and when pulmonary vascular resistance dropped, possibly requiring diuretics, which happens less commonly in Tetralogy of Fallot. If she otherwise remained stable and harbored a favorable prognosis, the repair of the Tetralogy of Fallot could have been performed during early infancy.
Most newborns with Tetralogy of Fallot are not cyanotic at birth. Typically there is a left to right shunt through the defect in the interventricular septum. Upon worsening of the subpulmonic stenosis, the right ventricular myocardium hypertrophies and the shunt through the interventricular septum may reverse and cause cyanosis. If the Tetralogy of Fallot is associated with situs inversus, the altered spatial orientation of the cardiac structures can present a challenge in patch closure of the VSD. The surgeon approaches the interventricular septum at a different angle in a patient with dextrocardia than in a patient with levocardia. Therefore, even though the conduction system is along the inferior margin of the VSD, there may be a higher probability of postsurgical heart block [2].
The prognosis is very poor for the combination of these findings. Giant encephalocele has a poor prognosis. The prognosis of tetralogy of Fallot depends on its severity. Generally the prognosis is good and patients live into adulthood. Isolated situs inversus has an excellent prognosis.
Treatment of the three combined conditions in the presented triad becomes extremely challenging. In this particular presented case, the patient ultimately did not undergo any of the above neurosurgical treatments, as the unstable respiratory status of the infant in the absence of ventilator support persisted, with an overall unfavorable prognosis.
Differential Diagnosis
The differential diagnosis can be broken down into that for each of the three entities (neural tube defects of occipital encephalocele and lipomyelomeningocele, Tetralogy of Fallot, and situs inversus). The diagnosis of Tetralogy of Fallot is evident if the four components of the disease are confirmed. The same applies to the diagnosis of situs inversus. The diagnostic considerations of the described triad therefore centers on the differential diagnosis of occipital encephalocele, which includes sinus pericranii, atretic cephalocele, cystic hygroma, teratoma, amniotic band syndrome, and various scalp masses with associated calvarial defect such as metastatic masses. The imager needs to confirm the presence of encephalocele by the presence of a bone defect through which extends herniated brain. The top differential diagnosis of lipomyelomeningocele includes lipoma, dorsal meningocele, and myelocele/myelomeningocele. Bony dysraphic defect and dorsal herniation of the thecal sac with or without the cord extending through the defect needs to be established.
Summary
We have presented a patient with neural tube defects of giant occipital encephalocele and lipomyelomeningocele with tethered cord, complete situs inversus, and Tetralogy of Fallot. To our knowledge, this is the first case report of such a combination. In addition, similar to existing rare genetic syndromes which include the finding of occipital encephalocele, there may be a genetic association not yet delineated among multiple neural tube defects, heterotaxia, and congenital heart disease.
TEACHING POINT
To date, there is only a single reported case of occipital encephalocele associated with congenital heart disease. This rare association of giant encephalocele in conjunction with lipomyelomeningocele, and additional findings of Tetralogy of Fallot and situs inversus totalis has never been described, raising the possibility of an undiscovered genetic association among multiple neural tube defects, heterotaxia, and congenital heart disease.
Table 1.
Summary of constellation of findings in this case of neural tube defect (NTD), Tetralogy of Fallot (TOF), and situs inversus (SI)
| Etiology: | NTD, TOF, SI: Congenital |
| Incidence: |
|
| Gender ratio: |
|
| Age predilection: | NTD, TOF, SI: At birth |
| Risk factors: |
|
| Treatment: |
|
| Prognosis: |
|
| Imaging Findings: |
NTD (encephalocele): Herniation of the brain, CSF, and meninges through a defect in the skull that is “closed” or covered with skin. MR Findings: T1 and T2-weighted sequences demonstrate characteristic signal of brain tissue and CSF. MRV characterizes dural venous anatomy in relation to the encephalocele. CT Findings: CT delineates the bone margins, demonstrates the bone defect, and the brain tissue. US findings: Delineates the encephalocele and will identify encephalocele in fetus. TOF: Conotruncal malformation of anterior malalignment of the infundibular septum resulting in a large VSD, varying degree of right ventricular outflow tract obstruction, overriding aorta, and right ventricular hypertrophy. MR findings: SSFP demonstrates the right ventricular hypertrophy and cine images may demonstrate the interventricular shunt. The ejection fraction of both ventricles may be calculated. MRA depicts the anatomy of the major vessels. Phase imaging permits calculation of the flow in the aortic valve and the degree of stenosis. CT findings: CTA depicts the anatomy of the major vessels and collaterals. US findings: The anatomy may be seen in the fetus. Color Doppler may demonstrate the direction of the interventricular shunt. Flow across the valve may be demonstrated. SI: All major organs are mirrored from their normal position. This abnormal anatomical configuration can be easily delineated on CT and on MR, however US has a limited field of view and can only effectively be utilized in the abdomen to evaluate single solid organs. |
Table 2.
Differential diagnosis of occipital encephalocele
| Diagnosis | MRI | CT | US |
|---|---|---|---|
| Encephalocele |
|
|
|
| Sinus Pericranii |
|
|
|
| Atretic Cephalocele |
|
|
|
| Cystic Hygroma |
|
|
|
| Teratoma |
|
|
|
| Amniotic band syndrome |
|
|
|
| Scalp mass (hemangioma, epidermal cyst, cephalohematoma, less common metastasis) |
|
|
ABBREVIATIONS
- CSF
Cerebral Spinal Fluid
- CT
Computerized Tomography
- CTA
Computerized Tomography Angiography
- DWI
Diffusion-Weighted Imaging
- FLAIR
Fluid-Attenuated Inversion Recovery
- MR
Magnetic Resonance
- MRA
Magnetic Resonance Angiography
- MRI
Magnetic Resonance Imaging
- MRV
Magnetic Resonance Venography
- NTD
Neural Tube Defect
- SI
Situs Inversus
- SSFP
Steady State Free Procession
- STIR
Short Tau Inversion Recovery
- T1WI
T1-weighted imaging
- T2WI
T2-weighted imaging
- TOF
Tetralogy of Fallot
- US
Ultrasonography
- VSD
Ventricular Septal Defect
REFERENCES
- 1.Mahapatra AK. Giant encephalocele: a study of 14 patients. Pediatr Neurosurg. 2011;47(6):406–11. doi: 10.1159/000338895. [DOI] [PubMed] [Google Scholar]
- 2.Dilorenzo M1, Weinstein S, Shenoy R. Tetralogy of fallot with dextrocardia and situs inversus in a 7-year-old boy. Tex Heart Inst J. 2013;40(4):481–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnamurthy S, Kapoor S, Sharma V, Prakash A. Tectocerebellar dysraphia and occipital encephalocele: an unusual association with abdominal situs inversus and congenital heart disease. Indian J Pediatr. 2008;75(11):1178–80. doi: 10.1007/s12098-008-0183-6. [DOI] [PubMed] [Google Scholar]
- 4.Scragg JN, Denny M. Dextrocardia, tetralogy of Fallot, and situs inversus; report of a case. S Afr Med J. 1952;26(52):1025–8. [PubMed] [Google Scholar]
- 5.Bharati AH, Naware A, Merchant SA. Absent pulmonary valve syndrome with tetralogy of Fallot and associated dextrocardia detected at an early gestational age of 26 weeks. Indian J Radiol Imaging. 2008;18(4):352–4. doi: 10.4103/0971-3026.43841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham KA, Cherian G, Rao VD, Sukumar IP, Krishnaswami S, John S. Tetralogy of Fallot in adults. A report on 147 patients. Am J Med. 1979;66(5):811–6. doi: 10.1016/0002-9343(79)91121-5. [DOI] [PubMed] [Google Scholar]
- 7.Evans WN, Acherman RJ, Collazos JC, Castillo WJ, Rollins RC, Kip KT, Restrepo H. Dextrocardia: practical clinical points and comments on terminology. Pediatr Cardiol. 2010;31(1):1–6. doi: 10.1007/s00246-009-9516-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen CP. Chromosomal abnormalities associated with neural tube defects (I): full aneuploidy. Taiwan J Obstet Gynecol. 2007 Dec;46(4):325–35. doi: 10.1016/S1028-4559(08)60002-9. [DOI] [PubMed] [Google Scholar]
- 9.Momma K, Takao A, Matsuoka R, Imai Y, Muto A, Osawa M, Takayama M. Tetralogy of Fallot associated with chromosome 22q11.2 deletion in adolescents and young adults. Genet Med. 2001 Jan-Feb;3(1):56–60. doi: 10.1097/00125817-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Vitale E, Brancolini V, De Rienzo A, Bird L, Allada V, Sklansky M, Chae CU, Ferrero GB, Weber J, Devoto M, Casey B. Suggestive linkage of situs inversus and other left-right axis anomalies to chromosome 6p. J Med Genet. 2001 Mar;38(3):182–5. doi: 10.1136/jmg.38.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andarabi Y, Nejat F, El-Khashab M. Progressive skin necrosis of a huge occipital encephalocele. Indian J Plast Surg. 2008;41(1):82–4. doi: 10.4103/0970-0358.41120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nath HD, Mahapatra AK, Borkar SA. A giant occipital encephalocele with spontaneous hemorrhage into the sac: A rare case report. Asian J Neurosurg. 2014;9(3):158–60. doi: 10.4103/1793-5482.142736. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez DF, Barone CM. Encephaloceles, meningoceles, and dermal sinuses. In: Albright AL, Pollack IF, Adelson PD, editors. Principles and Practice of Pediatric Neurosurgery. New York: Thieme Medical Publishers; 1999. p. 189. [Google Scholar]
- 14.Wininger SJ, Donnenfeld AE. Syndromes identified in fetuses with prenatally diagnosed cephaloceles. Prenat Diagn. 1994;14(9):839–43. doi: 10.1002/pd.1970140912. [DOI] [PubMed] [Google Scholar]
- 15.Sarris CE, Tomei KL, Carmel PW, Gandhi CD. Lipomyelomeningocele: pathology, treatment, and outcomes. Neurosurg Focus. 2012 Oct;33(4):E3. doi: 10.3171/2012.7.FOCUS12224. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Je BK, Kim SB, et al. Adult with sacral lipomyelomeningocele covered by an anomalous bone articulated with iliac bone: computed tomography and magnetic resonance images. Congenit Anom (Kyoto) 2012;52(2):115–8. doi: 10.1111/j.1741-4520.2011.00335.x. [DOI] [PubMed] [Google Scholar]
- 17.Nejmi K, Nebi Y, Ismail D, Siddik K. Prognostic factors in patients with occipital encephalocele. Pediatr Neurosurg. 2010;46:6–11. doi: 10.1159/000314051. [DOI] [PubMed] [Google Scholar]
- 18.Engel R, Buchan GC. Occipital encephaloceles with or without visual evoked potentials. Arch Neurol. 1974;30:314. doi: 10.1001/archneur.1974.00490340042009. [DOI] [PubMed] [Google Scholar]
- 19.Gallo AE. Repair of giant occipital encephaloceles with microcephaly secondary to massive brain herniation. Childs Nerv Syst. 1992;8(4):229–30. doi: 10.1007/BF00262854. [DOI] [PubMed] [Google Scholar]
- 20.Oi S, Saito M, Tamaki N, Matsumoto S. Ventricular volume reduction technique--a new surgical concept for the intracranial transposition of encephalocele. Neurosurgery. 1994;34(3):443–7. doi: 10.1227/00006123-199403000-00009. discussion 8. [DOI] [PubMed] [Google Scholar]
- 21.Bozinov O, Tirakotai W, Sure U, Bertalanffy H. Surgical closure and reconstruction of a large occipital encephalocele without parenchymal excision. Childs Nerv Syst. 2005;21(2):144–7. doi: 10.1007/s00381-004-1020-5. [DOI] [PubMed] [Google Scholar]
- 22.Garabedian C, Di Rocco F, Fallet-Bianco C, Zerah M, Jouannic JM. Prenatal repair of myelomeningocele: State of the art. J Gynecol Obstet Biol Reprod (Paris) 2013 May;42(3):227–31. doi: 10.1016/j.jgyn.2012.10.015. [DOI] [PubMed] [Google Scholar]