Abstract
We would like to report our experience of a rather rare complication that occurred in a 76-year old patient tree years after endovascular repair of a splenic artery pseudoaneurysm with a covered stent. Three years after stent insertion, the patient complained of mild abdominal pain and melena; it was revealed endoscopically that the covered stent has eroded the stomach wall and migrated into the stomach. The splenic artery is the most common location among the spectrum of potential presentation sites of visceral arteries aneurysms and pseudoaneurysms. Endovascular treatment with the use of coils or stents is the first option due to lower morbidity and mortality than open surgery. Endovascular repair may also lead to complications and patients need to be followed up in order to confirm aneurysm sealing, and exclude late complication. Minor stent graft migration may occur in the long term, however extra vascular migration is extremely rare.
Keywords: Abdominal pain, melena, gastroscopy, splenic artery, stent migration
CASE REPORT
A 73-year-old man was admitted the Emergency Department complaining of vomiting and abdominal pain for three days. He was also febrile and jaundiced. Endoscopic retrograde cholangio-pancreatography (ERCP) followed and revealed the presence of an obstruction of the ampulla of Vater; a plastic biliary stent was initially placed. Histology revealed that the lesion was malignant (adenocarcinoma). A Computed Tomography (CT) scan was used for staging (Figure 1) and the lesion was considered as resectable and a Whipple’s procedure followed. The postoperative recovery was uneventful and the patient was discharged. The patient was readmitted two weeks later due to severe abdominal pain, and presence of fresh blood in the surgical drains. CT revealed the presence of a peri-pancreatic collection suggesting post-operative pancreatitis. The collection was drained percutaneously and a somatostatin-based therapy was administered. The patient was again discharged however, two days later was readmitted once again due to further severe bleeding from the surgical drain. CT angiography was performed this time and revealed the presence of a 6 cm pseudoaneurysm at the origin of the splenic artery (Figure 2). Considering the increased morbidity of an attempt to control the bleeding intraoperatively, immediate endovascular repair was decided. Access from the right common femoral artery was obtained and selective catheterization of the splenic artery followed. A 45 cm 6Fr sheath (Radifocus, Terumo Europe) was advanced within the splenic artery and a 5×50mm self-expandable covered stent (Viabahn, W.L.Gore, Flagstaf, AZ, USA) was deployed (Figure 3). Immediate bleeding control followed. The patient underwent surgical debridement the next day and was discharged two weeks later. Control CT scan three months later confirmed successful exclusion of the pseudoaneurysm. However there was not flow within the stent graft’s lumen suggesting thrombosis, but this finding did not raise a concern at this stage, as the patient was stable and the spleen was perfused from collaterals (Figure 4). Two years later tumor progression occurred with lymph node involvement and vertebral metastasis and further three cycles of chemotherapy were administered. Three years after his initial admission, the patient was readmitted due to epigastric pain and melena; he was haemodynamically stable (Blood Pressure 135/70 mmHg; HR 73 b/min). A CT scan was performed that excluded bleeding, however revealed migration of the previously inserted stent graft within the stomach lumen (Figure 5). Endoscopy was performed and confirmed the finding (Figure 6). The patient was in a palliative pathway and the multidisciplinary board decided to leave the device in situ, given the poor general conditions and short life expectancy. The patient passed away 10 months later without any complications from the migrated stent graft.
Figure 1.
73-year-old man presenting an adenocarcinoma of the ampulla of Vater.
FINDINGS: an adenocarcinoma of the ampulla of Vater (white arrow) obstructing the choledochus. Note significant contrast enhancement of the tumor occluding the ampulla.
TECHNIQUE: axial arterial phase at 35 sec after automatic intravenous injection of 120 ml of Iopamidol 370 - Bracco S.p.A., Milan, Italy - with a flow rate of 3,5 ml/sec; Computed Tomography, helical mode, 2.5 mm slice thickness, fixed tube current of 100 mA (General Electric LightSpeed 16, Milwaukee, USA)
Figure 2.
73-year-old man presenting a six cm pseudoaneurysm of the splenic artery
FINDINGS: a six cm pseudoaneurysm (white arrows) between the liver and the gastric lesser curve showing contrast medium extravasation from the origin of the splenic artery, just anteriorly to the bifurcation (black arrow head). Note the surgical drain (white arrowhead) adjacent to the pseudoaneurysm.
TECHNIQUE: 10 mm MIP slice, axial arterial phase at 35 sec after automatic intravenous injection of 120 ml of Iopamidol 370 - Bracco S.p.A., Milan, Italy - with a flow rate of 3,5 ml/sec; Computed Tomography, helical mode, 2.5 mm slice thickness, fixed tube current of 100 mA (General Electric LightSpeed 16, Milwaukee, USA)
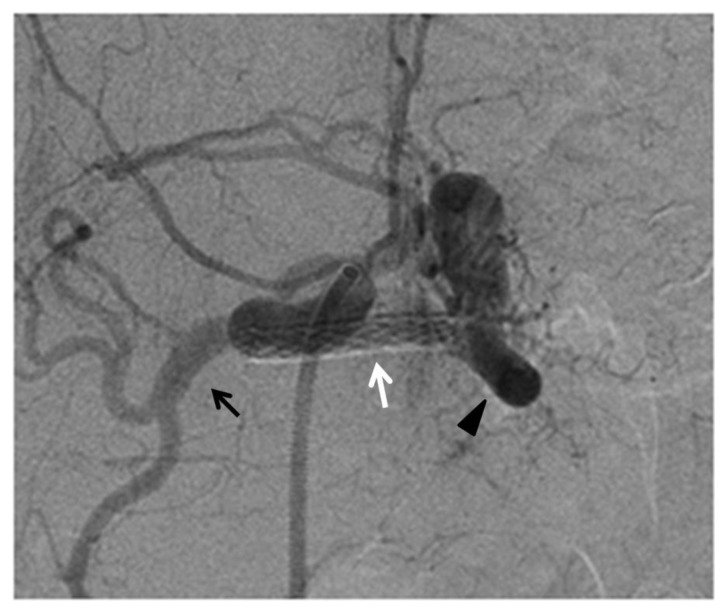
Figure 3.
76-year-old man post deployment of the covered stent in the splenic artery.
FINDINGS: angiography demonstrating the correct position of the covered stent (white arrow) with preservation of flow in the splenic artery (black arrow head) and absence of contrast medium extravasation and preservation of the patency of hepatic artery (black arrow).
TECHNIQUE: selective celiac axis angiography, in anterior-posterior view, after the deployment of the covered. Siemens AXIOM Artis C-arm. Hand injection 5 cc Omnipaque 350. kVp 64, mAs 10.
Figure 4.
73-year-old man presenting occlusion of the splenic artery stent, 3 months after the deployment to exclude the pseudoaneurysm.
FINDINGS: the splenic artery stent appears occluded at the three months follow-up CT scan. The stent graft is on the splenic artery pathway, and no end organ ischemia was noticed.
TECHNIQUE: axial arterial phase at 35 sec after automatic intravenous injection of 110 ml of Iobitridol 350 - Guerbet S.p.A., Villepinte, France - with a flow rate of 2,5 ml/sec; Computed Tomography, helical mode, 3 mm slice thickness, fixed tube current of 120 mA (General Electric LightSpeed 16, Milwaukee, USA)
Figure 5.
76-year-old man presenting the migration of the covered stent into the stomach.
FINDINGS: CT performed three years after stent deployment, showing the stent migration into the stomach: the splenic artery is not anymore depictable (white arrow head) and the device is not along its presumable path. The stent graft clearly “pierces” the gastric wall protruding more than half inside the lumen (black arrow).
TECHNIQUE: axial arterial phase at 35 sec after automatic intravenous injection of 120 ml of Iopamidol 370 - Bracco S.p.A., Milan, Italy - with a flow rate of 3,5 ml/sec; Computed Tomography, helical mode, 2.5 mm slice thickness, fixed tube current of 100 mA (General Electric LightSpeed 16, Milwaukee, USA)
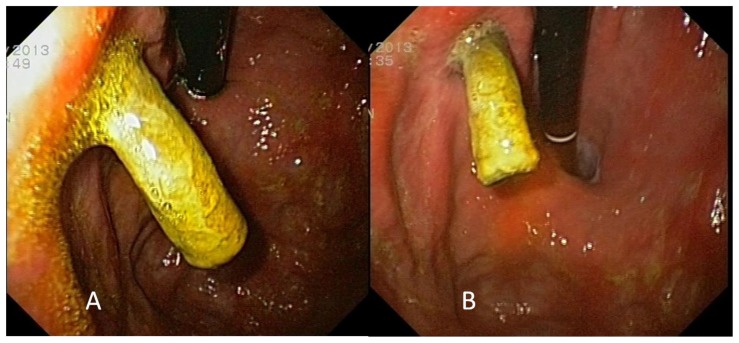
Figure 6.
76-year-old man presenting a covered stent protruding out of the stomach wall.
FINDINGS: endoscopy showing the stent migration into the stomach. The covered stent was protruding more than half inside the lumen through the lesser curvature, proximally to the cardias (A and B).
TECHNIQUE: sedation performed with 5 mg of midazolam. Olympus GIF-Q180 Gastroscope (Olympus, USA)
DISCUSSION
Etiology & Demographics
Splenic artery is the most common location for occurrence of pseudoaneurysms in visceral circulation [1–3]. Aneurysms of size smaller than 2 cm, with imaging features of chronicity (wall calcification) may be observed and left untreated for years if stable. Pseudoaneurysms, aneurysms larger than 2 cm or those that tend to grow in time need to be treated, due to the high risk of rupture [4].
The etiology of visceral artery aneurysms remains unclear; the origin is idiopathic in up to 40% of patients [1, 2]. The causes of visceral aneurysm include atherosclerosis, medial degeneration, infection, fibromuscular dysplasia and congenital anomalies [5]. Although arteriosclerosis is the most common cause of splenic artery aneurysm other causes like the degeneration of the media of the vessel, related to hormonal changes and increased splenic perfusion like in pregnancy may be considered [6]. Splenic artery pseudoaneurysm formation is less frequent than frank aneurysms and usually occurs post trauma or inflammatory conditions, like pancreatitis [3,4,7]. Independent of the specific aetiology, the natural history of splenic artery aneurysms appears to be the continuous expansion and the eventual rupture. Rupture of visceral artery aneurysms occurs in 3 to 8% of patients most of which are asymptomatic [3]. Rupture leads to a life-threatening, and often fatal bleeding. The mortality rate ranges from 10 to 25% (and may reach 75% if it occurs in pregnancy) [2,8].
Treatment & Prognosis
Endovascular treatment is an efficient and quick option in such cases [3–9]. The reported success rate of endovascular management of splenic aneurysms is more than 70% [10].
Treatment is performed either with the use of coils (also stent assisted coiling) or stents - mainly covered ones – and embolic agents as glue or Onyx® (Micro Therapeutics, Inc). The treatment choice is operator dependent based on the tortuosity of the target vessel, the location, morphology and dimension of the lesion and the intention to preserve the target vessel flow or not. There are two main techniques proposed using coils: the ‘’front and back door” and the “packing” technique; in the first coils are used to occlude the feeding and the draining arteries without deploying coils in the aneurysmal sac. In the latter coils within the sac of the aneurysm are deployed. There is a small chance of distal organ infarction with both techniques particularly if coil migration occurs [1,4]. The main advantage of using a stent-graft, in comparison with coil packing, is the preservation of the feeding artery and of the collateral branches. The risk of distal embolization with this technique is linked to the manipulation of the device (clot formation or air embolism on the carrier sheath) and is usually very low. The covered stent option is considered as the first option, particularly for lesions with a wide neck, however it is not always easy to advance a large bore sheath (6–7 Fr) within the tortuous splenic artery. In the case described, the covered stent option was retained as the more suitable technique due to the relatively proximal location of the lesion and by the fact that a 6Fr sheath was advanced within the splenic artery.
Follow-up is usually performed with CT that is non-invasive technique and allows evaluation of stent patency, end organ perfusion and exclusion of complications. Plain X-rays may be useful to evaluate the integrity and the approximate position of stent. In our case gastroscopy was used to confirm the stent’s position and with the intention to remove the migrated device.
Clinical & Imaging findings
Pseudoaneurysms frequently occur in the setting of acute or chronic pancreatitis, and the most common presenting symptom is abdominal pain [11]. Other symptoms include hematochezia, bleeding into the pancreatic duct and hematemesis [12]. Sizes may vary with a mean of about 4.8 cm (up to 17 cm), but there is no correlation of size with symptoms. Associated findings can aid identifying the aetiology such as pancreatitis, trauma, or postsurgical changes [13]. Splenic artery aneurysms are usually asymptomatic; however they could become symptomatic if they cause compression of other abdominal structures or if there is an imminent rupture. Splenic artery aneurysms are usually less than 3 cm in diameter, but aneurysms up to 30 cm have been reported. Peripheral calcification and mural thrombus may be present [11,14].
Most splenic artery aneurysms and pseudoaneurysm are detected incidentally during diagnostic imaging performed for other indications. If the splenic artery presents wall calcification the aneurysms could sometimes be detected even performing a plain abdominal X-ray. The use of ultrasonography (both b-mode and and Doppler) for the diagnosis of splenic artery aneurysms has also been reported [8,15] but is an operator-dependent technique and may be limited due to patient’s body habitus and bowel gas. Magnetic resonance angiography techniques have also been described [8,16]. Digital subtraction angiography (DSA) is still considered as the gold standard for the diagnosis of splenic artery aneurysm and pseudoaneurysms due to the fact that offers flow related information, however computed tomography (CT) offers rapid picture acquisition during the arterial phase, adds information about aetiology and offers accurate delineation of the lesion that is useful for treatment planning [12,17].
Differential Diagnoses
Clinical presentation of visceral stent migration is usually vague; often associated with abdominal pain or symptoms related to vascular occlusion. In the case reported symptoms appeared to be related to the stomach wall perforation, since collateral circulation supported end organs of the splenic artery. Usually stent graft migration is reported for aortic stents (e.g.: EVAR) than tend to migrate minimally distally leading to type IA endoleaks. Very few cases report significant or extra vascular migration. Migration of visceral stent grafts is also rare; Ferrero et al. reported just one case of migration of a celiac trunk stent among 32 cases of visceral stenting (3%), which required a re-intervention [18]. Negri et al. reported a case of migration three months after deployment of a celiac trunk stent into the splenic artery without end organ ischemia [19].
The stent, in its path from the splenic artery to the stomach, eroded the vessel wall, dissolved the retroperitoneal adipose tissue, perforated the posterior parietal peritoneal sheet by invading the lesser omentum and completely eroded the gastric wall. There are several factors that may have contributed to this migration. The cytotoxic effect of chemotherapy [20] probably damaged the vessel wall and also the others tissues, facilitating the migration and contributing to the inflammatory state [21] already promoted by the neoplastic condition. The chronic inflammatory condition was enhanced by the recent pancreatitis that was probably the main cause of the pseudoaneurysm development; another factor that probably led to stent migration was the short time laps between the pancreatitis, the percutaneous drainage and the endovascular exclusion with stent graft. However, the aneurysmal sac developed quickly and reached a diameter of 6 cm in few days, requiring a prompt treatment.
Although rare and unreported, intestinal perforation can represent a late complication of endovascular devices. Few cases of intestinal perforation by aortic dacron prosthesis migration have been described with a two- to three-years median interval from surgery to symptoms onset [22–23]. In our case, the poor general patient conditions as well as numerous and complex interventions, played a role in the splenic artery thrombosis, disruption, anatomic alterations and consequent stent dislocation.
Dienter et al. reported a lethal aortogastric fistula in a patient who had undergone an embolization of the celiac trunk aneurysm with coils and alcohol prolamine solution 10 years earlier. Acute hematemesis required urgent gastroscopy that showed the coils inside the stomach; patient died of a recurrent gastrointestinal bleeding two days later [24].
In conclusion, it is important to consider such possible late complications during patient follow-up; differential diagnosis should include the aforementioned complication in patients who have previously undergone endovascular treatment, referring gastrointestinal symptoms.
TEACHING POINT
Endovascular repair of splenic pseudoaneurysm is a useful tool but could be accompanied by late and unexpected complication as migration of the covered stent into the stomach; many factors could be involved in the pathogenesis as the inflammatory state of the patient (e.g.: previous surgery, chemotherapy, inflammatory diseases). Mild abdominal pain could be caused by such complication and physicians should be aware about this and should investigate for medical history of endovascular treatment.
Table 1.
Summary table for splenic artery stent graft migration.
| Etiology | Unknown (occlusion, chemotherapy, inflammatory conditions) |
| Incidence (among splenic artery aneurisms) | Rare/Uncommon |
| Gender ratio (M:F) | Unknown |
| Age predilection | 70–80 years |
| Risk factors | Unknown (occlusion, chemotherapy, inflammatory conditions) |
| Treatment | Observation/Surgical |
| Prognosis | Depending on the localization of the migrated stent |
| Findings on imaging | Incorrectly positioned/migrated stent |
Table 2.
Differential diagnosis table for migrated splenic artery stent graft.
| Splenic Artery Stent Graft Migration | Splenic Artery Stent Graft Rupture | Abdominal Foreign Body | |
|---|---|---|---|
| X - Ray | Stent graft localized in a different abdominal quadrant | Stent graft damaged or broken | Different shape and density |
| US |
|
|
|
| CT |
|
|
|
| MRI |
|
|
|
ACKNOWLEDGEMENTS
The authors thank Doctor Gianluigi Orgera who helped in images collection.
ABBREVIATIONS
- CT
Computed Tomography
- DSA
Digital Subtraction Angiography
- EVAR
Endovascular Aortic Repair
- ERCP
Endoscopic Retrograde Cholangio-Pancreatography
REFERENCES
- 1.Laganà D, Carrafiello G, Mangini M, Dionigi G, Caronno R, Castelli P, Fugazzola C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59(1):104–11. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Rossi M, Varano GM, Orgera G, Rebonato A, Laurino F, De Nunzio C. Wide-neck renal artery aneurysm: parenchymal sparing endovascular treatment with a new device. BMC Urol. 2014;14(1):42. doi: 10.1186/1471-2490-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi M, Rebonato A, Citone M, La Torre M, David V. Common hepatic artery aneurysm successfully treated with a celiac axis stent graft. Two years of follow up. European Journal of Radiology Extra. 2010;75( 3):125–8. [Google Scholar]
- 4.Gwon DI, Ko GY, Sung KB, Shin JH, Kim JH, Yoon HK. Endovascular management of extrahepatic artery hemorrhage after pancreatobiliary surgery: clinical features and outcomes of transcatheter arterial embolization and stent-graft placement. AJR Am J Roentgenol. 2011;196(5):W627–34. doi: 10.2214/AJR.10.5148. [DOI] [PubMed] [Google Scholar]
- 5.Grego FG, Lepidi S, Ragazzi R, et al. Visceral artery aneurysm: a single center experience. Cardiovasc Surg. 2003;11:19–25. doi: 10.1016/s0967-2109(02)00121-7. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Anno I, Yamaguchi M, Iida H, Orii K. Splenic artery aneurysm of the anomalous splenomesenteric trunk: successful treatment by transcatheter embolization using detachable coils. Cardiovasc Intervent Radiol. 2006 May-Jun;29(3):432–4. doi: 10.1007/s00270-005-0036-x. [DOI] [PubMed] [Google Scholar]
- 7.McDermott VG, Shlansky-Goldberg R, Cope C. Endovascular management of splenic artery aneurysm and pseudo-aneurysm. Cardiovasc Interv Radiol. 1994;17(4):179–184. doi: 10.1007/BF00571531. [DOI] [PubMed] [Google Scholar]
- 8.Rebonato A, Rossi M, Rebonato S, Cagini L, Scialpi M. Giant hepatic artery aneurysm: a fatal evolution. J Emerg Med. 2013;45(6):e217–9. doi: 10.1016/j.jemermed.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 9.Bratby MJ, Lehmann ED, Bottomley J, Kessel DO, Nicholson AA, McPherson SJ, Morgan RA, Belli AM. Endovascular embolization of visceral artery aneurysms with ethylene-vinyl alcohol (Onyx): a case series. Cardiovasc Intervent Radiol. 2006;29(6):1125–8. doi: 10.1007/s00270-005-0148-3. [DOI] [PubMed] [Google Scholar]
- 10.Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Interv Radiol. 2003;26(3):256–26. doi: 10.1007/s00270-003-1948-y. [DOI] [PubMed] [Google Scholar]
- 11.Lu M, Weiss C, Fishman EK, Johnson PT, Verde F. Review of visceral aneurysms and pseudoaneurysms. J Comput Assist Tomogr. 2015 Jan-Feb;39(1):1–6. doi: 10.1097/RCT.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol. 2007;188:992–999. doi: 10.2214/AJR.06.0794. [DOI] [PubMed] [Google Scholar]
- 13.Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg. 2003;38:969–974. doi: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 14.Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg. 2000;14:223–229. doi: 10.1007/s100169910039. [DOI] [PubMed] [Google Scholar]
- 15.Derchi LE, Biggi E, Cicio GR, Bertoglio C, Neumaier CE. Aneurysms of the splenic artery: noninvasive diagnosis by pulsed Doppler sonography. J Ultrasound Med. 1984;3:41–44. doi: 10.7863/jum.1984.3.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Pilleul F, Beuf O. Diagnosis of splanchnic artery aneurysms and pseudoaneurysms, with special reference to contrast-enhanced 3D magnetic resonance angiography: a review. Acta Radiol. 2004;45:702–708. doi: 10.1080/02841850410001358. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe TA, Nelson RC, Johnson GA, et al. Optimization of multiplanar reformations from isotropic data sets acquired with 16-detector row helical CT scanner. Radiology. 2006;238:292–299. doi: 10.1148/radiol.2381050404. [DOI] [PubMed] [Google Scholar]
- 18.Ferrero E, Ferri M, Viazzo A, et al. Visceral artery aneurysms, an experience on 32 cases in a single center: treatment from surgery to multilayer stent. Ann Vasc Surg. 2011 Oct;25(7):923–35. doi: 10.1016/j.avsg.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Negri S, Ferraro S, Piffaretti G, et al. An unusual case of stent migration after celiac trunk endovascular revascularization. Cardiovasc Intervent Radiol. 2012 Aug;35(4):950–3. doi: 10.1007/s00270-011-0303-y. [DOI] [PubMed] [Google Scholar]
- 20.Rohatgi S, Jagannathan JP, Rosenthal MH, Kim KW, Ramaiya NH, Krajewski KM. Vascular toxicity associated with chemotherapy and molecular targeted therapy: what should a radiologist know? AJR Am J Roentgenol. 2014 Dec;203(6):1353–62. doi: 10.2214/AJR.13.11967. [DOI] [PubMed] [Google Scholar]
- 21.Doll DC, Yarbro JW. Vascular toxicity associated with antineoplastic agents. Semin Oncol. 1992 Oct;19(5):580–96. Review. [PubMed] [Google Scholar]
- 22.Schwacha H, Kern WV, Wagner D. Perforation of a Dacron vascular endoprosthesis into the duodenum. Endoscopy. 2006;38( Suppl 2):E51. doi: 10.1055/s-2006-944682. [DOI] [PubMed] [Google Scholar]
- 23.Scheppach W, Polzien M, Kuesters W. Aortobifemoral prosthesis penetrating into the duodenal lumen. Clin Gastroenterol Hepatol. 2011;9(12):A28. doi: 10.1016/j.cgh.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Dinter DJ, Rexin M, Kaehler G, Neff W. Fatal coil migration into the stomach 10 years after endovascular celiac aneurysm repair. J Vasc Interv Radiol. 2007;18(1 Pt 1):117–20. doi: 10.1016/j.jvir.2006.10.003. [DOI] [PubMed] [Google Scholar]