Abstract
Osteosarcomas of the craniofacial bones account for fewer than 10% of all osteosarcomas. Primary osteosarcomas of the nasal cavity and paranasal sinus are rare (0.5–8.1% of the osteosarcomas occur in this location). Because of the rarity of this presentation, we report a case of osteogenic osteosarcoma arising de novo from the ethmoid bone in a 13 year old male who presented with discharge from the right eye and headaches. We describe the imaging features of this rare tumor and provide a brief review of the literature.
Keywords: Osteosarcoma, Paranasal sinus, Ethmoid sinus, Adolescent, Imaging
CASE REPORT
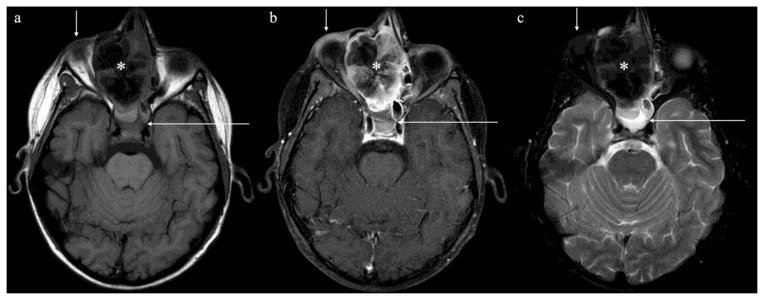
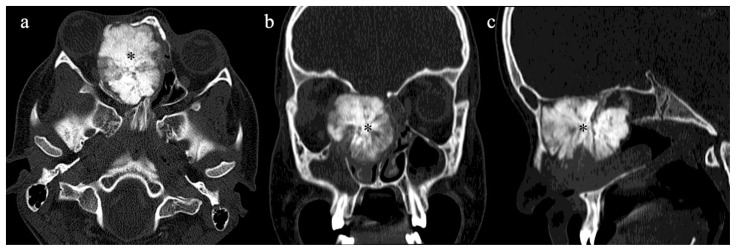
A 13 year old male previously healthy presented with dark discharge from his right eye and moderate headaches for two months, not relieved with over-the-counter medications. On physical exam, proptosis with inferolateral deviation of the right eye with a mass in the medial aspect of the orbit was noted. An MRI of the orbit was done. The pre–contrast and T2 weighted images revealed a predominantly hypointense expansile mass centered at the right ethmoid sinus that extended medially through the right lamina papyracea causing right-sided proptosis and inferiorly to the nasal cavity causing obstruction of the right frontal sinus outflow tract (Fig. 1a, 1c). After administration of IV gadolinium contrast, a radiating striated “sunburst” appearance of enhancement was noted (Fig. 1b, 2a, 2b). There was no invasion of the dura or brain parenchyma. 99m Tc diphosphonate bone scan demonstrated an intense radiotracer uptake in the nasal cavity corresponding to the tumor, with no evidence of distant metastasis (Fig. 3a, 3b). The bony character of the tumor was well demonstrated in the CT scan of the head (Fig. 4a, 4b, 4c). CT of the chest, abdomen and pelvis did not show any evidence of metastases.
Figure 1.
13 year old male with primary osteosarcoma of the ethmoid sinus. Protocol: Non-contrast and post contrast MRI images were acquired at 1.5 T Siemens Somaton. 8 ml of Gadopentetate dimeglumine was administered
1a) An axial precontrast MRI T1 shows a predominantly hypointense expansile mass centered in the right ethmoid sinus (asterisk) extending through the right lamina papyracea, causing right-sided proptosis (short arrow) and sphenoid sinus obstruction (long arrow) (TR 567 TE 15).
1b) Post contrast axial T1 weighted images with fat saturation showing heterogeneously enhancing tumor centered in the right ethmoid sinus and right nasal cavity, with hypoenhancing ossified areas (TR 518 TE 15).
1c) An axial fat suppressed T2 weighted axial images shows a predominantly hypointense expansile mass centered in the right ethmoid sinus (asterisk) extending through the right lamina papyracea, causing right-sided proptosis (short arrow) and sphenoid sinus obstruction (long arrow) (TR 5000 TE 96).
Figure 2.
13 year old male with primary osteosarcoma of the ethmoid sinus. Protocol: Postcontrast MRI images were acquired at 1.5 T Siemens Somaton. 8 ml of Gadopentetate dimeglumine was administered.
a) Coronal post contrast T1 weighted images demonstrate an expansile lesion of the right ethmoid sinus (asterisk) that extends inferiorly to the nasal cavity and obstructs the outlet of the frontal sinus (long arrow) and right maxillary sinus (short arrow). There is sunburst appearance of the septations after the administration of contrast. There is no enhancement in the meninges or brain parenchyma to suggest extension (TR 907 TE 15).
b) Sagittal post contrast T1 weighted images demonstrate an expansile lesion of the right ethmoid sinus (asterisk) that extends inferiorly to the nasal cavity and obstructs the outlet of the frontal sinus (long arrow).There is sunburst appearance of the septations after the administration of contrast. There is no enhancement in the meninges or brain parenchyma to suggest extension (TR 906 TE 15).
Figure 3.
13 year old male with primary osteosarcoma of the ethmoid sinus. Whole body diagnostic bone Scan, anterior and posterior in light (left) and dark (right) intensity after intravenous administration of 20.4 mCi of Tc-99 MDP (methylene diphosphonate). Images were acquired approximately 3 hours after injection of the radiotracer.
3a) Single intense radiotracer uptake in the nasal cavity (arrow) corresponding to the tumor.
3b) Bone scan, anterior view of upper body with intensity adjusted to show tumor detail, demonstrates the non-uniform, spherical uptake in the “hot spot” in the nasal cavity (arrow) consistent with the tumor.
Figure 4.
13 year old male with primary osteosarcoma of the ethmoid sinus. Protocol: Contrast enhanced CT after the administration of 50 cc of Iohexol 350. Images were acquired kVp 120, mAs 4, helical acquisition, Slice width 1.25mm.
4a) Axial view of a contrast CT of the sinus facial bones showing predominantly ossified mass in the right ethmoid sinus and right nasal cavity (asterisk).
4b) Coronal view of a contrast CT of the sinus facial bones showing predominantly ossified mass in the right ethmoid sinus and right nasal cavity (asterisk).
4c) Sagittal reformats of CT showing predominantly ossified mass in the right ethmoid sinus and right nasal cavity (asterisk).
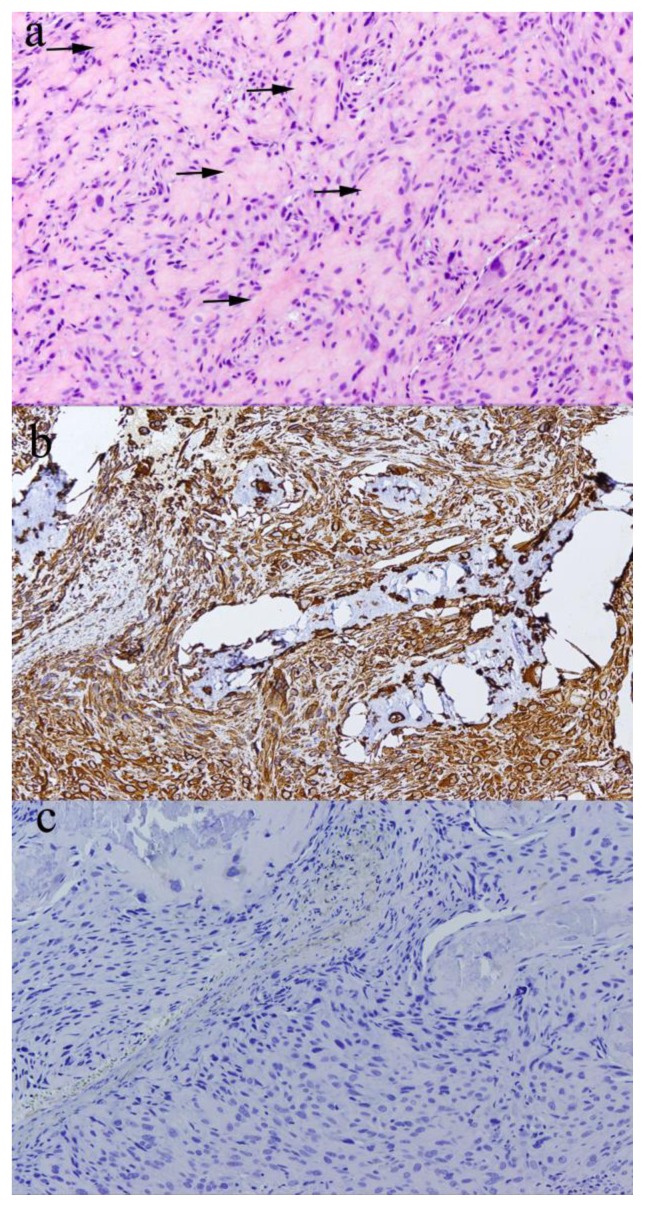
Preoperative endoscopic biopsy was done which showed “Sections from the sinus mass demonstrate pleomorphic cells with numerous mitoses and some atypical mitotic figures with osteoid in the background, some suspicious for malignant osteoid associated with osteosarcoma” (Fig 5a).
Figure 5.
13 year old male with primary osteosarcoma of the ethmoid sinus.
A: A hematoxylin and eosin stained section of the biopsy specimen shows pleomorphic spindle and ovoid cells surrounding foci of eosinophilic extracellular matrix material (arrows) reminiscent of lacelike malignant osteoid (100x).
B: The atypical cells stain strongly positive with an immunohistochemical stain for vimentin.
C: The vaguely whirled appearance of the atypical cells raised the possibility of an atypical meningioma, but this was ruled out with a negative immunohistochemical stain for epithelial membrane antigen.
The macroscopic surgical resection specimen revealed a well circumscribed mass arising from the right ethmoid bone measuring approximately 5.3 cm in the greatest dimension and was composed entirely of solid white bone with no areas of hemorrhage or necrosis. It showed tumor free margins. The microscopic evaluation revealed tumor cells with a vague whirling appearance, which raised the possibility of an atypical meningioma. The cells were positive for vimentin and negative for epithelial membrane antigen, arguing against meningioma (Fig. 5b, 5c). In conjunction with the radiological appearance, high-grade osteogenic osteosarcoma was diagnosed.
The patient received twelve cycles of neoadjuvant chemotherapy prior to craniofacial resection and adjuvant chemotherapy after surgery. We have followed this patient for the last 24 months; he is receiving chemotherapy and has been free of local recurrence and distant metastases.
DISCUSSION
Etiology & Demographics
Osteosarcomas of the head and neck represent a small percentage of all osteosarcomas with studies reporting incidence between 0.5–8.1percent [1, 2]. These tumors usually present in the third to fourth decades of life, later than the presentation of osteosarcoma arising in the long-bones and usually occur as secondary tumors after radiation therapy, thorium oxide exposure, chemotherapy, inherited predispositions to development of osteosarcoma, or arise from a preexisting benign bone disease such as Paget’s disease, bone infarcts, osteomyelitis, trauma [1,2,3,4,5,6,7,8,9,10, 11]. No gender predominance has been described [1, 12, 13, 14, 15]. The most frequent site of location for craniofacial osteosarcomas is the mandible and maxilla [2, 6, 9, 11, 12, 15, 16].
Osteosarcomas can be divided into a number of subtypes according to degree of differentiation, location within the bone, and histological variants. These subtypes vary in imaging findings, demographics and biological behavior, and include: intramedullary: which is the most common encompassing 80 percent of the cases and include: conventional high-grade, telangiectatic osteosarcoma, and low-grade osteosarcoma. Surface or juxtacortical is the second most common encompassing 10–15%. These include intracortical osteosarcoma, parosteal osteosarcoma and periosteal osteosarcoma. Finally, the extraskeletal osteosarcoma represents 5% of the cases.
Osteosarcomas of the ethmoid sinus are rare, particularly in pediatric patients [5, 6]. In our review, we found only 14 reported cases of ethmoidal osteosarcoma in the English and Japanese literature [16].
In a 2001 publication based on cases retrieved from the Otorhinolaryngic Head & Neck Tumor Registry of the Armed Forces Institute of Pathology (AFIP), Gadwal et al reported twenty-two cases of osteosarcomas of the head and neck in patients 18 years of age or younger, diagnosed between 1970 and 1997, which excluded those that were radiation-induced or those arising after chemotherapy or associated with known syndromes [1]. Our case with de novo osteosarcoma arising in the ethmoid sinus is notable because this is one of the rare pediatric patients with primary osteosarcoma of the head and neck.
Osteosarcomas of the paranasal sinus tend to be in patients over 30 years of age [4, 12, 15]. Primary pediatric osteosarcoma of the head and neck is rare, as was mentioned by Kirby et al in their article in 2011[2, 11]. With only a handful of case reports and a small study series (up to 7 patients) reported in the English language literature [1]. According to Maes P et al osteogenic osteosarcoma of the face is one of the rarest tumors encountered in childhood oncology [17]. In 1979, Huvos reported approximately 412 published cases of osteogenic sarcoma of craniofacial bones in the literature after registering them for 60-year period (1921–1981) and found that patients with de novo osteogenic osteosarcoma were considerably younger than those with secondary lesions [14]. Our patient was 13 year old when diagnosed.
Like the discussion case, nearly all the ethmoid sinus osteosarcomas are high grade [5, 12].
Approximately 35–50% of tumors are categorized as osteoblastic, like the tumor in our patient; with chondroblastic or fibroblastic subtypes being the other subtypes [12, 15, 18]. Hematogenous metastases occur less frequently in high-grade sinonasal osteosarcoma than in osteosarcomas of long bones, although metastases to the lungs and other bones, as well as lymph node metastases have been reported [5]. The main problem of craniofacial osteosarcomas is extension to the neighboring bones and anatomical structures including the nasopharynx, orbit and cranium [5, 12, 15].
Clinical & Imaging Findings
The clinical presentation is non-specific: pain, epistaxis, paresthesia, swelling, lacrimation, nasal obstruction and displacement of the eye; such as in our patient [5, 12, 15].
Like osteosarcoma in general, craniofacial osteosarcomas exhibit variable imaging features depending on the extent of bone destruction, soft tissue extension and matrix composition [5,10].
Radiologic studies are critical for evaluation of the primary tumor and detection of metastatic disease. Imaging evaluation for patients with confirmed osteosarcoma, in addition to radiographs of the primary site, include magnetic resonance imaging (MRI) and/or computed tomography (CT) scan of the primary site. Staging studies should include whole body bone scan, chest x ray and CT scan of the chest. Conventional radiographs represent the first method of radiographic evaluation of the head and neck osteosarcomas; however, they are of limited value because of the superimposed bony structures [18]. Typically they demonstrate an aggressive bone tumor with cortical and medullary destruction with wide transition zone, permeative or moth-eaten appearance, aggressive periosteal reaction (sunburst type and Codman triangle; the lamellated (onionskin) reaction is less frequently seen. CT scanning may be helpful locally when the radiographic appearances are confusing, particularly in areas of complex anatomy. CT provides excellent detection of tumor calcification, cortical involvement, and, in most instances, soft-tissue and intramedullary extension [18]. Computed tomography is of great value as a guide to biopsy. MRI is accurate for local staging, particularly for evaluation of intraosseous tumor extension and soft-tissue involvement. Soft tissue components of the tumor demonstrate intermediate signal intensity on T1 and high signal intensity on T2. Mineralized components demonstrate low signal intensity on T1 and T2. Solid components enhance after the administration of intravenous contrast. Peritumoral edema shows high signal intensity on T2. Scattered regions of hemorrhage will have variable signal. However, CT and plain films are superior to MR in detecting the matrix calcifications and bone destruction or reaction [18]. 99m Tc diphosphonate bone scans have documented high sensitivity for demonstration of primary and metastatic bone deposits because of the increased osteoblastic activity induced by the tumor [19]. As many as 20% of osteosarcoma patients will have radiographically detectable metastases at diagnosis, with the lung being the most common site [20, 21].
The radiologic studies in our patient demonstrated an expansile lesion centered in the ethmoid sinus showing a “sunburst enhancement of the striations” representing tumor bone as is frequently described with osteosarcomas of the extremities. Multiple lesions including benign and malignant tumors may have similar pathologic and radiologic findings, making the diagnosis difficult.
Treatment & Prognosis
Treatment options include surgery, neoadjuvant and adjuvant chemotherapy but similar to osteosarcoma of the extremities, adequate surgical resection is considered a mainstay of treatment [7, 15]
For the purposes of treatment, there are only two stages of high-grade osteosarcoma, either localized or metastatic. The osteosarcomas of head and neck have less risk of distant metastases but a higher rate of local recurrence which may be due to difficulty in achieving wide surgical margins because of anatomic and cosmetic reasons [9]. Patients with localized disease have a much better prognosis than those with overt metastatic disease. Resectability of the tumor is an important prognostic factor because osteosarcoma is relatively resistant to radiation therapy [9, 13, 21]. Surgery is followed by appropriate adjuvant therapy as warranted. According to Patel et al surgical free margins appear to be the only significant predictors of overall and disease-specific survival, and the patient prognosis does not vary if the osteosarcoma arises de novo or as a second malignant neoplasm[22]. However, some authors consider that the most important good prognostic variable for patients with osteosarcoma of the extremity is tumor necrosis evident following preoperative chemotherapy [13, 23, 24]. The tumor in our patient did not respond to neoadjuvant therapy given the absence of necrosis in the surgical specimen. Local recurrence is common, but according to Gadwall SR et al, the presence of local recurrence does not change the prognosis [1, 12, 15]. Jasnau et al in their cohort found a local failure rate of approximately 50%, which was similar to the observed rate by most others [15]. The National Cancer institute says that approximately 50% of relapses occur within 18 months of therapy termination, and only 5% of recurrences develop beyond 5 years [23]. In our patient, the tumor was high grade with free margins and it has not recurred locally 24 months after the resection.
After administration of preoperative chemotherapy, surgical resectability and the degree of tumor necrosis influence outcome. In general, prognostic factors in osteosarcoma have not been helpful in identifying patients who might benefit from treatment intensification or who might require less therapy while maintaining an excellent outcome [23].
Prognosis is more favorable for jaw lesions: approximately 30–40% 5-year survival rate versus 20% for non-jaw lesions [1, 24, 25]. There are some data that suggest that this prognosis is increasing with better adjuvant therapies [1]. Some authors agree that high grade osteosarcomas of the head and neck have a poorer outcome than osteosarcomas of the peripheral skeleton [1].
Differential Diagnoses
The differential diagnosis of osteosarcoma tumors clinically, histopathologically and radiographically is broad including but not limited to benign and malignant tumors and infectious processes. Tumors such as chondrosarcomas, fibrous dysplasia, osteoblastomas and osteomyelitis may mimic osteosarcomas [Table 2].
Table 2.
Differential diagnosis for Primary Osteosarcoma of the ethmoid sinus.
| Osteosarcoma | Fibrous Dysplasia | Osteoblastoma | Osteomyelitis | |
|---|---|---|---|---|
| Plain Films |
|
|
|
|
| CT |
|
|
|
|
| MRI |
|
|
|
|
| Bone scan (Tc99m) |
|
|
|
|
The presence of chrondroid matrix in chondrosarcomas may be indistinguishable from a less aggressive osteosarcoma on noncontract CT [5]. Regions of bone density may be observed in these tumors because of localized ossification.
Fibrous dysplasia was considered in the differential diagnosis. These patients also may present with proptosis and often present within the first two decades of life such as our patient; however, this is a benign process. Radiologically, fibrous dysplasia may also mimic a malignant process. It causes expansion of the involved bone but maintains a thinned, intact cortical boundary; osteosarcomas destroy cortical boundaries [25]. Another diagnostic consideration was primary squamous cell carcinoma of the nasal cavity and paranasal sinus but is not typically a disorder of childhood. Osteoblastomas may have similar pathologic findings as osteosarcoma; however, the presence of atypical cells and destructive radiologic appearance of such osteosarcomas helps to distinguish them from osteoblastomas [1]. Other malignant neoplasms such as melanoma, adenoid cystic carcinoma, adenocarcinoma, lymphoma and undifferentiated carcinoma can occur in the sinonasal cavities but, again, are diseases of adulthood. Osteomyelitis should be included in the differential diagnosis; nonetheless, no infection was found in our patient [Table 2]. In the pediatric age group, rhabdomyosarcoma can occur in the sinonasal cavities, but these present as enhancing soft tissue masses on imaging.
In conclusion, ethmoid sinus osteosarcoma is a rare disease. The presentation of primary osteosarcoma arising de novo in the ethmoid sinus as a solitary mass in a pediatric patient as opposed to the typical long bone location is rare. Our patient illustrates that osteosarcoma of the sinus should be considered when a sinonasal tumor occurs in a child.
TEACHING POINT
Primary osteosarcomas of the ethmoid sinus are rare with no differences in terms of prognosis if the tumor arises de novo or a second malignancy.
Table 1.
Summary table for Primary Osteosarcoma of the ethmoid sinus.
| Incidence | Head and neck: 0.5–8.1% |
| Gender Predilection | No gender predominance |
| Age Predilection | Third to fourth decades of life |
| Risk factors | Radiation therapy, thorium oxide exposure, chemotherapy, inherited predispositions such a Rothmund-Thomson syndrome, preexisting benign bone disease such as Paget’s disease. |
| Treatment | Surgery, neoadjuvant and adjuvant chemotherapy. Resistant to radiation therapy |
| Prognosis | Localized disease has better prognosis that metastatic disease. Prognosis depends of resectability of the tumor and the presence of tumor necrosis following preoperative chemotherapy. |
| Imaging Findings | Plain films: Cortical and medullary destruction and aggressive periosteal reaction, wide transition zone, tumor matrix ossification. CT: Its main role is to assist in biopsy and staging. Adds little to plain radiography. Except in lytic lesions in which small amount of mineralized material may be in-apparent on both plain film and MRI. MRI: Local staging: intraosseous tumor extension and soft tissue involvement. T1: soft tissue non-mineralized component : intermediate signal intensity; mineralized / ossified components : low signal intensity; peri-tumoral edema : intermediate signal intensity; scattered regions of hemorrhage will have variable signal (see ageing blood on MRI), enhancement : solid components enhance T2: soft tissue non-mineralized component : high signal intensity; mineralized / ossified components : low signal intensity; peri-tumoral edema : high signal intensity Bone Scan: Increased radiotracer uptake of the tumor and metastases. |
ACKNOWLEDGEMENTS
To Andrey Escolar MD for his collaboration with the figures.
ABBREVIATIONS
- 99m Tc
Metastable nuclear isomer of technetium-99
- AFIP
Armed Forces Institute of Pathology
- C+
Contrast
- CT
Computer Tomography
- Gd
Gadolinium
- IV
Intravenous
- MRI
Magnetic resonance Imaging
REFERENCES
- 1.Gadwal SR, Gannon FH, Fanburg-Smith JC, Becoskie EM, Thompson LD. Primary osteosarcoma of the head and neck in pediatric patients: a clinicopathologic study of 22 cases with a review of the literature. Cancer. 2001;91(3):598–605. [PubMed] [Google Scholar]
- 2.Kirby EJ, Zhou HH, Morales L., Jr Primary osteosarcoma of the skull. J Craniofac Surg. 2011;22( 6):2399–2405. doi: 10.1097/SCS.0b013e318231fe9b. [DOI] [PubMed] [Google Scholar]
- 3.Kanazawa R, Yoshida D, Takahashi H, Matsumoto K, Teramoto A. Osteosarcoma arising from the skull-case report. Neurol Med Chir. 2003;43(2):88–91. doi: 10.2176/nmc.43.88. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Kim YJ, Chu YC, Lee JW, Jeon YS, Lee KH, Cho SG, Kim MY. Primary osteosarcoma arising from the middle turbinate in a pediatric patient. Clin Exp Otorhinolaryngol. 2012;5(4):237–239. doi: 10.3342/ceo.2012.5.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlychou M, Ostlere SJ, Kerr R, Athanasou NA. Low-grade osteosarcoma of the ethmoid sinus. Skeletal Radiol. 2007;36(5):459–462. doi: 10.1007/s00256-006-0231-0. [DOI] [PubMed] [Google Scholar]
- 6.Kohanawa R, Tabuchi K, Okubo H, Nagata M, Hara A. Primary osteogenic sarcoma of the ethmoid sinus: a case report. Auris Nasus Larynx. 2005;32(4):411–413. doi: 10.1016/j.anl.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Chan LL, Czerniak BA, Ginsberg LE. Radiation induced Osteosarcoma after bilateral Childhood Retinoblastoma. AJR 200. 174(5):1288. doi: 10.2214/ajr.174.5.1741288. [DOI] [PubMed] [Google Scholar]
- 8.Alzahrani M, Robier A, Pointreau Y, Bakhos D. A rare case of radiation-induced osteosarcoma of the ethmoid sinus. Case Rep Otolaryngol. 2011;2011:786202. doi: 10.1155/2011/786202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagat D, Lone A, Bhardwaj S, Choudhary A. Osteogenic Sarcoma of Lower Jaw. JK Science. 2004;6:37–39. No PMID. [Google Scholar]
- 10.Chaudhary M, Chaudhary SD. Osteosarcomas of the jaws. J Oral Maxillofac Pathol. 2012;16(2):233–238. doi: 10.4103/0973-029X.99075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadley C, Gressot LV, Patel AJ, Wang LL, Flores RJ, Whitehead WE, Luerssen TG, Jea A, Bollo RJ. Osteosarcoma of the cranial vault and skull base in pediatric patients. J Neurosurg Pediatr. 2014;13(4):380–385. doi: 10.3171/2013.12.PEDS13359. [DOI] [PubMed] [Google Scholar]
- 12.Park YK, Ryu KN, Park HR, Kim DW. Low-grade osteosarcoma of the maxillary sinus. Skeletal Radiol. 2003;32( 3):161–164. doi: 10.1007/s00256-002-0577-x. [DOI] [PubMed] [Google Scholar]
- 13.Van den Berg H, Schreuder WH, de Lange J. Osteosarcoma: A Comparison of Jaw versus Nonjaw Localizations and Review of the Literature. Sarcoma. 2013;2013:316123. doi: 10.1155/2013/316123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huvos AG, Sundaresan N, Bretsky SS, Butler A. Osteogenic sarcoma of skull: a Clinicopathologic study of 19 patients. Cancer. 1985;56( 5):1214–1221. doi: 10.1002/1097-0142(19850901)56:5<1214::aid-cncr2820560543>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Jasnau S, Meyer U, Potratz J, Jundt G, Kevric M, Joos UK, Jürgens H, Bielack SS Cooperative Osteosarcoma Study Group COSS. Craniofacial osteosarcoma. Experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol. 2008;44(3):286–294. doi: 10.1016/j.oraloncology.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Park HR, Min SK, Cho HD, Cho SJ, Lee JH, Lee Y, Park YK. Osteosarcoma of the ethmoid sinus. Skeletal Radiol. 2004;33(5):291–294. doi: 10.1007/s00256-003-0742-x. [DOI] [PubMed] [Google Scholar]
- 17.Maes P, Brichard B, Vermylen C, Cornu G, Ninane J. Primary and secondary osteosarcoma of the face: a rare childhood malignancy. Med Pediatr Oncol. 1998;30( 3):170–4. doi: 10.1002/(sici)1096-911x(199803)30:3<170::aid-mpo8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Lee YY, Van Tassel P, Nauert C, Raymond AK, Edeiken J. Craniofacial osteosarcomas: plain film, CT, and MR findings in 46 cases. AJR Am J Roentgenol. 1988;150:1397–1402. doi: 10.2214/ajr.150.6.1397. [DOI] [PubMed] [Google Scholar]
- 19.Mettler F, Guiberteau M. Essentials of Nuclear Medicine Imaging. 5th ed. Philadelphia, USA: Saunders/Elsevier; 2006. Skeletal System; pp. 243–292. [Google Scholar]
- 20.Osteosarcoma. Sarcoma Foundation of America; [Accessed: May 21rst, 2014]. Sarcoma Subtypes. Available at: http://www.curesarcoma.org/index.php/patient_resources/subtypes/osteosarcoma. [Google Scholar]
- 21.Staging and site information about Osteosarcoma and Malignant Fibrous Histiocytoma (MFH) of Bone Treatment. National Cancer Institute at the National Institutes of Health; [Accessed: May 25th, 2014]. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/osteosarcoma/HealthProfessional/page3. [Google Scholar]
- 22.Patel SG, Meyers P, Huvos AG, Wolden S, Singh B, Shaha AR, Boyle JO, Pfister D, Shah JP, Kraus DH. Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer. 2002;95(7):1495–1503. doi: 10.1002/cncr.10849. [DOI] [PubMed] [Google Scholar]
- 23.General Information about Osteosarcoma and Malignant Fibrous Histiocytoma (MFH) of Bone. National Cancer Institute at the National Institutes of Health; [Accessed: May 20th, 2014]. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/osteosarcoma/HealthProfessional. [Google Scholar]
- 24.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12(2):423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 25.Yeşilova E, Akgünlü F, Dolanmaz D, Yaşar F, Şener S. Osteosarcoma: A Case Report. Eur J Dent. 2007;1( 1):60–63. [PMC free article] [PubMed] [Google Scholar]