Abstract
Background.
Weakness predisposes seniors to a fourfold increase in functional limitations. The potential for age-related degradation in nervous system function to contribute to weakness and physical disability has garnered much interest of late. In this study, we tested the hypothesis that weaker seniors have impairments in voluntary (neural) activation and increased indices of GABAergic inhibition of the motor cortex, assessed using transcranial magnetic stimulation.
Methods.
Young adults (N = 46; 21.2±0.5 years) and seniors (N = 42; 70.7±0.9 years) had their wrist flexion strength quantified along with voluntary activation capacity (by comparing voluntary and electrically evoked forces). Single-pulse transcranial magnetic stimulation was used to measure motor-evoked potential amplitude and silent period duration during isometric contractions at 15% and 30% of maximum strength. Paired-pulse transcranial magnetic stimulation was used to measure intracortical facilitation and short-interval and long-interval intracortical inhibition. The primary analysis compared seniors to young adults. The secondary analysis compared stronger seniors (top two tertiles) to weaker seniors (bottom tertile) based on strength relative to body weight.
Results.
The most novel findings were that weaker seniors exhibited: (i) a 20% deficit in voluntary activation; (ii) ~20% smaller motor-evoked potentials during the 30% contraction task; and (iii) nearly twofold higher levels of long-interval intracortical inhibition under resting conditions.
Conclusions.
These findings indicate that weaker seniors exhibit significant impairments in voluntary activation, and that this impairment may be mechanistically associated with increased GABAergic inhibition of the motor cortex.
Key Words: Dynapenia, Sarcopenia, Muscle, Weakness, Transcranial magnetic stimulation.
Although some loss of strength is common with aging, the extent of strength loss varies greatly. In fact, around 30% of older women and 15% of older men in the United States self-report that they are unable to lift or carry 10 pounds, and ~50% of women and 40% of men report difficulty in stooping, crouching, or kneeling (1). Weakness predisposes seniors to a fourfold increase in functional limitations as well as a twofold increase in mortality (2). Identifying the factors contributing to such extensive physical impairments is necessary to develop targeted interventions.
For three decades the scientific and medical communities largely assumed that age-related loss of muscle size (ie, sarcopenia) was the primary determinant of weakness (3). It was recently reported that the decline in muscle strength in seniors—observed longitudinally over 5 years—occurs more rapidly than the concomitant loss of mass, and that the change in muscle size explains less than 10% of the between-subject variability in the change in strength (4). Further, maintaining or gaining muscle mass does not necessarily prevent aging-related declines in strength (4). Collectively, these findings indicate that loss of strength is modestly associated with loss of mass in seniors. Accordingly, the development of optimal strategies to ameliorate age-related weakness requires the identification of anatomical and/or physiological mechanisms of weakness other than simple muscle size.
Degradation in nervous system function is one potential contributor to weakness. Numerous studies have been conducted to identify age-related changes in anatomical and physiological properties of the spinal motor neurons (5–11) as well as the motor cortex (12–15). Similarly, studies have sought to examine the influence of aging on the nervous system’s ability to “activate” muscles by examining the amplitude characteristics of the electromyogram (EMG) signal during volitional contractions (15–17) or by electrically stimulating the motor nerve and quantifying the “added force” via the interpolated twitch technique or variation thereof (henceforth referred to as a measure of “voluntary activation” [VA]) (see Clark and Taylor (18) for review). Collectively, these studies demonstrate that aging results in altered motor neuron properties, such as decreased excitability (6), lower discharge rates (8), and a lower incidence of doublet discharges (7). Further, these studies have reported an interrelationship between the number of functioning motor units and strength (11), as well as a slower rate of EMG rise during a leg press task that was associated with the loss of power (16).
The effects of aging on maximal VA, which reflects the nervous system’s ability to fully activate skeletal muscle (19), are not clear as there are discrepant findings (see Clark and Taylor (18) for review). Similarly, there are differing reports of the effects of aging on measures of intracortical excitability (20–29). Although these discrepancies may reflect heterogeneity within the aged population, no studies have examined whether weaker seniors (defined here as those in the lowest tertile of strength relative to weight) have a lower maximal VA or altered motor cortical excitability relative to their stronger counterparts. Identification of impairments in VA is important to determine as to what extent weakness in seniors is due to neural factors, and to what extent neurological interventions could improve strength. Simply measuring VA does not provide insight into the specific neurophysiological mechanisms of weakness per se; however, transcranial magnetic stimulation (TMS) may help gain this insight.
TMS can be used to measure aspects of cortical excitability in vivo. Single-pulse TMS can be used to assess corticospinal excitability by eliciting motor-evoked potentials (MEPs) and silent periods (SP). Paired-pulse TMS involves combining a conditioning stimulus with a test stimulus at different interstimulus intervals and allows a more direct evaluation of intracortical excitability (ie, excitability of intracortical interneuron networks within the motor cortex) (30). Paired-pulse TMS can be used to quantify a number of different outcomes, such as intracortical facilitation (ICF), short-interval intracortical inhibition (SICI), and/or long-interval intracortical inhibition (LICI). It is generally thought that SICI is mediated by GABAA receptors (31,32), LICI is mediated by GABAB receptors (31,33), and ICF is mediated by excitatory glutamatergic interneurons and N-methyl-d-aspartate receptors (32,34). In general, SICI and ICF are mediated within the primary motor cortex (M1). LICI is commonly suggested to be mediated within M1 (34,35), although recent evidence suggests that it can also be influenced by spinal mechanisms during voluntary contractions (36). A limited number of studies have examined age-related changes in measures of intracortical inhibition and facilitation with largely discrepant findings reported (20–29); accordingly, we sought to clarify the discrepant findings in the extant literature.
The purposes of this study were to (i) determine whether, and to what extent, seniors exhibit differences in wrist flexor VA capacity and measures of corticospinal and intracortical excitability in comparison to young adults, and (ii) determine whether, and to what extent, weaker seniors exhibit differences in wrist flexor VA capacity and measures of corticospinal and intracortical excitability in comparison to stronger seniors. We hypothesized that seniors, and weak seniors in particular, have decreased VA and that they exhibit cortical hypoexcitability due to increased GABAergic inhibition.
Methods
General Overview of the Study Design
A group of young adults and seniors underwent an orientation and familiarization session followed by a testing session involving the assessment of neuromuscular function of the nondominant arm. In addition to measuring wrist flexion strength (ie, maximal voluntary isometric contraction or MVC), we utilized electrical stimulation to measure the amplitude of the maximal compound muscle fiber action potential (M max), and single-pulse TMS to measure resting motor threshold, MEP) amplitude, and SP duration during isometric wrist flexion contractions at 15% and 30% of strength. Paired-pulse TMS was used to measure ICF and SICI and LICI.
Subjects
Forty-six young adults (age range: 18–30 years; mean age 21.2±0.5 years; 20 women and 26 men) and 42 seniors (age range: 60–88 years; mean age 70.8±5.9 years; 27 women and 15 men) completed the study (see Supplementary Table 1 in online supplement for complete descriptive statistics). All subjects were free of major medical disease and disorders, and specific exclusion criteria and permitted medications are detailed in the online supplement (see Supplementary Material). We conducted a short physical performance battery (SPPB) to obtain a clinical characterization of physical function status of our older adult subjects (37). It should be noted that ~80% of our study participants would generally be considered to be high functioning (SPPB scores >11), with ~ 20% having SPPB scores in the 8–10 range (mean SPPB score: 11.3±0.9). All study participants were instructed to not consume alcohol (abstain for 24 hours) or caffeine (abstain for 4 hours) prior to the testing sessions. The Ohio University Institutional Review Board approved the study, and all participants provided informed consent.
Muscle Strength and VA
For complete details of the strength and VA testing protocol (including illustration) please see the online supplement (Supplementary Material). In brief, wrist flexion forces were quantified with the subjects seated in a Biodex dynamometer with visual feedback provided. To assess wrist flexion strength, subjects performed a minimum of three MVCs with verbal encouragement and a 1–2 minute rest period between MVCs. The highest value was considered the MVC.
VA was quantified using a doublet interpolation technique that involved delivering electrical stimulation (0.2ms pulses) to the median nerve in the cubital fossa. Stimuli were administered at increasing intensities until the amplitude of the compound muscle fiber action potential evoked in flexor carpi radialis reached a plateau (M max). The intensity was subsequently increased 20% above that eliciting M max. Next, a supramaximal 100-Hz electrical doublet was delivered while the subject performed a 4–5 seconds MVC and again 1–2 seconds after the MVC. The increase in force immediately following the stimulation during the MVC was expressed relative to that produced by the doublet after the MVC, and VA was calculated as follows: %VA = (1 − [Evoked Force During MVC/Evoked Force Following MVC]) × 100.
Transcranial Magnetic Stimulation
For complete details of the TMS protocol see the online supplement (Supplementary Material). In brief, EMG signals were recorded from the flexor carpi radialis muscle. Single-pulse stimuli were delivered using a 70-mm figure-of-eight focal coil. The stimulation location that elicited the largest MEP was identified and marked. Resting motor threshold was determined, and then the MEP amplitude and SP duration were evaluated using single-pulse TMS with the stimulation intensity set to 130% of resting motor threshold. These measures were elicited while the subjects performed brief (~ 5 seconds) voluntary contractions equal to 15% and 30% MVC (eight trials per contraction intensity). The peak-to-peak (p–p) amplitude of the MEPs was calculated and expressed as a percentage of the M max. The SP duration was also quantified.
We also performed paired-pulse TMS at rest to quantify ICF, SICI, and LICI. To quantify SICI, ICF, and LICI the second (test) stimulus was set at an intensity that, when it was given alone, evoked an MEP of 0.4–1.0 mV p–p amplitude. For SICI and ICF quantification, the intensity of the first (conditioning) stimulus was set to 70% of MT, and for LICI quantification, the first (conditioning) stimulus was set at the same intensity as the second (test) pulse. The interstimulus intervals for assessing SICI, ICF, and LICI were 3, 15, and 100ms. Eight trials of each of these four conditions (test pulse given alone, ICF, SICI, and LICI trials) were performed in randomized blocks and averaged.
ICF was operationally defined as: ICF = (conditioned test MEP/test MEP) × 100, such that a higher value is indicative of higher levels of facilitation.
SICI was operationally defined as: SICI = 100 − ([conditioned test MEP/test MEP] × 100), such that a higher value is indicative of higher levels of inhibition.
LICI was operationally defined as: LICI = 100 − ([conditioned test MEP/test MEP] × 100), such that a higher value is indicative of higher levels of inhibition.
Statistical Analyses
For complete details of the statistical analysis see the online supplement (Supplementary Material). In brief, analysis of variance (ANOVA) procedures with Sidak post hoc tests were used to examine differences between the younger adults and the seniors. In addition, we also stratified the seniors into tertiles by relative strength (ie, strength to body mass) to compare stronger seniors (ie, those in the upper two tertiles) to the weaker seniors (ie, those in the lower tertile). ANOVA procedures were used to examine differences here as well. Gender was entered as a covariate for all analyses, and, where appropriate, within-subject factors were added to the model (eg, contraction intensity). A Mann–Whitney U-test was used for the VA-dependent variable due to it having a ceiling effect, and we also report the median and interquartile range (IQR) for VA to aid in interpretation. Alpha was set to 0.05. Data are presented as means ± standard error of the mean.
Results
Muscle Strength and VA
Young adults versus older adults
There were no differences in absolute strength between the young adults and the seniors (19.1±0.9 vs 16.5±1.0 N•m; p = .07); however, when expressed relative to body mass, the young adults were 20% stronger than the seniors (Figure 1A; p = .01). There were no differences in VA between young adults and seniors (Figure 1C presents means; p = .11; median for young adults was 100 with an IQR of 5.4 and the median for older adults was 100 with an IQR of 12.2). Descriptive characteristics of study participants are provided in the online supplement (see Supplementary Material).
Figure 1.
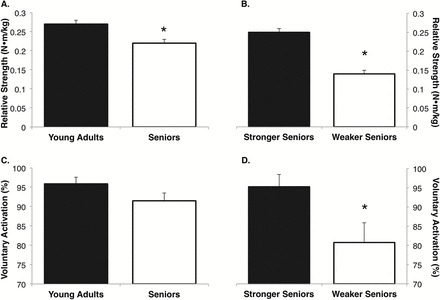
Seniors exhibited ~20% less relative wrist flexor strength in comparison to young adults (A), with the weakest tertile of seniors being 44% weaker than the stronger seniors (B). Mean group differences were not observed between seniors and young adults for voluntary activation (VA) (C); however, the weakest tertile of seniors demonstrated a 20% deficit in VA, which was a significantly greater impairment when compared to the stronger seniors (D). *Significantly different from comparison group (ie, young adults vs seniors or stronger seniors vs weaker seniors), p < .05.
Stronger seniors versus weaker seniors
The weaker seniors were 39% weaker than the stronger seniors in absolute terms (17.6±0.8 vs 10.7±1.2 N•m; p < .01), and ~44% weaker relative to mass (Figure 1B; p < .01). The weaker seniors exhibited 16% lower VA when compared with the stronger seniors (Figure 1D presents means; p = .03; median for weaker seniors was 87.7 with an IQR of 34.0 and the median for stronger seniors was 100 with an IQR of 5.4). Descriptive characteristics of study participants are provided in the online supplement (see Supplementary Material).
TMS-Based Outcomes of Cortical and Corticospinal Excitability
Young a.dults versus older adults
No differences were observed between young adults and seniors for the MEP amplitude during contractions of 15% and 30% of MVC (Figure 2A; contraction intensity × age group interaction p = .11). With regard to the SP duration, a contraction intensity × age group interaction was observed (p = .05), with follow-up analyses indicating that the younger adults had a reduction in their SP duration with increasing contraction intensity, but seniors exhibited no change, which ultimately resulted in the seniors having a 9% longer SP at the 30% MVC intensity in comparison to young adults (Figure 2C; p = .02). With regard to the paired-pulse measures, group differences were not observed between young adults and seniors in ICF (p = .88), SICI (p = .95), or LICI (p = .33) (Figure 3).
Figure 2.
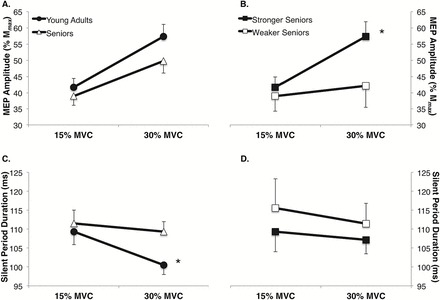
Group differences were not observed between seniors and young adults in the motor-evoked potential (MEP) amplitude at contraction intensities equal to 15% and 30% of maximum voluntary contraction (MVC) force (A); however, a group × paradigm interaction was observed indicating that the weakest tertile of seniors demonstrated ~20% smaller MEPs during the 30% MVC task when compared with the stronger seniors (ie, those in the top two tertiles of relative muscle strength) (B). Seniors exhibited ~10% longer silent periods (SP) during the 30% MVC task in comparison to young adults (C), with no differences being observed in the SP duration between the weakest tertile of seniors and the stronger seniors (D). *Significantly different from comparison group (ie, young adults vs seniors or stronger seniors vs weaker seniors) (p < .05).
Figure 3.
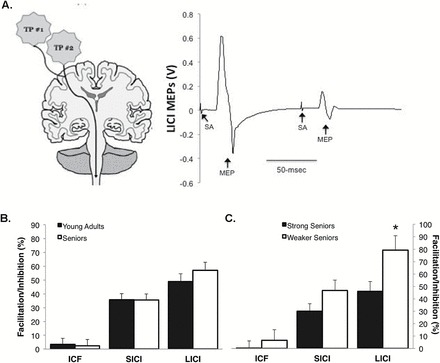
(A) Example of long-interval intracortical inhibition (LICI; ensemble average of eight trials) obtained from a 77-y-old female. To quantify LICI, two test pulses (TP) were delivered at an interstimulus interval of 100ms, which results in the second motor-evoked potential (MEP) being inhibited in comparison to the first MEP. The example trace illustrates a subject with a high level of LICI (76.9%). (B and C) Group differences were not observed between seniors and young adults in intracortical facilitatory (ICF) and short- and long-interval intracortical inhibition (SICI and LICI) when obtained under resting conditions (B); however, the weakest tertile of seniors demonstrated nearly twofold higher levels of LICI compared with the stronger seniors (ie, those in the top two tertiles of relative muscle strength) (C). *Weaker seniors have > LICI than strong seniors (p < .05). Note: higher value of ICF is indicative of higher levels of facilitation; higher values of SICI and LICI are indicative of higher levels of inhibition.
Stronger seniors versus weaker seniors
A contraction intensity × strength group (ie, stronger vs weaker seniors) interaction was observed for MEP amplitude (Figure 2B; p < .01) with follow-up testing indicating that the stronger seniors increased their MEP amplitude with increasing contraction intensity (p < .01), whereas the weaker seniors did not (p = .23). No differences were observed between the stronger and weaker seniors for SP duration (Figure 2D; contraction intensity × age group interaction, p = .11). With regard to the paired-pulse measures, mean group differences were not observed between the stronger and weaker seniors for ICF (p = .51) and SICI (p = .14); however, the weaker seniors demonstrated nearly twofold higher levels of LICI compared with the stronger seniors (Figure 3; p = .03).
Discussion
We sought to determine how much of the weakness in seniors, and weak seniors in particular, is due to global impairments in the nervous system’s ability to fully activate muscle. On the surface, the lack of any significant difference in VA between young adults and seniors seems to indicate that weakness observed in seniors is not due to nervous system impairment. However, weaker seniors exhibited a significant reduction in VA in comparison to stronger seniors and this suggests that in many seniors weakness is, to some extent, attributable to the inability of the nervous system to fully activate skeletal muscle. It should be noted that the individuals in this “weaker senior” tertile were generally higher functioning (ie, of the 14 seniors stratified into the weaker senior tertile only four scored below 11 on the SPPB). Thus, based on our findings, it is plausible that lower-functioning seniors and/or those with clinically significant weakness (eg, those whose strength is below a certain threshold that impairs physical function, Manini et al. (2)) could show even lower levels of VA. This finding suggests that many of the discrepancies in the extant literature on the effects of aging on VA can likely be attributed to the heterogeneity of neuromuscular function (and perhaps physical function) observed in seniors. Many studies have suggested that there are no age-related impairments in VA (see Clark and Taylor (18) for review). We would have drawn this conclusion as well, had we not analyzed our senior cohort with stratification by relative strength. To our knowledge, this is the first study to perform such an analysis, though Harridge and colleagues (38) did report reduced VA capacity in the leg extensor muscles of relatively weak seniors aged 85–97 years without a stronger comparison group. Similarly, these findings are conceptually supported by studies indicating that aging results in altered motor neuron properties, such as decreased spinal excitability (6), lower mean motor unit discharge rates (8), a lower incidence of doublet discharges (7), an interrelationship between number of functioning motor units and muscle strength (11), as well as a slower rate of EMG rise during a leg press task that was associated with the age-related loss of muscle power (16). Together, these reports and our present findings clearly indicate that the nervous system can serve as a key contributor to weakness in the elderly.
With respect to excitability and inhibition, the TMS results yielded several notable and novel findings. Specifically, we observed that (i) seniors, and weaker seniors in particular, exhibit an attenuated increase in corticospinal excitability (interpreted based on the SP and MEP data) with increasing contraction intensity, and (ii) weaker seniors exhibit significantly higher levels of LICI when compared to stronger seniors.
The amplitude of an MEP evoked by a single suprathreshold TMS pulse to the motor cortex provides a composite index of excitability of the entire voluntary motor pathway, as the size of the response depends upon both cortical and spinal excitability (39,40). With increasing contraction intensities in the low-to-moderate force range (as performed herein), the MEP amplitude has been shown to increase (41,42). This increase in response has been attributed to enhanced excitability of cortical and spinal neurons through increased voluntary drive both to and from the motor cortex and with consequent increased descending drive to recruit motor neurons in order to increase muscle activation (43,44). Similarly, when evoked during a voluntary contraction, the MEP is followed by an SP, observed as a transient cessation of ongoing EMG activity consistent with an interruption in volitional drive and hence, withdrawal of descending input to the spinal motor neurons (45). In nonfatiguing contractions, the SP duration has been attributed to an initial short period of spinal refractoriness (~50ms) combined with a longer period of cortical inhibition (up to ~200ms) (44,45). To terminate the SP, volitional excitatory input to the motor cortex neurons must overcome the waning inhibition. Thus, stronger voluntary contractions with greater volitional excitation after the SP are associated with reduced SP durations as seen for the younger adults in our study. There were two significant findings in our dataset with respect to these parameters. First, seniors did not exhibit a reduction in the SP duration as contraction intensity increased. Second, weaker seniors did not exhibit the characteristic increase in the MEP amplitude observed with increasing contraction intensity. Thus, our data suggest that older adults, and weaker seniors in particular, are unable to modulate their corticospinal excitability to the same extent during different levels of contraction strength.
Unfortunately, because single-pulse TMS responses are mediated at both the cortical and spinal levels it is difficult to determine the site at which differences in these parameters are mediated. Paired-pulse TMS protocols, however, provide a strategy to more directly evaluate intracortical excitability (30). Our results show that weaker seniors exhibited higher levels of LICI, suggesting that age-related weakness may be mechanistically associated with GABAB receptor-mediated inhibition. This finding is consistent with prior studies of experimentally induced weakness in young adults that resulted in impairments in VA (46–48), where deficits in VA were found to be correlated with higher levels of LICI (48). However, whether our findings are due to impaired structural (anatomical) or functional (physiological) differences cannot be ascertained based on the current findings, and considering the widespread and complex neurobiological changes observed with advancing age it seems probable that it is a combination of the two (12–15).
To date, a relatively limited number of studies have examined age-related changes in measures of intracortical inhibition and facilitation with largely discrepant findings being reported (20–29). For instance, some studies observed an age-related decrease in inhibition (in the hand musculature) (23–25), some studies from a mix of muscle groups (elbow, leg, and hand) observed no age-related effects (20,22,28), and others from the wrist musculature observed an age-related increase in inhibition (26,29). It seems probable that the discrepancies can be attributed to a number of potential factors, such as (i) muscle group-specific differences, (ii) basic methodological differences (eg, stimulator type or stimulator waveform, Peinemann et al. (25)), and/or (iii) the large degree of heterogeneity in health status among older adults. As our current findings from a single muscle group show that when data from all older subjects are pooled, statistical analysis detects few functional or physiological differences associated with aging, but when we analyze our data based on cohorts determined from relative strength values, physiological differences become apparent; we believe the inconsistency between studies may be due to the heterogeneity issue. However, further work is required to more fully address these discrepancies.
Our results suggesting that weaker seniors have higher levels of intracortical inhibition are consistent with studies on immobilization (48) and resistance exercise in young adults (49) that provide support for the idea of high levels of intracortical inhibition being linked to weakness. However, the results contrast with the recent findings from Plow and colleagues (20). These investigators observed no differences between younger and older (~74 years) adults for SICI and an increase in interhemispheric inhibition in older adults in the elbow flexors, and further found that higher levels of voluntary EMG were associated with higher levels of SICI and interhemispheric inhibition. Thus, they suggest that, for older adults, high levels of intracortical inhibition are associated with the ability to more fully activate musculature (which is required for higher levels of strength). Clearly, further work is needed to clarify the discrepant findings.
There are several limitations of our work that should be noted. First, it is a cross-sectional design, and thus must be interpreted accordingly. Second, the data are derived from a distal forearm muscle that acts to flex and abduct the hand, and caution is urged when extrapolating findings to other muscle groups. Third, we expressed muscle strength relative to body weight as a way to try to control for differences in body stature and muscle mass on strength values. Conceptually, one could argue that a better approach would be to express the strength values relative to a more precise estimate of muscle size from the muscle group involved in the strength testing (eg, MRI-derived cross-sectional muscle mass of the wrist flexors). Unfortunately, we did not have measures of this nature available. Finally, although this study examined “weaker seniors,” it did not actually systematically examine group differences in seniors with clinically significant weakness (or physical function impairments) per se, and the findings should be interpreted within this context. Similarly, although we statistically controlled for the effect of gender, the majority of study participants classified as “weaker seniors” were women, and it is possible that our findings are confounded by sex differences in the measured outcomes.
Summary Comments and Conclusions
In recent years it has become increasingly accepted that weakness in seniors is attributable, in part, to degradation in nervous system function. Herein, we quantified VA, a measurement of one’s ability, or lack thereof, to maximally activate a muscle voluntarily and, using a cross-sectional design, compared values between young adults to those obtained from independent living, moderate-to-high functioning seniors. Additionally, we also compared the “stronger seniors” (top two tertiles for relative strength) to the “weaker seniors” (lowest tertile for relative strength). Our main finding here was that, although there were no systematic differences in VA between young adults and seniors, there were clear and stark differences between the stronger seniors and the weaker seniors, with the weaker seniors only being able to achieve VA levels of, on average, ~80%. This finding suggests that impairments in central motor drive (ie, motor unit recruitment and rate coding) contribute substantially to the weakness observed in our weaker seniors. Thus, interventional strategies designed to enhance motor function of the nervous system have the potential to meaningfully increase muscle strength in this cohort of individuals (eg, mental imagery, Clark et al. (50)). However, other age-related factors are also likely to contribute to weakness (eg, impaired excitation–contraction coupling, dysfunction of the neuromuscular junction, muscle wasting, impaired force transmission, etc.), and thus truly optimal interventional strategies to mitigate weakness may need to consider multiple mechanisms. Our findings regarding VA do not provide insight into the specific neuroanatomical and/or neurophysiological mechanisms of weakness per se. However, our TMS-based findings suggest that weaker seniors exhibit reduced corticospinal excitability at higher contraction intensities and exhibit higher levels of LICI under resting conditions. Although we cannot definitively link these differences to the motor cortex, the findings pertaining to LICI do suggest motor cortical involvement. Thus, this finding contributes to the growing body of literature reinforcing the role of the motor cortex as a key contributor to muscle strength/weakness, which is an extension beyond the more historically viewed scope in movement coordination and skill acquisition. Accordingly, these findings indicate the need for scientists and clinicians to consider neurological mechanisms as key contributors to weakness in older adults and to identify rational strategies to enhance neurological function in older adults.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported in part by the following NIH grants to B.C.C.: R01AG044424 from the NIA, R15HD065552 from the NICHD, R01AT006978 from the NCCAM, and R21AR063909 from the NIAMS.
Supplementary Material
References
- 1. Louie GH, Ward MM. Sex disparities in self-reported physical functioning: true differences, reporting bias, or incomplete adjustment for confounding? J Am Geriatr Soc. 2010;58:1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 4. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scaglioni G, Ferri A, Minetti AE, et al. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol (1985). 2002;92:2292–2302. [DOI] [PubMed] [Google Scholar]
- 6. Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. [DOI] [PubMed] [Google Scholar]
- 7. Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795. [DOI] [PubMed] [Google Scholar]
- 8. Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf). 2010;200:45–55. [DOI] [PubMed] [Google Scholar]
- 9. Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. [DOI] [PubMed] [Google Scholar]
- 10. Fling BW, Knight CA, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res. 2009;197:125–133. [DOI] [PubMed] [Google Scholar]
- 11. Kaya RD, Nakazawa M, Hoffman RL, Clark BC. Interrelationship between muscle strength, motor units, and aging. Exp Gerontol. 2013;48:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. [DOI] [PubMed] [Google Scholar]
- 13. Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–254. [DOI] [PubMed] [Google Scholar]
- 14. Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. [DOI] [PubMed] [Google Scholar]
- 15. Reid KF, Pasha E, Doros G, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark DJ, Pojednic RM, Reid KF, et al. Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68:1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark DJ, Reid KF, Patten C, et al. Does quadriceps neuromuscular activation capability explain walking speed in older men and women? Exp Gerontol. 2014;55:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol (1985). 2009;107:354–355. [DOI] [PubMed] [Google Scholar]
- 20. Plow EB, Cunningham DA, Bonnett C, et al. Neurophysiological correlates of aging-related muscle weakness. J Neurophysiol. 2013;110:2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plow EB, Varnerin N, Cunningham DA, et al. Age-related weakness of proximal muscle studied with motor cortical mapping: a TMS study. PLoS One. 2014;9:e89371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevens-Lapsley JE, Thomas AC, Hedgecock JB, Kluger BM. Corticospinal and intracortical excitability of the quadriceps in active older and younger healthy adults. Arch Gerontol Geriatr. 2013;56:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marneweck M, Loftus A, Hammond G. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci Res. 2011;70:408–414. [DOI] [PubMed] [Google Scholar]
- 24. Lysianne B, Alan C, Nathalie G, Sylvain H, Christian M. Age-related changes in intracortical inhibition are mental-cognitive state-dependent. Biol Psychol. 2014;101:9–12. [DOI] [PubMed] [Google Scholar]
- 25. Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. [DOI] [PubMed] [Google Scholar]
- 26. Kossev AR, Schrader C, Däuper J, Dengler R, Rollnik JD. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333:83–86. [DOI] [PubMed] [Google Scholar]
- 27. Oliviero A, Profice P, Tonali PA, et al. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. [DOI] [PubMed] [Google Scholar]
- 28. Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500. [DOI] [PubMed] [Google Scholar]
- 29. McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothwell JC, Day BL, Thompson PD, Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol. 2009;587(Pt 1):11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ziemann U. Pharmacology of TMS. Suppl Clin Neurophysiol. 2003;56:226–231. [PubMed] [Google Scholar]
- 33. McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. [DOI] [PubMed] [Google Scholar]
- 34. Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498(Pt 3):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNeil CJ, Martin PG, Gandevia SC, Taylor JL. Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res. 2011;209:287–297. [DOI] [PubMed] [Google Scholar]
- 37. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 38. Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. [DOI] [PubMed] [Google Scholar]
- 39. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. [DOI] [PubMed] [Google Scholar]
- 40. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. [DOI] [PubMed] [Google Scholar]
- 41. Gelli F, Del Santo F, Popa T, Mazzocchio R, Rossi A. Factors influencing the relation between corticospinal output and muscle force during voluntary contractions. Eur J Neurosci. 2007;25:3469–3475. [DOI] [PubMed] [Google Scholar]
- 42. Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res. 1997;117:472–478. [DOI] [PubMed] [Google Scholar]
- 43. McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol. 2011;589(Pt 14):3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rothwell JC. The fatigued spinal cord. J Physiol. 2009;587(Pt 23):5517–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. [DOI] [PubMed] [Google Scholar]
- 46. Kawakami Y, Akima H, Kubo K, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7–12. [DOI] [PubMed] [Google Scholar]
- 47. Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3wk of human forearm cast immobilization. J Appl Physiol (1985). 2008;105:868–878. [DOI] [PubMed] [Google Scholar]
- 48. Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve. 2010;42:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol (Oxf). 2012;206:109–119. [DOI] [PubMed] [Google Scholar]
- 50. Clark BC, Mahato NK, Nakazawa M, Law TD, Thomas JS. The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J Neurophysiol. 2014;112:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


