Abstract
Background
The DSM uses one set of abuse and dependence criteria to assess multiple substance use disorders (SUDs). Most SUD research aggregates across these symptoms to study the behavior of SUD as a static construct. We use an alternative approach that conceptualizes symptoms as directly interacting variables in psychopathological networks. We apply network models to symptom-level data to investigate the unique roles of individual symptoms and their interactions in SUD.
Methods
We analyzed 11 DSM III-R/IV abuse and dependence criteria in a sample of 2405 adult twins who reported use of at least one illicit substance six or more times from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD). We estimated a symptom network for each substance class as well as a global network collapsed across all substance classes. We examined similarities and differences across the 6 networks in terms of symptom-to-symptom connections and symptom centrality.
Results
The global network model revealed several interesting symptom connections, such as a strong predictive relation between tolerance and more-than-planned substance use. The most central symptom was using a drug more than planned. In addition, several interesting differences across substances emerged, both in the strength of symptom connections as well as the centrality of symptoms to each network.
Conclusions
When analyzed as networks, abuse and dependence symptoms do not function equivalently across illicit substance classes. These findings suggest the value of analyzing individual symptoms and their associations to gain new insight into the mechanisms of SUD.
Keywords: substance abuse, substance use disorders, network analysis, symptom interactions
Graphical abstract
1. INTRODUCTION
Drug abuse and dependence is a common and increasing worldwide public health concern (World Health Organization, 2010). In the US, life-time prevalence estimates of substance use disorders (SUD) range from 2–3% for illicit substances to 8% for alcohol use, and 12-month rates of substance abuse or dependence increase from 7% to 20% during adolescence (Merikangas and McClair, 2012).
Recent research in psychopathology indicates that the analysis of individual symptoms can reveal crucial insights obfuscated by other analytic strategies (Fried and Nesse, 2015; Smeets et al., 2014). A central tenet of symptom-based approaches is that interactions among symptoms may be central to understanding how disorders arise, sustain themselves, and are cured (Borsboom and Cramer, 2013; Buu et al. 2012; Cullen et al., 2013; Fergus et al., 2015; Fried, 2015; Jacobsen et al., 2001). A useful way to examine such symptom-level effects is to apply a network model, which uses pairwise interactions among symptoms to represent a disorder as a web of mutually influencing symptoms (Borsboom and Cramer, 2013). These models have been successfully applied to a number of disorders such as posttraumatic stress disorder (McNally et al., 2014) and major depression (Fried et al., 2015).
The network framework is an appropriate and useful conceptual approach to analyzing data whenever relations among symptoms can be plausibly interpreted as interacting directly with each other. Similar to other disorders, there is evidence that SUD symptoms may arise in a causal sequence; for example, drinking more alcohol than planned is frequently the first symptom of alcohol use disorder to arise (Buu et al., 2012), which aligns with the finding that impaired control over alcohol use is an important predictor of problem drinking in adolescents (Leeman et al., 2012). To date, no research has investigated such symptom interactions. A network model of SUD can give an overview of the connection patterns among symptoms, revealing which symptoms are most closely related to each other, and which symptoms are most central to the disorder. In addition, network analyses allow us to compare networks across several substance classes, and to locate important differences in the symptom-to-symptom pathways that may exist due to distinct pharmacologic and psychological properties of the substance and/or different patterns of use (Degenhardt et al., 2001; Koob and Le Moal, 2006).
In the remainder of the paper, we present and interpret three cross-sectional network analyses of substance abuse and dependence symptoms. First, we examine a psychopathological network of symptom data averaged over 6 illicit substance classes (cannabis, sedatives, stimulants, cocaine, opioids, and hallucinogens) in 2,405 individuals. We investigate the pairwise connections among 11 symptoms, and estimate measures of symptom centrality to identify which symptoms may be most important in the maladaptive behavior patterns of SUD. Second, we compute symptom networks for each of the substance classes separately. Our aim here is to explore the important differences and similarities of substance classes based on a network representation, and what these differences can tell us about the interconnectivity patterns of SUD symptoms. Finally, we estimate the variance of symptom-to-symptom connections across substance classes (i.e., how much does the strength of the association between symptom pairs vary across the six classes) to identify which of these connections vary most widely across substances.
2. METHOD
2.1. Sample
Data for the analyses carried out in this study come from twins who participated in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD). Initial eligibility was determined through successful matching of birth records, if twin members were Caucasian and born between 1940 and 1974 in Virginia, USA. Detailed information about substance use and related behaviors were obtained for 2 different data collection samples. Female-female twins participating in the third follow-up (Wave 4, N = 1,928 individuals interviewed by phone in 1995 – 1997) and male-male and male-female twins from the first follow-up (Wave 2, N = 5,602 individuals personally interviewed in 1994–1998) served as the sample pool of twins with valid substance use data. These interviews included assessments of lifetime drug use and items worded according to the DSM abuse and dependence criteria for six categories of substances that were administered using an adaptation of the Structured Clinical Interview (SCID; Spitzer et al., 1987). Drug classes were defined as follows: cannabis (e.g., marijuana and hashish); sedatives (e.g., quaalude, Seconal and Valium); stimulants (e.g., speed, ecstasy and Ritalin); cocaine (intranasal and crack); opioids (e.g., heroin and morphine); and hallucinogens (e.g., LSD and PCP). Of the sample pool of 7530 twins (44% female, age range 20–63, mean age = 36.8, SD = 8.9), 2405 reported having used at least one of the six substances more than 6 times during his or her life and were therefore retained for analysis.
The eleven SUD criteria are presented in Table 1. Each participant, based on his responses to the usage items, was asked to respond to some or all of the 4 abuse and 7 dependence criteria for each substance class using a 3 point response scale. The response options included two positive choices (e.g., “definitely” and “probably”) and one negative response (“no”). The individual symptoms were always asked for the time period in the respondent’s life when they were using that drug class the most. For the analyses reported here, responses were recoded into binary variables1 by collapsing over the two positive response options.
Table 1.
Substance abuse and dependence criteria used to determine diagnostic status for each substance use disorder
| Variable | Criterion |
|---|---|
| … did you often use it when you were doing something important like being at school or work or taking care of children? | |
| A1 | … did you stay away from work or school or miss appointments because you were using it? |
| A2 | … did you ever use it in a situation in which it might have been dangerous? |
| A3 | … did you have legal problems or traffic accidents because you were using it? |
| A4 | … did your use of it cause problems with other people such as family members, friends, or people at work? |
| D1 | … did you find that you needed to use a lot more in order to (get high/feel its effects) than you did when you first started using it? |
| D2 | … did you ever have withdrawal symptoms – that is feeling sick when you cut down or stopped using it? |
| … did you often use it to keep from getting sick (with withdrawal symptoms)? | |
| D3 | … did you often find that when you started using it, you ended up taking much more than you had planned? |
| D4 | … did you try to cut down or stop using it? |
| D5 | … did you spend a lot of time taking it or recovering from using it, or doing whatever you had to do to get it? |
| D6 | … did you use it so often that you would use it instead of working or spending time on hobbies or with your family or friends? |
| D7 | … did your use of it cause physical problems or make you depressed or very nervous? |
Note. The question stem for all items was, “During that time when you were using [drug] the most, … ”. Variables A1 and D2 were formed by collapsing two highly similar items; if either item was positively endorsed, the collapsed item was scored as endorsed.
2.1.1. Missing Data
The analysis sample for each substance class included only those participants who reported having used the substance 6 or more times. These participants were asked to indicate whether they had ever used the substance at least 11 times during a single month. Participants who reported not having used a particular substance 11 times in a month were administered the set of abuse items (i.e., A1 – A4), and were then administered the set of dependence items (i.e., D1 – D7) only if they positively endorsed at least one of the abuse symptoms. For all analyses reported here, missing values generated by this imposed structured skip out were set to zero, indicating an implied negative response for each skipped item. Participants who reported having used a substance 11 times in a month were administered all abuse and dependence items. Table 2 displays the number of participants falling into each of these categories (i.e., 6 or more lifetime uses, endorsement of at least one abuse criterion, and 11 or more uses in a month) for each substance class. In addition to the structured skip-related missingness, 41 individuals had additional item-level missing data; these cases were deleted.
Table 2.
Sample size for each substance
| Substance Class
|
||||||
|---|---|---|---|---|---|---|
| Skip Criterion | can | sed | sti | coc | opi | hal |
| > 6 lifetime uses; no abuse criteria endorsed | 853 | 140 | 174 | 233 | 71 | 142 |
| > 6 lifetime uses; ≥1 abuse criterion endorsed | 425 | 118 | 257 | 218 | 44 | 159 |
| > 11 uses within one month | 952 | 100 | 246 | 188 | 84 | 49 |
|
| ||||||
| Total N | 2230 | 358 | 677 | 639 | 199 | 350 |
Note. can = cannabis, sed = sedatives, sti = stimulants, coc = cocaine, opi = opioids, hal = hallucinogens. Participants who reported more than 6 lifetime uses but endorsed no abuse criteria were not administered the dependence criteria; zeroes were imputed on all dependence criteria for these participants. Participants who endorsed at least one abuse criterion, and those who reported more than 11 uses within one month were administered all criteria.
2.2. Network analyses
Symptom networks consist of nodes (symptoms) and edges (connections among symptoms). In this report, edges represent the conditional pairwise relations between two variables controlling for all other symptoms in the network. This means that the whole network can be interpreted as a joint partial correlation structure among a set of items. We performed three analyses.
2.2.1. Individual Substance Class Networks
First, we used the Ising model estimation procedure (van Borkulo et al., 2014) to estimate one network for each substance class, based on the total sample of users for each class (see Table 2). An Ising model can be understood as estimating partial correlations among a set of binary items. More technically, it is a probabilistic model in which the joint distribution over the 11 SUD criteria is represented using threshold parameters (related to the marginal probability of endorsement of any individual item) and pairwise association parameters (edge weights; related to the associations between items). The association parameters are similar to partial correlation coefficients for continuous normally distributed variables: they are unique (partial) associations between pairs of variables controlling for all other variables. Having more edges in the model leads to a more complex model with possibly many spurious connections that are not present in the population. Van Borkulo et al.’s (2014) method estimates a regularized Ising model by applying 1-regularized logistic regressions that constrain many of the small coefficients to zero (Ravikumar et al., 2010). A penalty parameter, selected using the extended Bayesian Information Criterion, determines the extent to which coefficients are shrunk to zero. The smaller the sample size, the stronger the penalty and the more sparse the resulting network will be (i.e., the fewer edges it will have) in order to identify only the relevant relations between symptoms. A fuller explanation of the Ising model can be found in van Borkulo et al. (2014).
Because sample size varied across substance classes, there was a concern that the resulting networks would not be comparable due to differential sparsity (e.g., in the opioid network with a sample of only N = 195, edges are much more easily set to 0 than in the cannabis network with N = 2,216). To address this concern, we used a bootstrapping procedure to draw 500 samples of size N = 500 each, with replacement, from the item data of each substance class. We produced a network for each bootstrapped sample and averaged across them to create a set of substance class networks based on the equated bootstrap sample sizes. The bootstrapped networks were very similar to the ones originally obtained directly from the data, and we therefore present the original networks here. The bootstrapped networks are available in the supplementary materials.
2.2.2. Cross Substance Class Network
To understand what a general network across all six SUDs would look like, we averaged each of the 55 edges over the six separate substance networks. This analysis results in a single aggregate cross-substance network with each substance network being given equal weight.
2.2.3. Cross Substance Class Variability Network
To determine whether the six individual networks differed from each other, we correlated the network edges to obtain an index of the degree of similarity across substances. We then constructed a network to visualize the variability of the edges across substance classes using the standard deviation of each edge across the six substance class networks.
2.2.4. Centrality
We computed three measures of symptom centrality (Boccaletti et al., 2006) for both the cross substance class network and the individual substance class networks. Centrality can be understood to reflect how connected and thus potentially clinically relevant a symptom is in a network. Network models make the assumption that the pattern of relations among symptoms is due to direct, bidirectional causal pathways among variables; to the extent that this assumption is true, intervening on a highly central symptom will affect other nodes both directly and indirectly (e.g., via paths through other symptoms), pushing the entire network into a healthier state (Borsboom and Cramer, 2013).
Betweenness centrality is based on the concept of the shortest path length connecting any two symptoms. If a network is seen as a grid that can be traversed, then any two symptoms are connected by either a direct path or a path that travels via other symptoms. A symptom with high betweenness centrality is one that lies along the shortest path connecting other symptoms. Closeness centrality is an index of how close a symptom is to every other symptom, on average. A node that is connected to every other node has high closeness. Finally, node strength is, for each symptom, the sum of the edge weights of the edges connecting it to each other symptom. All centrality estimates were standardized.
2.2.5. Visualization
The R-package qgraph (Epskamp et al., 2012) was used to visualize all networks. Thicker edges represent stronger relations, and red edges represent negative relations. The positioning of the 11 SUD criteria nodes in relation to each other also reflects the strength of edges in the global SUD network; that is, nodes that are depicted closer together are more strongly related. The node placement in all other graphs was fixed to be equal to that of the global SUD network, for ease of comparison.
3. RESULTS
3.1. Demographics
2,405 participants were included in the final sample. Of these, all were Caucasian, 35% were female, and the average age was 34.7 (SD = 7.3, range = 20–57). 58% of participants used a single substance class, while 20% used two substance classes, 9% three, 5% four, 4% five, and 3% all six.
3.2. Cross-substance network
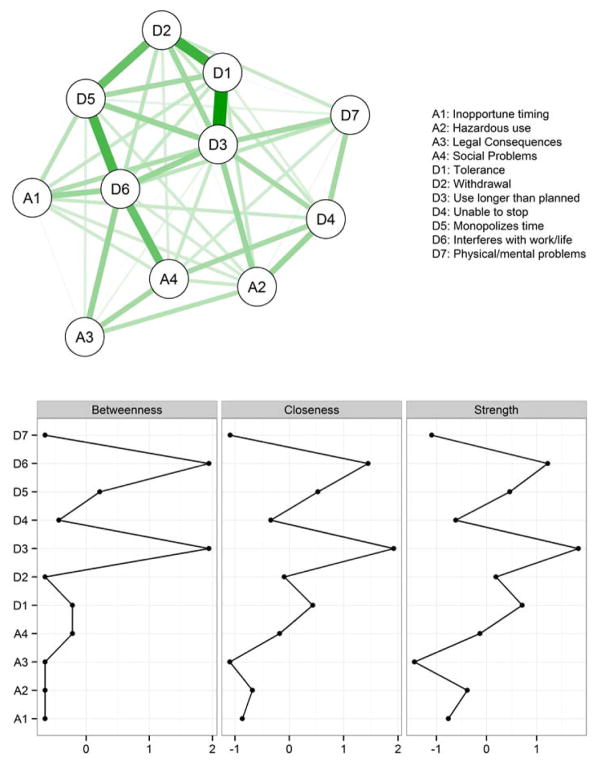
Figure 1 depicts the results of the global SUD network. Each node in the network depicts a symptom, and each edge represents bidirectional partial relations between symptoms, controlling for all other associations in the network. For example, there is a very strong positive connection between using a substance more than planned (D3) and tolerance (D1), controlling for all other associations. This link suggests that, across substances, using a drug more or longer than one planned to is a good predictor of drug tolerance, and vice versa. In contrast, using a substance more than planned is only weakly related to legal consequences (A3), suggesting that knowing whether someone has used a drug more than planned is not very informative about legal consequences resulting from her drug use, or vice versa.
Figure 1.
Network of abuse and dependence symptoms across all substances classes (cannabis, sedatives, stimulants, cocaine, opioids, and hallucinogens). Upper: Line thickness represents the strength of pairwise symptom connections; green paths represent positive relations. Lower: standardized centrality measures for each node. For the full item wording of each symptom, see Table 1.
Below the network diagram, Figure 1 depicts the results of the three centrality measures for each symptom. The pattern of symptom centrality is similar––in each case, symptom D3 (substance used more/longer than planned) is the most central symptom in the network. This implies that to predict whether a person is likely to have a host of other symptoms related to abuse and dependence, D3 provides the most information. As our network is based on cross-sectional lifetime use data when using the most, however, it cannot be determined whether D3 is more cause or consequence (or both) of the other nodes in the network. The centrality of D3 implies that an intervention targeting this symptom would have the greatest potential to affect the status of an individual’s SUD, whereas a low centrality symptom such as D7 (physical and mental problems as a result of substance use) would be of limited therapeutic use.
3.3. Individual substance class networks
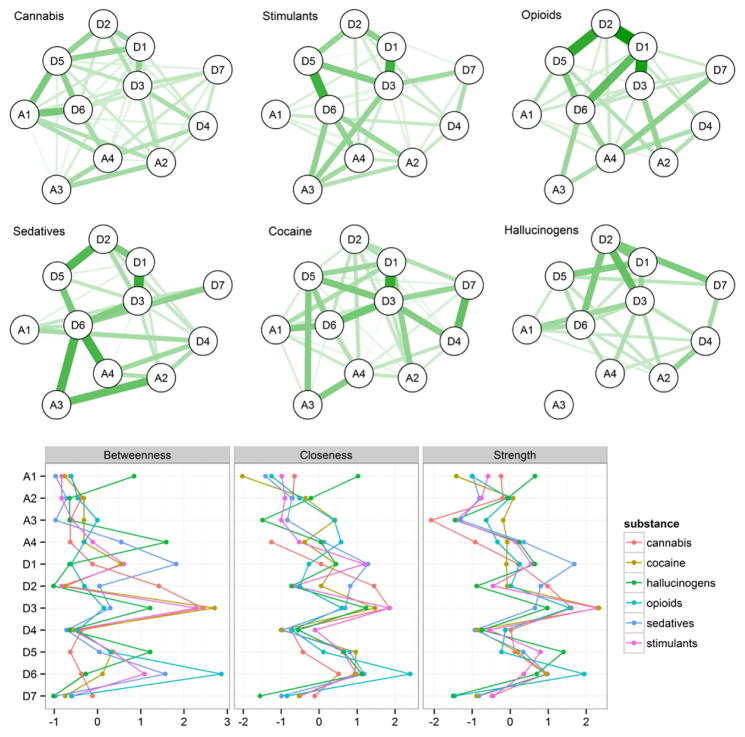
To explore the unique patterns of symptom interactions within each substance class, we estimated networks for each substance class separately. Figure 2 presents the six estimated networks (top) and centrality measures (bottom) for each substance class. Examination of these six networks reveals some noteworthy similarities as well as marked differences. For instance, the association between D4 – A2 (unable to stop – hazardous use) is present across substances, and all edges are small to moderate, implying a consistent pattern across substance classes. In contrast, the edge between A2 – A3 (hazardous use – legal consequences) ranges from absent for opioids, cocaine, and hallucinogens to strong for sedatives. That is, the connection between one’s hazardous use of an illicit substance and legal consequences of use depends on the type of substance, perhaps due to the contexts in which these substances are taken.
Figure 2.
Symptom networks for individual substances. Upper: Line thickness/darkness indicates the strength of pairwise connections. All six networks use the same graphical standardization, which means that the strength of the edges can be compared across networks. Lower: standardized centrality measures for each symptom within each substance network.
Moving from edges to centrality estimates (Figure 2; bottom), some nodes are consistently more central or peripheral to all SUDs, whereas others vary considerably. D3 (use more than planned) is central within most substance classes, suggesting that this criterion is both highly predictive of the status of other nodes in the network as well as being a good candidate for intervention, regardless of substance class. In contrast, D4 (inability to stop) is not central to any substance network. Other symptoms, such as D1 (tolerance) show differential importance across substance classes: whereas one’s tolerance of sedatives is the best predictor of an elevated sedative-use network, tolerance of hallucinogens is uninformative about the status of one’s hallucinogen use. The most central node (as indicated by betweenness centrality, though the three centrality measures are typically in agreement) for cannabis, cocaine, and stimulants is D3 (use more than planned), for hallucinogens it is A4 (social problems), for opioids it is D6 (interferes with work/life), and for sedatives it is D1 (tolerance).
3.4. Cross-substance variability network
To further understand the differences between the six substance networks in Figure 2, we derived an index of network similarity by correlating edge strength across substance networks. There are 55 edges per network, each of which has a weight, representing its connection strength. Table 3 presents the correlation matrix of these weights across substances.
Table 3.
Correlations of network edge weights across substance classes
| can | sed | sti | coc | opi | |
|---|---|---|---|---|---|
| sed | 0.54 | ||||
| sti | 0.41 | 0.64 | |||
| coc | 0.54 | 0.35 | 0.43 | ||
| opi | 0.49 | 0.58 | 0.52 | 0.29 | |
| hal | 0.44 | 0.25 | 0.20 | 0.37 | 0.26 |
Note. can = cannabis, sed = sedatives, sti = stimulants, coc = cocaine, opi = opioids, hal = hallucinogens.
The correlations of edge weights are, as expected from the differences in Figure 2, low to moderate, ranging from .20 for the association between hallucinogen and stimulant networks to .64 for the association between networks of stimulants and sedatives.
A second way to examine the differences across networks is to inspect the variability of edge weights across the 6 substances. Figure 3 presents a network in which each edge represents variability in connection strength across the 6 substances (i.e., thicker edges = higher cross-substance variability) rather than average connection strength. In this network, the edges A2 – A3 (hazardous use – legal consequences) and D2 – D5 (withdrawal – monopolizes time) are highly variable across substances, whereas A2 – D4 (hazardous use – unable to stop) is stable across substances, consistent with Figure 2.
Figure 3.
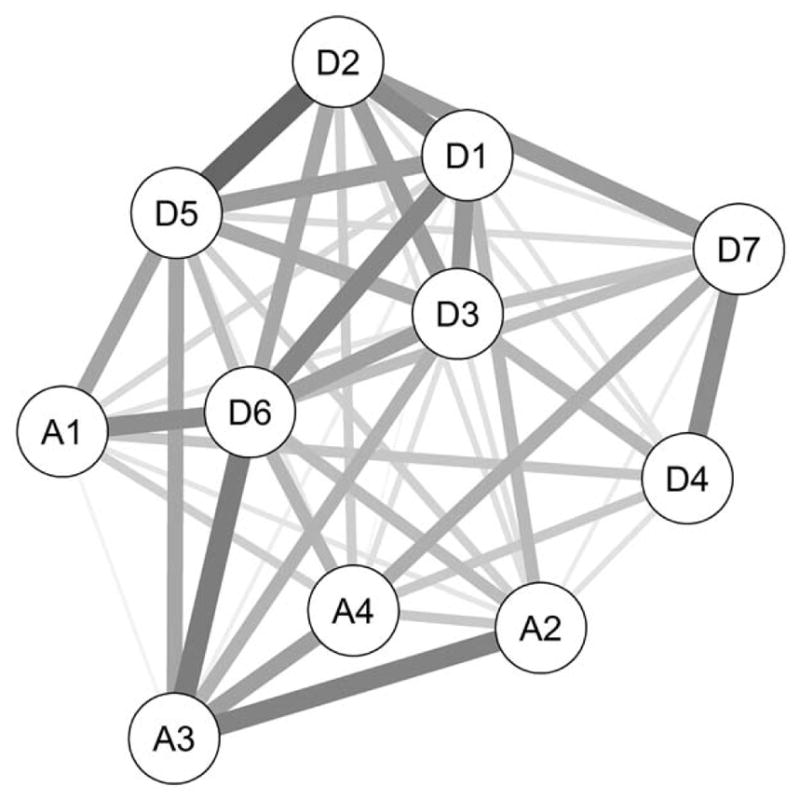
Each edge represents the standard deviation across the six edge weights of each individual substance network. Thicker/darker edges imply a higher variability of a given edge across substance classes.
4. DISCUSSION
Most research on SUD uses aggregated symptom-level data: symptom scores are transformed into a diagnostic category, a sum-score (e.g., Grant et al., 2015), or a latent variable (e.g., Gillespie et al., 2007). But theorists and clinicians also recognize that there are important differences among individual symptoms; symptoms may behave differently, they may be indicative of different developmental stages of a disorder, and they may have direct effects on other symptoms, leading a disordered system to intensify, sustain itself, or heal (DiFranza et al., 2002; Koob and Le Moal, 1997). Network models allow us to represent disorders as a dynamic system of symptom-to-symptom interactions for the first time, consistent with the tacit understanding many clinicians and patients have about SUD.
The goals of this report were twofold. First, on the level of SUD in general, we examined the associations among symptoms of abuse and dependence and investigated whether particular symptoms were more central to the overall pattern of SUD criteria. In a global network formed by averaging the network connections across six substance classes (cannabis, sedatives, stimulants, cocaine, opioids, and hallucinogens), all symptoms were positively connected, and some symptoms (e.g., used more/longer than planned) were highly central to the disorder.
Second, we compared the networks of individual substance classes. This comparison revealed similarities and differences between substances with respect to the pattern and strength of connections between symptom pairs. These comparisons suggested that the most central symptoms differ across substances. This result implies that specific abuse and dependence criteria have differential clinical relevance for different substance classes.
4.1. Novel insights provided by the network perspective
The network analyses presented here are, to our knowledge, the first such analyses to be applied to a population-based sample of self-report data on SUD, and thus are primarily exploratory. We see the main value of fitting networks models to cross-sectional data of large samples in generating hypotheses about the clinical importance of particular symptoms and symptom interactions for future research to follow up on. From this perspective, our study points to several interesting relations and effects among symptom criteria across substance classes.
First, the network framework allows us to examine the full symptom-level data without collapsing them into a composite or latent variable that may contain substantially less information. Of note, abuse and dependence criteria are substantially inter-related. This is assumed in the factor model literature (Lynskey and Agrawal, 2007), and consistent with the updated DSM in which abuse and dependence criteria are grouped together. In contrast to the perspective of one general liability underlying all SUDs, however, the network approach does not assume that symptoms are measurements of an underlying disorder. Instead, symptoms are viewed as important variables in their own right that may provide crucial insight into what is actually happening in patients’ lives.
The most central symptom emerging from our cross-substance network analyses, as well as three out of the six individual symptom networks, was using a substance more than planned (D3). Buu et al. (2011) reported that this same symptom was the most frequently reported initial symptom of alcohol use in adolescents, suggesting that its centrality may indicate its status as a gateway symptom: losing control over how much of a drug one takes, or how long one takes it, may precipitate a host of other abuse and dependence symptoms. In addition, our analyses revealed a strong interaction between using a substance more than planned (D3) and tolerance (D1). This result aligns with Buu et al.’s finding that these two symptoms tend to be the first symptoms that appear in the development of problematic alcohol use. These two symptoms may be key to understanding the development and maintenance of SUDs. Future studies should investigate the functional properties of these symptoms, similar to work in depression (Fried and Nesse, 2015) and psychosis (Bentall et al., 2014; Coltheart et al., 2011).
Second, our findings suggest that symptoms differ in their functional properties across substance classes. One such functional property is centrality, which summarizes the probability that symptoms trigger other symptoms and thus predict a negative clinical course. We have shown that different symptoms are central to different substance networks; for instance, the extent to which a drug interferes with work and life in general (D6) is especially central for opioids compared to other SUDs. This sort of specificity is consistent with previous findings that psychometric properties of these symptoms such as item difficulty and discrimination differ across substance classes (Gillespie et al., 2007), and suggest follow-up research to further explore functional differences of abuse and dependence criteria.
Third, symptoms pairs vary in whether and how strongly they relate to each other, depending on the type of substance consumed. From a purely pharmacological perspective, several of these results are novel. For example, the correlation of edge weights is high between stimulants and sedatives, despite quite different pharmacologic properties, and low between cocaine and stimulants despite similar biological effects on brain dopamine systems (Koob and Le Moal, 2006). At least two other important factors may influence the structural properties of the specific substance networks: the social context in which drugs are taken, and the psychological experiences sought by the drug user. For example, stimulants are sometimes abused for their effects on attention and to reduce fatigue while cocaine is most commonly consumed for its strong hedonic effect (Koob and Le Moal 2006). Further research may clarify the meaning of these findings and elucidate the degree to which they result from biological versus social or psychological processes.
Fourth, there may be particular pathways common to all substances; for example, we identified strong associations between using substances longer than planned, withdrawal, and tolerance. Interestingly, the variability across substances for these symptom-to-symptom associations was only moderate. This suggests a number of tentative path configurations that may be involved in drug abuse that should be tested in prospective research, for instance in individuals at a high risk for relapse. While this path configuration of three symptoms was present for all individual substance classes (and most pronounced for opioids), differences between substance class networks suggest that more drug-specific pathways may also exist. For example, the sedatives network featured strong connections between hazardous use, legal consequences, social problems, and interfering with work/life. The present cross-sectional analysis cannot reveal which symptoms are causes, consequences, or both; however, the network approach gives new insight into relations among symptoms, which insight is not possible when modeling sum scores or latent variables.
4.2. Limitations
The present analyses offer a first look at what information network models can provide about SUD, but these results should be interpreted in light of a number of limitations. Most critically, modeling cross-sectional data cannot reveal the causal nature of the connections between pairs of symptoms, leaving it unclear which symptoms cause which others, and leaving open the possibility of feedback loops among symptoms. To uncover how a dynamic system of symptoms behaves, intensive longitudinal symptom data will be necessary (Wichers, 2013).
A related limitation is that the present networks aggregate symptom-level data across participants (i.e., inter-individual differences), revealing patterns of partial correlations based on aggregating across the entire sample. These relations may or may not hold at the level of the individual; that is, inter-individual symptom interactions may not translate to intra-individual interactions. For example, when the SUD system varies dramatically across individuals, the group network will reflect the average over many individual networks, rather than one that describes any given individual. Furthermore, conclusions drawn about effective interventions (e.g., based on symptom centrality) assume that the same symptoms that are central in the inter-individual network will also be central in the network of a given individual. Network analyses of subgroups can begin to address this problem, when it is possible to identify subgroups of participants who are likely to have similar networks (e.g., patients who remit vs. those who persist in their disorder, van Borkulo et al., 2015). To estimate individual patient networks, intensive longitudinal data from individuals is required (Molenaar, 2013).
The network approach makes the assumption that all variables relevant for the network are included in the analysis. If an important variable that has strong connections to two nodes in the network is omitted, this omission may substantially alter the relationships among these two nodes as well as others. One possibly relevant symptom that is missing in these data is craving, which was included in the DSM-5 SUD criteria.
The skip structure built into the substance use section of the interview relies on the assumption that participants who reported not using a particular substance at least 11 times in a month and did not endorse any of the abuse symptoms would not have positively endorsed any dependence symptoms. For these participants in the network analyses conducted in this study, dependence symptoms were coded as zero. Although unlikely, it is possible that someone could endorse some dependence symptoms in the absence of abuse symptoms and without ever having used a substance 11 times in a month.
Our dataset is limited in that participants were ethnically homogeneous (i.e., they are White Virginians), which is both helpful to our analyses (subgroups may have introduced additional variability into the network, and we lack power to investigate relevant subgroup differences) and also a detriment: the network results may not generalize to other subpopulations.
Finally, we have 4,406 SUD cases in 2405 participants, implying that about 42% of the study population were in multiple substance use categories. For one, this means that a network such as the cannabis network is not a network of individuals who used only cannabis, but a network of individuals who use either only cannabis, or cannabis and one or more of the other five substances. To control for this dependency we could drop all participants with multiple use from the data; however, the sample would be nearly cut in half, and individual substance samples would be too small to estimate Ising models. Moreover, excluding multiple users results in somewhat artificial samples and potentially decreased generalizability of the results, because nearly half of all individuals do use more than one substance. A second possibility to address this feature of these data is to randomly assign all multiple users to one of their multiple substance classes, leading to 2,405 observations for 2,405 individuals; this way, all individuals are retained in the analysis. However, individual substance networks become substantially smaller, and especially for the already small samples such as opioids (N = 195) this leads to samples in which an Ising model cannot be reliably estimated. In sum, multiple use remains a substantive and methodological challenge for future studies.
Highlights.
Abuse and dependence symptoms are modeled as directly interacting variables in a network
Network analysis reveals pairwise symptom interactions that traditional analyses obscure
Abuse and dependence symptoms do not function equivalently across 6 illicit substance classes
Acknowledgments
Role of Funding Source
This work was supported by the European Research Council (MR; FP7/2007-2013 no. 631145, and DB; consolidator grant no. 647209), the Research Foundation Flanders (EIF; G.0806.13), the Belgian Federal Science Policy within the framework of the Interuniversity Attraction Poles program (EIF; IAP/P7/06), a grant from the University of Leuven (EIF; GOA/15/003), and the National Institutes of Health (KSK; grants RO1DA037558 and R01DA03005).
We would like to express our gratitude to all participants of the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders study.
Footnotes
There are two reasons for dichotomizing the responses. First, the category ‘probably’ was, on average, much less endorsed than the other two, leading to small cell optimization problems. Second, the behavior of potentially skewed polytomous variables in network models is not well understood at present.
Contributors
Mijke Rhemtulla, Department of Psychology, University of Amsterdam, The Netherlands
Eiko I. Fried, Faculty of Psychology and Educational Sciences, University of Leuven, Belgium
Steven H. Aggen, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, VA, USA; Department of Psychiatry, Virginia Commonwealth University, Richmond, VA, USA
Francis Tuerlinckx, Faculty of Psychology and Educational Sciences, University of Leuven, Belgium
Kenneth S. Kendler, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, VA, USA; Department of Psychiatry, Virginia Commonwealth University, Richmond, VA, USA; Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, VA, USA
Denny Borsboom, Department of Psychology, University of Amsterdam, The Netherlands
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentall RP, de Sousa P, Varese F, Wickham S, Sitko K, Haarmans M, Read J. From adversity to psychosis: pathways and mechanisms from specific adversities to specific symptoms. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1011–1022. doi: 10.1007/s00127-014-0914-0. [DOI] [PubMed] [Google Scholar]
- Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D. Complex networks: structure and dynamics. Phys Rep. 2006;424:175–308. [Google Scholar]
- Borsboom D, Cramer AOJ. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Langdon R, McKay R. Delusional belief. Annu Rev Psychol. 2011;62:271–298. doi: 10.1146/annurev.psych.121208.131622. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96:1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: network visualizations of relationships in psychometric data. J Stat Softw. 2012;48:1–18. [Google Scholar]
- Fried EI, Nesse RM. Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Med. 2015;13:1–11. doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Prescott CA, Aggen SH, Kendler KS. Factor and item-response analysis DSM-IV criteria for abuse of and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opioids. Addiction. 2007;102:920–930. doi: 10.1111/j.1360-0443.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology Of Addiction. Elsevier Inc; London, UK: 2006. [Google Scholar]
- Lynskey MT, Agrawal A. Psychometric properties of DSM assessments of illicit drug abuse and dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Psychol Med. 2007;37:1345–1355. doi: 10.1017/S0033291707000396. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL. Epidemiology of substance use disorders. Hum Genet. 2012;131:779–789. doi: 10.1007/s00439-012-1168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar P, Wainwright MJ, Lafferty JD. High-dimensional Ising model selection using 1 -regularized logistic regression. Ann Stat. 2010;38:1287–1319. [Google Scholar]
- Smeets F, Lataster T, Viechtbauer W, Delespaul P. Evidence that environmental and genetic risks for psychotic disorder may operate by impacting on connections between core symptoms of perceptual alteration and delusional ideation. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu122. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-III-R-Patient Version (SCID-P,4/1/87) New York State Psychiatric Institute; New York: 1987. [Google Scholar]
- van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, Waldorp LJ. A new method for constructing networks from binary data. Sci Rep. 2014;4:1–10. doi: 10.1038/srep05918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC. The dynamic nature of depression: a new micro-level perspective of mental disorder that meets current challenges. Psychol Med. 2013;616:1–12. doi: 10.1017/S0033291713001979. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Atlas on substance use (2010): resources for the prevention and treatment of substance use disorders. World Health Organization; Geneva: 2010. [Google Scholar]