Abstract
Pancreatic panniculitis is a rare cutaneous presentation in patients with pancreatic pathology. While it presents as cutaneous inflammation with painful and erythematous nodules which demonstrate ulceration, imaging features of this pathology are seldom described. The common sites of involvement are the extremities. It demonstrates characteristic histological features of lobular panniculitis with ghost cells. MR imaging with its excellent soft tissue contrast can be helpful in confirming the diagnosis, demonstrating imaging features of fat necrosis with surrounding inflammation as demonstrated in our patient.
Keywords: panniculitis, pancreatitis, magnetic resonance imaging, nodules, subcutaneous fat tissue
CASE REPORT
A 76 year-old Chinese male with past medical history of diabetes mellitus, hypertension and hyperlipidemia was admitted to our hospital for complaints of bilateral lower limb swelling with rashes for duration of one week. The patient also gave a history of vomiting and mild abdominal pain one week prior. Clinical examination revealed redness and swelling of both lower limbs with tender erythematous nodular cutaneous lesions, some of which were discharging serous fluid (Figs 1A and B). He had a normal white cell count of 7.6 × 109 /L (normal range of 3.3–9.3 ×109 /L) but showed a raised C-reactive protein level of 133.6 mg/L (normal range 0.0–5.0 mg/L). The liver function tests revealed raised levels of alanine aminotransferase 67 U/L (normal range 17–63 U/L) and alkaline phosphatase 527 U/L (normal range 38–126 U/L). The patient underwent a punch biopsy of the skin which showed a lobular panniculitis with characteristic necrosis of adipocytes in the form of ghost cells. (Figure 9) These ghost cells were anucleate and had amorphous granular material within. Based on the pathognomonic findings of the skin biopsy, a diagnosis of pancreatic panniculitis was made. Serum amylase level was markedly elevated at >2000 U/L (normal range 36–128 U/L). Serum lipase was also elevated at > 400 U/L (normal range 15–50 U/L). Blood cultures for aerobic and anaerobic bacteria were negative. T-Spot tuberculosis test was negative.
Figure 1.
76 year-old Chinese man with pancreatic panniculitis.
Photograph of the patient’s lower limb showing erythematous nodular lesions. One of these nodules was discharging serous fluid (arrow).
Figure 9.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Adipocytes within fat lobules showed fat necrosis with characteristic “ghost cells”, which are anucleate and contain amorphous granular debris within them
TECHNIQUE: Skin biopsy, Hemotoxylin and Eosin stain × 200
He initially underwent a computed tomography (CT) examination of the abdomen and pelvis which demonstrated an oedematous and bulky pancreas with surrounding inflammatory stranding and a dilated pancreatic duct in keeping with changes of acute pancreatitis (Figure 2). Multiple gallstones were also noted (Figure 3). The common bile duct was dilated (Figure 4). There was no intrahepatic ductal dilatation. There was no scan evidence of complication such as pseudocyst formation, necrotizing pancreatitis or splenic vein thrombosis. As there was concern for superimposed cellulitis and deep seated abscess formation in both lower extremities, the patient underwent a contrast enhanced Magnetic Resonance Imaging (MRI) of bilateral lower extremities. MRI showed multiple nodular lesions scattered within the subcutaneous fat in both lower limbs with fairly symmetrical appearance. Majority of the lesions measured 1 to 2 cm in size and demonstrated heterogeneous T1 weighted (T1w) signal with some foci of both high T2w and T1w signals, similar to the surrounding fat. These lesions demonstrated avid uptake after intravenous administration of gadolinium-based contrast media. There was also subcutaneous soft tissue oedema with fluid over the superficial fascia in keeping with superimposed cellulitis (Figure 6). No deep seated abscess or evidence of necrotizing fasciitis such as deep fascial fluid collections was identified.
Figure 2.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Axial image showed an oedematous pancreas with peri-pancreatic inflammatory changes (arrows) and dilated pancreatic duct (arrowhead) as well as common bile duct (black arrow).
TECHNIQUE: Contrast-enhanced axial CT scan of the abdomen, 3mm thickness, SIEMENS SOMATOM definition AS+ scanner, 120kV, 168mAs with 90 ml of intravenous Omnipaque 350 was performed.
Figure 3.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Axial image showed several gallstones (arrow) with pericholecystic fluid and fat stranding in keeping with superimposed cholecystitis.
TECHNIQUE: Contrast-enhanced axial CT scan of the abdomen, 3mm thickness, SIEMENS SOMATOM definition AS+ scanner, 120kV, 168mAs with 90 ml of intravenous Omnipaque 350 was performed.
Figure 4.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Axial image showed dilated common bile duct (arrowhead). No mass is noted at the ampulla of Vater (arrow).
TECHNIQUE: Contrast-enhanced axial CT scan of the abdomen, 3mm thickness, SIEMENS SOMATOM definition AS+ scanner, 120kV, 168mAs with 90 ml of intravenous Omnipaque 350 was performed.
Figure 6.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Axial T2-weighted with fat suppression image of lower limb revealed heterogeneous hyperintense subcutaneous lesion (arrow), subcutaneous oedema and fluid in the superficial fascia (arrowhead).
TECHNIQUE: Multiplanar MR imaging of the lower limbs was performed on a GE MEDICAL SYSTEMS 1.5 Tesla MR scanner, TE 88.23, TR 4980 before administration of 10 ml intravenous Dotarem.
The patient however was discharged early against medical advice with oral antibiotics. Oral antibiotic was given in view of underlying cellulitis and as empirical antibiotic for gallstone pancreatitis.
DISCUSSION
Etiology & Demographics
Panniculitis refers to a group of disorders characterised by inflammation of the subcutaneous fat. Panniculitis may be idiopathic, but may also be secondary to infection, trauma, cold, systemic diseases such as connective tissue disorders, lymphoproliferative disease, and sarcoidosis. Pancreatic panniculitis is a rare variant of panniculitis, occurring in approximately 0.3–3 percent of all patients with pancreatic diseases [1,2], most commonly alcohol-related pancreatitis and pancreatic malignancy (usually acinar cell carcinoma, less frequently islet cell carcinoma) [3]. Other associated pancreatic disorders include post-traumatic pancreatitis, pancreatic pseudocyst, pancreas divisum, vascular pancreatic fistulae, and rarely subclinical pancreatic disease or high serum lipase levels of unknown origin [3,4,5]. There are also well documented cases of pancreatic panniculitis with normal serum lipase level [6].
The pathogenesis of pancreatic panniculitis is not fully known. A widely-accepted mechanism would be the release of the pancreatic enzyme trypsin into the circulation, resulting in increased capillary and lymphatic permeability [2]. This results in crossing of pancreatic lipase and amylase from the circulation into the subcutaneous fat causing fat necrosis and saponification. Fat saponification together with pannicular inflammation results in a lobular panniculitis with ghost cells – necrotic, anucleate adipocytes with granular amorphous material, which is characteristic of pancreatic panniculitis. This theory is supported by the fact that these enzymes are often found in high levels within the areas of panniculitis. Onset of disease can therefore be correlated to the elevated serum lipase and amylase [7]. However, pancreatic panniculitis has also been reported with normal enzyme levels, suggesting an additional unknown factor in the pathogenesis [3].
Clinical & Imaging Findings
Clinical features include local cutaneous inflammation with painful, erythematous to violaceous subcutaneous nodules 1–2 cm in size that spontaneously ulcerate to discharge thick oily brown material. Most common sites of manifestation are the distal parts of the lower extremities, mainly around the ankles and pretibial regions of the legs. Involvements of areas such as breasts, buttocks, thighs and abdomen have been described [3]. Skin manifestations can precede abdominal symptoms by up to 1–7 months in 40% of cases. In milder cases, the nodule can be single and resolve spontaneously while fatal outcome has been documented rarely. Besides the skin, there can be involvement of the peri-articular, abdominal and bone marrow fat.
Histopathological examination is required for diagnosis of this entity from deep skin biopsy [8]. Panniculitis is generally characterised histologically by the location of inflammatory process. Inflammation occurring mainly in the fibrous septa of the subcutis is classified as septal panniculitis while inflammation primarily in the fat lobules is classified as lobular panniculitis.
In the very early stage of pancreatic panniculitis, a septal pattern of panniculitis can be seen characterized by lymphoplasmacytic infiltration along the fibrous septa surrounding the subcutaneous fat lobules and around the dermal blood vessels. However, the characteristic histological subtype is that of lobular panniculitis without vasculitis and with marked necrosis of adipocytes. This is characterized by predominant neutrophilic lobular inflammatory infiltrate surrounding collection of “ghost adipocytes”, which are necrotic adipocytes with no nuclei and finely granular and basophilic material in the cytoplasm [4]. Fat necrosis and calcified ghost adipocytes are less evident in older lesions which consist of more foamy histiocytes and multinucleated giant cells, similar to that of a chronic granulomatous process. The liquefactive necrosis of adipocytes clinically manifests as spontaneous discharge of oily brown material through ulcerations.
Although diagnosis of pancreatic panniculitis hinges on clinical and histological examination, MRI has increasingly been utilised in diagnosis as skin inflammation and ulceration in the limbs can be confused with cellulitis or necrotizing fasciitis. Short Tau Inversion Recovery (STIR) or fat-supressed sequences are important in demonstrating inflammation in panniculitis, seen as focal or nodular areas of increased signal [9,10]. In our patient, the localization of the lesions on MRI to the subcutaneous fat is helpful in demonstrating that the disease process is localized to involvement of just the subcutaneous fat tissues. The symmetrical appearance of the lesions in both lower limbs would be consistent with that of a more diffuse disease process such as panniculitis although limb infections can infrequently occur bilaterally. Minimal or no intrinsic contrast enhancement is seen in fat necrosis [9]. However peripheral enhancement can be seen due to underlying fibrous tissue or granulation tissue [9]. The usual appearance of these lesions is that of a nodular mass-like lesion. MRI is also important in early detection of marrow changes due to necrosis of bone marrow fat [10,11,12].
Treatment & Prognosis
Therapeutic approach to this entity requires treatment of the underlying pancreatic diseases, and it usually resolves with resolution of pancreatic inflammation. Chronic or recurrent disease would be suggestive of recurrent pancreatitis and may reflect presence of an underlying carcinoma. This should prompt the physician to investigate further for pancreatic carcinoma.
Differential Diagnosis
The main differential diagnosis to consider in this disease entity is cellulitis. Cellulitis is diagnosed when there is skin thickening and subcutaneous oedema on T2 weighted images. The subcutaneous tissue or superficial fascia or both may show contrast enhancement on MRI. Cellulitis has more diffuse involvement as compared to panniculitis which typically presents as nodular or focal area of increased signal intensity on MRI that is confined to the subcutaneous tissue. As in this case, the patient has both panniculitis and cellulitis.
The other alternative consideration with patient presenting with lower limb swelling and erythema would be necrotizing fasciitis. In necrotizing fasciitis, MRI shows involvement of deep fasciae with fluid collections, thickening and enhancement after contrast administration. Fascial gas can be present.
CONCLUSION
Pancreatic panniculitis is a rare cutaneous presentation of pancreatitis. While it remains a clinical and histological diagnosis, we present pertinent Magnetic Resonance (MR) imaging features which could help in identifying pancreatic panniculitis. When evaluating patients for lower limb inflammation and infection on MRI, such features should prompt further investigation for underlying pancreatic pathology. In such instances, skin biopsy may be avoided.
TEACHING POINT
MRI features of pancreatic panniculitis in the lower limbs are usually nodular areas of T1w hypointense and T2w hyperintense signal intensity, showing no or immediate uptake of the intravenously administered contrast media, corresponding to focal areas of inflammation in the subcutaneous fat tissue. MRI can be a useful imaging tool to differentiate between this disease entity from cellulitis or necrotizing fasciitis. It is also important in defining the disease extent.
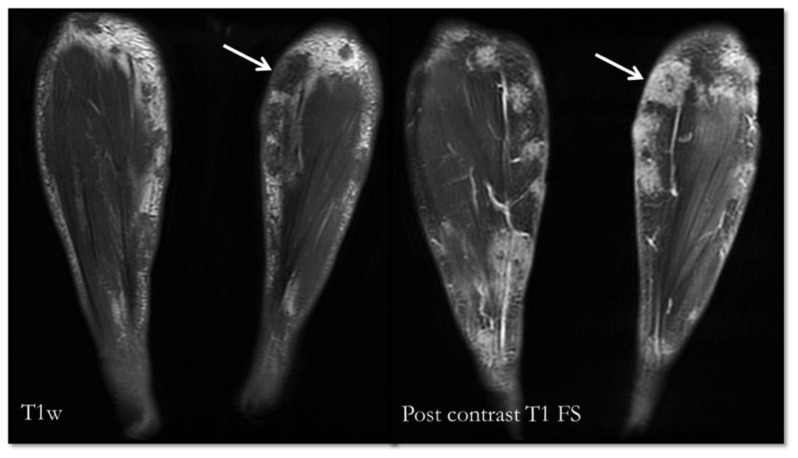
Figure 5.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Pre and post contrast-enhanced axial T1-weighted with fat suppression images of lower limb revealed the nodular enhancing T1w hypointense lesions in the subcutaneous plane.
TECHNIQUE: Multiplanar MR imaging of the lower limbs was performed on a GE MEDICAL SYSTEMS 1.5 Tesla MR scanner, TE 13.28, TR 540 before and after administration of 10 ml intravenous Dotarem.
Figure 7.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Pre contrast coronal T1-weighted and post contrast-enhanced coronal T1-weighted with fat suppression images of both lower limbs demonstrate symmetrical appearance of nodular T1w hypointese enhancing lesions.
TECHNIQUE: Multiplanar MR imaging of the lower limbs was performed on a GE MEDICAL SYSTEMS 1.5 Tesla MR scanner, TE 12.78, TR 520 before and after administration of 10 ml intravenous Dotarem.
Figure 8.
76 year-old Chinese man with pancreatic panniculitis.
FINDINGS: Pre contrast coronal Fast Spin Echo Inversion Recovery (FSEIR) and post contrast-enhanced coronal T1-weighted with fat suppression images of both lower limbs demonstrate no abnormal bone marrow signal or abnormal enhancement to suggest bone marrow necrosis.
TECHNIQUE: Multiplanar MR imaging of the lower limbs was performed on a GE MEDICAL SYSTEMS 1.5 Tesla MR scanner, TE 12.78, TR 520 after administration of 10 ml intravenous Dotarem (T1w), TE 30.78, TR 5800 (FSEIR).
Table 1.
Summary table for pancreatic panniculitis.
| Etiology | Pancreatic diseases |
| Incidence | 0.3–3% of all patients with pancreatic disease |
| Gender Ratio | Unknown |
| Age Predilection | Unknown |
| Risk Factors | Unknown |
| Treatment | Treat underlying pancreatic diseases |
| Prognosis | Dependent on the underlying pancreatic diseases Poor in cases associated with pancreatic carcinoma |
| Findings on Imaging | MRI: Nodular or focal areas of T1w hypointense and T2w hyperintense signal with or without contrast enhancement, confined to subcutaneous fat tissue. |
Table 2.
Differential diagnosis table for panniculitis.
| Radiography | MRI | Ultrasound | |
|---|---|---|---|
| Panniculitis | None or soft tissue swelling | Focal or nodular areas of T1w hypointense and T2w hyperintense signal in the subcutaneous fat tissue. | Nodular hypoechoic area in subcutaneous tissue |
| Cellulitis | None or soft tissue swelling | Diffuse skin thickening and subcutaneous oedema. Superficial fascial thickening or fluid may be seen. | Diffuse skin thickening and subcutaneous oedema (ill-defined anechoic area between subcutaneous fat) |
| Necrotizing fasciitis | Soft tissue gas | Involvement of deep fasciae with fluid collection, thickening and post contrast enhancement. Presence of fascial gas. | Difficult to visualized deep structure but if present it is seen as fluid between muscle plane or deep fascial gas. |
ABBREVIATIONS
- CT
computed tomography
- FSEIR
fast spin echo inversion recovery
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- STIR
short tau inversion recovery
- T1w
T1 weighted
- T2w
T2 weighted
REFERENCES
- 1.Requena Luis, Yus Evaristo Sanchez. Panniculitis. Part II. Mostly lobular Panniculitis. J Am Acad Dermatol. 2001 Sep;45(3):325–361. doi: 10.1067/mjd.2001.114735. [DOI] [PubMed] [Google Scholar]
- 2.Rongioletti F, Caputo V. Pancreatic Panniculitis. G Ital Dermatol Venereol. 2013 Aug;148( 4):419–25. [PubMed] [Google Scholar]
- 3.Madarasingha NP, Satgurunathan K, Fernando R. Pancreatitc panniculitis: A rare form of panniculitis. Dermatology online journal. 2009 Mar 15;15(3):17. [PubMed] [Google Scholar]
- 4.Dahl PR, Su WP, Cullimore KC, Dicken CH. Pancreatic panniculitis. J Am Acad Dermatol. 1996 Aug;35(2 Pt1):282–3. doi: 10.1016/0190-9622(95)91385-8. [DOI] [PubMed] [Google Scholar]
- 5.Millns JL, Evans HL, Winkelmann RK. Association of islet cell carcinoma of pancreas with subcutaneous fat necrosis. Am J Dermatopathol. 1979 Fall;1(3):273–809. doi: 10.1097/00000372-197900130-00015. [DOI] [PubMed] [Google Scholar]
- 6.Berman B, Conteas C, Smith B, Leong S, Hornbeck L., III Fatal pancreatitis presenting with subcutaneous fat necrosis. Evidence that lipase and amylase alone do not induce lipocyte necrosis. J Am Acad Dermatol. 1987 Aug;17(2Pt2):359–364. doi: 10.1016/s0190-9622(87)70213-8. [DOI] [PubMed] [Google Scholar]
- 7.Förström TL, Winkelmann RK. Acute generalized panniculitis with amylase and lipase in the skin. Arch Dermatol. 1975 Apr;111(4):497–502. doi: 10.1001/archderm.1975.01630160087010. [DOI] [PubMed] [Google Scholar]
- 8.Zheng JZ, Gong J, Xiang GM, Mai G, Liu XB. Pancreatic panniculitis associated with acinar cell carcinoma of the pancreas: a case report. Ann Dermatol. 2011 May;23( 2):225–228. doi: 10.5021/ad.2011.23.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan LP, Gee R, Keogh C, Munk PL. Imaging features of fat necrosis. AJR Am J Roentgenol. 2003 Oct;181(4):955–959. doi: 10.2214/ajr.181.4.1810955. [DOI] [PubMed] [Google Scholar]
- 10.Walsh M, Jacobson JA, Kim SM, Lucas DR, Morag Y, Fessell DP. Sonography of fat necrosis involving the extremity and torso with magnetic resonance imaging and histologic correlation. J Ultrasound Med. 2008 Dec;27(12):1751–1757. doi: 10.7863/jum.2008.27.12.1751. [DOI] [PubMed] [Google Scholar]
- 11.Price-Forbes AN, Filer A, Udeshi UL, Rai A. Progression of imaging in pancreatitis panniculitis polyarthritis (PPP) syndrome. Scand J Rheumatol. 2006 Jan-Feb;35(1):72–74. doi: 10.1080/03009740500228073. [DOI] [PubMed] [Google Scholar]
- 12.Haller J, Greenway G, Resnick D, Kindynis P, Kang HS. Intraosseous fat necrosis associated with acute pancreatitis: MR imaging. Radiology. 1989 Oct;173(1):193–195. doi: 10.1148/radiology.173.1.2781007. [DOI] [PubMed] [Google Scholar]