Abstract
Objective. To evaluate whether patients with RA who belong to the spectrum of fibromyalgic RA (FRA) have an impaired response to treatment measured by traditional activity scores.
Methods. Patients from the ESPOIR cohort were analysed. This prospective cohort included 813 patients with early arthritis not initially receiving DMARDs. Among the 697 patients who met RA classification criteria, we studied two groups, one with and the other without FRA. The following endpoints were compared at 6, 12 and 18 months using a mixed linear regression model: 28-joint DAS (DAS28), Simple Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI) and HAQ. In addition, attainment of low disease activity (LDA; DAS28 <3.2) and remission (DAS28 <2.6, SDAI <3.3, CDAI <2.8) at these time points was analysed.
Results. Patients with FRA (n = 120) had higher DAS28, SDAI, CDAI and HAQ scores than patients with RA and no fibromyalgic characteristics (n = 548). DAS28 and other DASs started out higher in subjects with FRA, and while they improved to a similar extent to in the isolated RA group, they remained consistently higher among FRA patients. Achievement of LDA and remission was significantly less likely in subjects with FRA.
Conclusion. Patients with FRA and RA will have a similar response to treatment according to the decrease in indexes of disease activity, but may miss the target of remission or LDA.
Keywords: rheumatoid arthritis, therapy, outcome measures, disease activity scores, fibromyalgia, DMARDs, fibromyalgic RA, treat to target, early rheumatoid arthritis
Rheumatology key messages
Traditionally used activity scores may overestimate inflammatory activity in patients with fibromyalgic RA.
Treat to target may need to be modified in fibromyalgic RA patients.
Introduction
RA is a chronic inflammatory disease that produces pain and physical limitation, severely compromising functioning [1,2]. A treat-to-target strategy has been shown to improve outcomes and is advocated in RA. This strategy is based on the use of activity scores to define remission/low disease activity (LDA) and adjustment of treatment according to these aims. The 28-joint DAS (DAS28) has been the most used score [3]. This score gives a particularly high weight to the tender joint count (TJC) vs swollen joint count (SJC). The Simple Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI) do not give differential weights to the two joint counts.
Remission in RA is an achievable target, but patients may have residual disease activity [4]. There is limited capacity to anticipate who will require aggressive management in RA [5]. Given the cost and toxicity of treatment, it is crucial to determine who will truly benefit from intense therapy.
FM often coexists with RA. The prevalence of FM in the general population is 2.7–5.1% and in RA it is 10–20% [6,7]. Concern has been raised regarding the validity of RA activity scores in patients with coexistent RA and FM. Cross-sectional studies have shown that FM patients have higher DAS28 and worse functional impairment [8–11]. Regarding fibromyalginess without meeting FM criteria, a study by Wolfe et al. [12] showed that with increasing FM symptoms, clinical variables become more abnormal.
Tender points have been traditionally used to diagnose FM [13]. However, measuring them is time consuming and is not always performed in RA clinics. An index was developed by Pollard et al. [14] to identify fibromyalgic RA (FRA). They showed that a TJC/SJC ≥7 had 83% sensitivity and 80% specificity, validating it in a replicate cohort with high sensitivity and specificity for FM (72% and 98%).
Worse disease activity in FRA could be a consequence of a blunted response to RA treatment or it could be due to the effect of fibromyalgic symptoms on activity measures. To our knowledge, no longitudinal studies in treatment-naive patients have been carried out to address this question. In this prospective study we hypothesized that patients with FRA have an impaired response to treatment measured by traditionally used scores.
Materials and methods
Study subjects
We addressed our questions using the ESPOIR prospective early arthritis cohort. This cohort includes patients aged 18–70 years, with arthritis duration <6 months and no previous DMARDS or steroids. The protocol of the ESPOIR cohort study was approved by the ethics committee of Montpellier. Participants provided informed consent before entering the ESPOIR cohort. We included only patients with RA defined by the 1987 ACR and/or 2010 ACR/EULAR classification criteria. Participants with missing values of DAS28, SDAI or CDAI at baseline or at all follow-up visits were excluded. Data belonging to the ESPOIR cohort are deidentified and do not need any further ethical approval for analysis.
The diagnosis of FRA was defined at baseline as having a TJC/SJC ≥7 [14]. Our two study groups for comparison were patients with FRA and patients with RA who did not meet FM criteria (isolated RA).
Differences in demographics at baseline between these groups were explored. Smoking status was defined as ever or never smokers and BMI was categorized as obese or non-obese (BMI ≥30 or <30 kg/m2, respectively). In addition, a comparison of RA characteristics and treatment was performed.
Study outcomes
The main outcome was response to treatment measured using the DAS28 and its core components, SDAI, CDAI and HAQ. As secondary outcomes, an analysis was performed of the attainment of LDA (DAS28 ≤3.2) and remission (DAS28 ≤2.6, CDAI ≤2.8, SDAI ≤3.3). Treatment in the two groups and the modified total Sharp score (mTSS) were also analysed. All outcomes were compared at 6, 12 and 18 months of follow-up.
Power calculation
We had power of >90% to detect a DAS28 difference of 0.5, considering a s.d. of 1.3 using 120 subjects with FRA and 548 without, three visits per subject and an intraclass correlation coefficient of 0.6 for repeated measures. Power continued to be >90% after excluding patients with at least one missing visit.
Statistical analysis
Baseline characteristics were compared using two-sample t-test and chi-square test. Continuous outcomes were analysed using a mixed linear regression model, adjusting for baseline value, gender, age and smoking status. An estimation of the adjusted average of outcomes was performed using least squares means.
Categorical variables were compared using a log binomial regression. Relative risks (RRs) were obtained using a generalized estimating equation model. A level of significance of 0.05 was used. SAS 9.3 (SAS, Cary, NC, USA) was used to perform statistical calculations.
Results
Baseline characteristics
There were 697 subjects with RA at baseline among the 813 cohort participants. Of these patients, 29 were excluded because they had activity measures missing in all follow-up visits. Therefore 668 subjects were the focus of the analysis (supplementary Fig. S1, available at Rheumatology Online). Loss to follow-up, considered as missing all visits, was low (3.6%) and there was no difference between these patients and the patients analysed.
Subjects had a mean age of 48.3 years, 76.1% were female, 92.1% were Caucasian, 14.4% were obese and 47.3% were smokers. Half of the participants were seropositive (54.6% RF, 45.7% ACCP).
At baseline, patients had active disease with a mean DAS28 of5.32, CDAI 28.77, SDAI 31.0 and HAQ 1.03. The average SJC was 7.99 and the average TJC was 9.36. Inflammatory parameter mean values were elevated, with an ESR of 30.6 mm/h and CRP of 21.4 mg/dl. Erosions were present in 63.6% of subjects.
FRA was present in 120 (17.96%) patients. There was no significant difference in baseline demographic characteristics according to the presence of FRA. However, FRA patients met ACR/EULAR 2010 RA criteria more frequently than patients without FRA (97.5% vs 92.5%, P = 0.04) and had a lower frequency of seropositivity (RF = 54.6% vs 40% and CCP = 45.7% vs 29.2%).
RA activity scores and HAQ were higher in FRA patients (DAS28 = 6.02 vs 5.16, CDAI 37.94 vs 26.76, SDAI 39.78 vs 29.09, HAQ 1.25 vs 0.98) with a significant P-value in each comparison (<0.0001). Similarly, TJC and global health (GH) evaluation were higher in this group. On the other hand, ESR and the mTSS score were significantly lower in FRA subjects. There was no difference in treatment (supplementary Table S1, available at Rheumatology Online). Patients with missing values at any visit did not have significant differences in baseline characteristics, with 68 subjects presenting at least one missing visit.
Activity scores in FRA patients over time
In a multivariate analysis, patients with FRA had a higher DAS28 (P < 0.0001). DAS28 started out higher in subjects with FRA, and while it improved to a similar extent in both groups, it remained consistently higher among FRA patients. In none of the visits in FRA patients did the average DAS28 score reach LDA. Similarly, TJC and GH showed an important decrease after treatment, but remained higher in patients with FRA (Fig. 1). On the other hand, there was no difference between groups during follow-up in SJC, mTSS and inflammatory parameters (Table 1). The SDAI, CDAI and HAQ showed similar behaviour to DAS28 (Table 1).
Fig. 1.
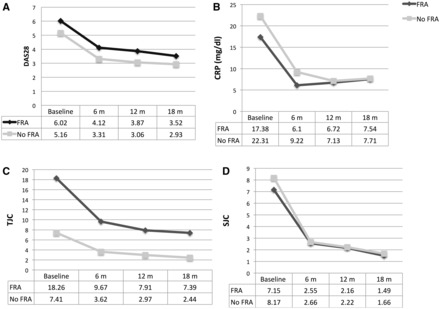
DAS28 and core measures of disease activity at different time points grouped by the presence of fibromyalgic RA
(A) 28-joint DAS (DAS28), (B) CRP, (C) tender joint count (TJC and (D) swollen joint count (SJC). FRA: fibromyalgic RA.
Table 1.
Comparison of RA activity scores and radiological scores over follow-up according to the presence of FRA
| FRA | No FRA | Difference in adjusted scores | P-valuea | |
|---|---|---|---|---|
| DAS28 | 3.50 | 3.05 | 0.45 | <0.0001 |
| SDAI | 16.09 | 11.54 | 4.55 | <0.0001 |
| CDAI | 14.98 | 10.75 | 4.23 | <0.0001 |
| HAQ | 0.63 | 0.45 | 0.17 | 0.0002 |
| SJC | 2.32 | 2.18 | 0.14 | 0.5476 |
| TJC | 7.97 | 3.27 | 2.37 | 0.0001 |
| PtGH VAS | 3.82 | 3.01 | 0.81 | <0.0001 |
| PhGH VAS | 2.88 | 2.38 | 0.51 | 0.0044 |
| CRP | 0.75 | 0.84 | 0.09 | 0.4147 |
| ESR | 13.99 | 15.05 | 1.06 | 0.3602 |
| SHARP | 7.33 | 7.68 | 0.34 | 0.3125 |
aEstimates presented correspond to the least squares means using a mixed regression model including the 6-, 12- and 18-month visit, adjusting for baseline score, gender, age and smoking status. CDAI: Clinical Disease Activity Index; DAS28: 28-joint DAS; FRA: fibromyalgic RA; PhGH: physician global health; PtGH: patient global health; SDAI: Simplified Disease Activity Index; SHARP: Sharp–van der Heijde score; SJC: swollen joint count, TJC: tender joint count; VAS: visual analogue scale.
The overall achievement of LDA was significantly less likely in subjects with FRA, with an RR of 0.77 (95% CI 0.63, 0.94). Also, there was less attainment of remission according to the DAS28 and SDAI in this study group [RR 0.61 (95% CI 0.46, 0.81) and 0.65 (95% CI 0.43, 0.97), respectively]. FRA patients also had a lower risk of achieving CDAI remission (RR = 0.70, with a borderline P = 0.06).
Association between FRA and therapy
We did not find an association between FRA and analgesics (P = 0.07), NSAIDS (P = 0.98), CSs (P = 0.4785), synthetic DMARDS (monotherapy P = 0.98, combined P = 0.9205) or biologic DMARDS (monotherapy P = 0.2928, combined P = 1.222).
Discussion
In this cohort, patients with RA and FRA had higher baseline activity scores than those with isolated RA. Although FRA patients improved with treatment to a similar extent to patients without FRA, they maintained higher scores at all time points. TJC also continued to be higher in FRA patients. In contrast, SJC, acute phase reactant levels and mTSS were higher in patients with isolated RA at baseline, but there was no difference at follow-up. Therefore activity scores and core measurements decreased after treatment in both groups, reflecting that response to therapy existed in all patients, but TJC and activity scores values remained higher in FRA patients.
One other study addressed response to treatment in RA patients with chronic widespread pain (CWP), a condition in the same spectrum as FM. They found worse activity scores in CWP subjects [15]. However, patients with active RA without FRA could have met their definition of CWP, so CWP could have reflected active RA, and the number of RA patients in this subset was higher than the usual estimate for FM.
Our baseline findings are concordant with previous cross-sectional studies [8–12]. One important question is whether activity scores measure RA activity or a mixture of RA and FM-like symptoms in FRA. The reliability of the DAS28 has been shown to be inferior in patients with FM [16].
It could be argued that our finding of a higher baseline DAS28 is secondary to a definition of FRA, which emphasizes TJC, also heavily weighted by the DAS28. However, the CDAI and SDAI weigh TJC and SJC equally, and these scores were also higher in patients with FRA. Still, they include TJC and are influenced by pain perception [17]. The HAQ measures functional limitation, so it is probably affected by the symptoms generated by FRA per se and not necessarily RA.
At first impression, higher activity scores after treatment in the FRA group can be interpreted as poor response to therapy, but the decrease in scores was similar in both groups (Fig. 1). Therefore patients with FRA do respond to treatment, but maintain higher scores. This could have two explanations. First, FRA could be assessed as continuing to have activity due to pain secondary to FRA. On the other hand, patients with FRA have central sensitization that may affect their response to RA therapy [18]. At study initiation, the existence of a higher TJC is related to our definition of FRA. However, at follow-up the TJC and SJC decreased, but the TJC continued to be higher in FRA patients, as did the TJC–SJC difference. Finally, mTSSs were not higher in patients with FRA, supporting the hypothesis that activity scores in this group do not reflect only RA activity, although follow-up may be too short for radiological changes.
Regardless of how they were defined, remission and LDA were less frequent in patients with FRA (Fig. 1). This could lead to escalating RA treatment intensity. Treatment for arthritis is aimed at controlling inflammation, and in patients with FRA these scores may reflect pain and not necessarily inflammation. A possibility is to establish a less stringent target for this group.
McWilliams et al. [19] created a score called the DAS28-P, which focuses on TJC and patient GH and has been shown to predict bodily pain. Although it needs further validation, this could be used in patients with FRA.
Our FRA definition could generate misclassification of patients with high disease activity. However, given the diagnostic performance of the TJC–SJC measure [14], it is unlikely since there are few false positives (i.e. the specificity is high). Further, maintenance of the TJC–SJC discordance in these patients argues against this misclassification. It is possible that some persons with FRA were not captured by our approach, but this would not invalidate our findings. Given the low proportion of loss to follow-up (3.6%), this is unlikely to represent a source of bias.
Conclusion
In conclusion, this study indicates that the DAS fails to decrease to LDA or remission levels in FRA subjects. However, RA inflammation responds as scores drop and the residual activity measured could correspond mainly to residual pain related to FRA. We must reconsider the use of a treat-to-target strategy, as it is currently defined, in patients with FRA.
Supplementary Material
Acknowledgements
An unrestricted grant from Merck Sharp and Dohme was allocated for the first 5 years of the ESPOIR cohort. Two additional grants from INSERM were obtained to support part of the biological database belonging to the ESPOIR cohort. The French Society of Rheumatology, Pfizer, AbbVie and Roche-Chugai also supported the ESPOIR cohort study. We also wish to thank Nathalie Rincheval, who did expert monitoring and data management, and all the investigators who recruited and followed patients: F. Berenbaum (Paris–Saint Antoine), M.C. Boissier (Paris–Bobigny), A. Cantagrel (Toulouse), B. Combe (Montpellier), M. Dougados (Paris–Cochin), P. Fardelonne and P. Boumier (Amiens), B. Fautrel and P. Bourgeois (Paris–La Pitié), R.M. Flipo (Lille), P. Goupille (Tours), F. Liote (Paris– Lariboisière), X. le Loet and O. Vittecoq (Rouen), X. Mariette (Paris–Bicetre), O. Meyer (Paris–Bichat), A. Saraux (Brest), T. Schaeverbeke (Bordeaux) and J. Sibilia (Strasbourg).
Funding: The analysis of this study was supported by the National Institutes of Health (grant AR47785).
Disclosure statement: C.G.-V. has received honoraria from AbbVie, BMS, Janssen, MSD, Nordic Pharma, Pfizer, Roche-Chugai and UCB and has received a research grant from Pfizer. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Allaire S, Wolfe F, Niu J, Lavalley MP. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Care Res 2008;59:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstappen SMM, Bijlsma JWJ, Verkleij H, et al. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Care Res 2004;51:488–97. [DOI] [PubMed] [Google Scholar]
- 3.Schoels M, Knevel R, Aletaha D, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010;69:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma MH, Scott IC, Kingsley GH, Scott DL. Remission in early rheumatoid arthritis. J Rheumatol 2010;37:1444–53. [DOI] [PubMed] [Google Scholar]
- 5.Katchamart W, Johnson S, Lucy Lin H-J, et al. Predictors for remission in rheumatoid arthritis patients: a systematic review. Arthritis Care Res 2010;62:1128–43. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Cathey MA, Kleinheksel SM. Fibrositis (fibromyalgia) in rheumatoid arthritis. J Rheumatol 1984;11:814–18. [PubMed] [Google Scholar]
- 7.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995;38:19–28. [DOI] [PubMed] [Google Scholar]
- 8.Toms J, Soukup T, Bradna P, Hrncir Z. Disease activity composite indices in patients with rheumatoid arthritis and concomitant fibromyalgia. J Rheumatol 2010;37:468. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo A, Ojeda S, Francisco F, et al. Fibromyalgia in patients with rheumatoid arthritis is associated with higher scores of disability [letter]. Ann Rheum Dis 2002;61:660–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranzolin A, Tavares Brenol JOC, Bredemeier M, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Care Res 2009;61:794–800. [DOI] [PubMed] [Google Scholar]
- 11.Coury F, Rossat A, Tebib A, et al. Rheumatoid arthritis and fibromyalgia: a frequent unrelated association complicating disease management. J Rheumatol 2009;36;58–62. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Michaud K, Busch RE, et al. Polysymptomatic distress in patients with rheumatoid arthritis: understanding disproportionate response and its spectrum. Arthritis Care Res 2014;66:1465–71. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 14.Pollard L, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology 2010;49:924–8. [DOI] [PubMed] [Google Scholar]
- 15.Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 2013;40:1977–85. [DOI] [PubMed] [Google Scholar]
- 16.Leeb BF, Andel I, Sautner J, Nothnagl T, Rintelen B. The DAS28 in rheumatoid arthritis and fibromyalgia patients. Rheumatology 2004;43:1504–7. [DOI] [PubMed] [Google Scholar]
- 17.Rintelen B, Haindl PM, Maktari A, et al. SDAI/CDAI levels in rheumatoid arthritis patients are highly dependent on patient’s pain perception and gender. Scand J Rheumatol 2008;37:410–3. [DOI] [PubMed] [Google Scholar]
- 18.Cagnie B, Coppieters I, Denecker S, et al. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014;44:68–75. [DOI] [PubMed] [Google Scholar]
- 19.McWilliams DF, Zhang W, Mansell J, Young A, Walsh DA. Predictors of change in bodily pain in early rheumatoid arthritis: an inception cohort study. Arthritis Care Res 2012;64:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


