Abstract
Background.
We describe the recruitment of men for The Testosterone (T) Trials, which were designed to determine the efficacy of T treatment.
Methods.
Men were eligible if they were ≥65 years, had an average of two morning total T values <275ng/dL with neither value >300ng/mL, and had symptoms and objective evidence of mobility limitation, sexual dysfunction, and/or low vitality. Men had to be eligible for and enroll in at least one of these three main trials (physical function, sexual function, vitality).
Results.
Men were recruited primarily through mass mailings in 12 U.S. communities: 82% of men who contacted the sites did so in response to mailings. Men who responded were screened by telephone to ascertain eligibility. Of 51,085 telephone screens, 53.5% were eligible for further screening. Of 23,889 initial screening visits (SV1), 2,781 (11.6%) men were eligible for the second screening visit (SV2), which 2,261 (81.3%) completed. At SV2, 931 (41.2%) men met the criteria for one or more trials, the T level criterion and had no other exclusions. Of these, 790 (84.6%) were randomized; 99 (12.5%) in all three trials and 348 (44%) in two trials. Their mean age was 72 years and mean body mass index (BMI) was 31.0kg/m2. Mean (standard deviation) total T (ng/dL) was 212.0 (40.0).
Conclusion:
Despite the telephone screening to enrollment ratio of 65 to 1, we met the recruitment goals for each trial. Recruitment of symptomatic older men with low testosterone levels is difficult but feasible.
Key Words: Testosterone treatment, Recruitment, Hypogonadal men, Physical function, Vitality, Sexual function, Randomized clinical trials.
Introduction
Complex randomized clinical trials present recruitment challenges because of many stringent inclusion and exclusion criteria. Recruitment delays can affect project timelines and budget. Furthermore, failure to meet recruitment goals will diminish statistical power and the trial’s capacity to identify safety signals. Trial eligibility criteria must be directed at identifying those most likely to benefit from the intervention and excluding those more likely at risk from the intervention. Recruitment challenges are a major barrier to clinical research (1). A recent report noted that 25% of randomized trials were discontinued, 40% because of poor recruitment (1).
Recruitment can be especially challenging in elderly populations (2). Older persons have more comorbidities (3), take more medications and have more frequent hospitalizations, all of which may disqualify them. Sensory deficits, cognitive impairment, caregiving responsibilities, and access to transportation may interfere. Yet, if the research is to inform clinical practice, the study population should represent the target population to the extent possible.
In this report, we describe the recruitment and screening results of the Testosterone (T)Trials, a coordinated set of seven randomized trials designed to determine the efficacy of T administration in symptomatic older men with low T (4). Men had to be eligible for at least one of the three main trials: physical function, sexual function, or vitality. Thus, for this report, we focus on these three trials. The major consideration in participant selection was setting the eligibility criterion for serum T low enough to ensure that men were unequivocally T deficient, but not so low as to preclude sufficient enrollment or generalizability of results.
Methods
The TTrials are being conducted at 12 U.S. medical centers (Supplemental Material A) and were approved by the Institutional Review Boards at each center. All men provided written informed consent.
The study design for the TTrials has been published (4). Men were eligible if they were ≥65 years of age (no upper limit) and reported symptoms and had objective evidence of mobility limitation (Physical Function Trial), diminished libido (Sexual Function Trial), and/or low vitality (Vitality Trial). Initially, we included men whose serum testosterone was <250ng/dL between 8 and 10 am at both screening visits (SV1 and SV2). Men could enroll in as many of the main trials for which they qualified. Subsequently, men were enrolled in the cognitive function, anemia, cardiovascular, and/or bone trials if they met specific additional enrollment criteria.
Study Recruitment Goals
The recruitment goals were 275 for sexual function, 420 for vitality, and 388 for physical function. The original plan was to close enrollment into a trial once its goal was met. Later this plan was revised to complete enrollment in the cardiovascular and bone trials.
Eligibility Criteria for Individual Trials
Eligibility for the Physical Function Trial required reported difficulty walking one-quarter mile and/or walking up one flight of stairs and a gait speed <1.0 m/s on the 6-minute walk test (4). To qualify for the Sexual Function Trial, men had to report decreased libido, have a score ≤20 on the Derogatis Inventory of Sexual Function questionnaire (5) and have a partner willing to have sexual intercourse at least twice per month. For the Vitality Trial, men had to report decreased energy and score <40 on the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale (5). Standardized instructions were given for all tests. All examiners were certified. Regular monitoring was carried out through in-person quality assurance evaluations.
Changes to Eligibility Criteria
After 6 months of screening, the ratio of eligible to interested men was lower than predicted. To improve the ratio, some screening criteria were modified but in ways that would not significantly change the desired characteristics of the population. The T level criteria were changed at Month 1 to a total T level <275ng/dL at SV1, <300ng/dL at SV2 and an average T level <275ng/dL. After 13 months, the gait speed criterion for the Physical Function Trial was changed from <1.0 to <1.2 m/s on the rationale that it would improve eligibility yet still be associated with reduced survival (6–8).
Exclusion Criteria
Exclusion criteria have been previously described (4). At the telephone screen, we excluded men reporting a history of prostate cancer. At SV1, we excluded men with 35% (initially >30%) risk for any prostate cancer or >7% risk of high-grade prostate cancer (9). We excluded men with diagnosed but untreated sleep apnea, myocardial infarction, or stroke within the previous 3 months, angina not controlled by treatment, or BMI >37kg/m2 (initially, BMI > 35kg/m2). We also excluded men reporting alcohol or substance abuse within the previous year. At SV2, we excluded men with a prostate nodule or severe lower urinary tract symptoms by the International Prostate Symptom Score questionnaire (score >19) (10), uncontrolled hypertension, or selected abnormal laboratory tests [see ref. (4) for complete list of exclusions].
Recruitment Strategies
Each site used strategies based on their previous experience. A recruitment committee developed materials for study-wide use. It met bimonthly and monitored enrollment carefully. We developed an initial brochure (Supplementary Material B), which was later redesigned to target men more likely to have mobility limitations (Supplementary Material C).
Screening and Randomization
Toll-free numbers were established at most clinical centers. Men who contacted the center were screened for eligibility over the phone. Men who were eligible and interested were asked to come to the research clinic to complete the screening consent form and have blood drawn (SV1). Men with a total T level of <275ng/dL and a low risk of prostate cancer were asked to return for the second screening visit. At SV2, men had a second blood draw for T level and were evaluated for objective evidence of mobility limitation, sexual dysfunction, or low vitality; urinary tract symptoms and other medical exclusions; and had a digital rectal exam. Eligible men were invited to complete randomization.
Results
A summary of the TTrials recruitment strategies (Table 1) shows that direct mail brochures were the most successful: 82% of men who contacted the site did so in response to mailings. A variety of mailing lists was used, and multiple mailings to the same geographical areas were sent. Response rates were generally <4% but were 12% in older veterans and 16% in a registry of older volunteers. The characteristics of respondents, however, varied by type of mailing list. For example, sites using a Veteran’s Affairs mailing list noted that exclusions due to comorbidities were higher compared with a general population-based mailing. The volunteers on the registry were healthier, less likely to report physical or sexual dysfunction or low vitality and therefore, not eligible. Investigator talks at community events and/or assisted care facilitates were held infrequently with no estimated yield. The most common advertising medium was print, but five sites used a 30-second television advertisement, mostly posted around the 12-noon news to target this age group. Advertisements on the Chicago Cubs radio network were particularly successful at Northwestern. The estimated cost per telephone screen ranged from $38 (mass mailing) to $105 (print), Table 1.
Table 1.
Testosterone Trial Recruitment Sources and Strategies
| Percent of Telephone Screens (%) | Estimated Cost per Telephone Screen ($) | |
|---|---|---|
| Mass mailing | 82 | 38 |
| Voter registration lists | ||
| Veteran’s administration lists | ||
| Commercial mailing lists | ||
| Local HMO membership | ||
| Private patient registries | ||
| Local agencies who service people over age 65 | ||
| Volunteer registries | ||
| Advertisements | ||
| 3.20 | 105 | |
| Television | 1.30 | 46 |
| Radio (paid and public service) | 4.20 | 51 |
| Internet (ClinicalTrials.gov) | 0.30 | 0 |
| Flyers/posters in community, senior organizations, MD offices | 0.70 | |
| Friend/family referral | 0.70 | |
| Presentation by investigators: information and education programs | * | |
| Senior housing | ||
| Assisted living | ||
| Local physicians | ||
| Community groups | ||
| Collaboration with physicians and medical groups | ||
| Other | 7.80 | |
*No estimated yield.
We randomized 790 men between June 2010 and June 2013. An overview of the screening-to-randomization funnel is shown in Figure 1. In total, 51,085 telephone screening interviews were completed and 32,341 men (63.3%) were eligible to proceed to SV1. Major reasons for exclusion at the telephone screen were history of prostate cancer or other cancer, no self-reported impairment, BMI >37, high cardiovascular risk, and age <65. Of those deemed eligible for SV1 over the telephone, 23,889 completed SV1 and 4,700 were eligible by T level (9.2% of the total telephone screens; 19.7% of those who attended SV1). Of these, 1,616 men were excluded because of a high prostate cancer risk. Of the 2,852 eligible for SV2, 2,261 completed it (4.4% of total telephone screened). Of these, 515 were found ineligible due to lack of evidence of qualifying symptoms, 391 were ineligible due to T level and a variety of other reasons, leaving, 931 (1.8% of total telephone screened; 41% of the SV2’s completed) eligible for randomization. Ultimately, 790 (1.5% of total telephone screened) were randomized; two were randomized in error, for a final enrollment of 788 men. The telephone screen-to-randomization ratio was 65 to 1.
Figure 1.
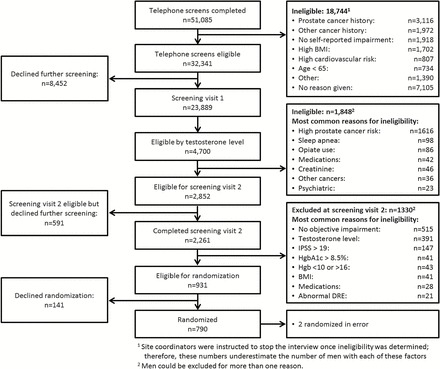
Testosterone trials screening to recruitment funnel. Percentages calculated as percent of total number of telephone screens. IPPS = International Prostate Symptom Score; DRE = digital rectal exam.
The screening yields differed by site (Table 2). The yields for telephone screen ranged from 37% to 68%. Yields were less variable for SV1, ranging from 17% to 24%). The University of Washington site relied heavily on electronic medical records for targeted recruitment, but the percent eligible at SV1 was similar to other clinics that used mass mailings for example, University of Minnesota. The percent of men at SV2 eligible to be randomized ranged from 27% to 69%; the total number randomized ranged from 44% to 84%.
Table 2.
Testosterone Trial Screening Yields Study-Wide and for Each Clinical Site
| Site | Telephone Screen | SV1 | SV2 | Randomization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Eligible | % Eligible Telephone Screen | Total | Eligible | % Eligible SVI** | Total | Eligible | % Eligible SV2 | Total | % Randomized of Telephone Screen* | |
| Albert Einstein University | 3,733 | 2,087 | 56 | 1,574 | 301 | 19 | 133 | 58 | 44 | 52 | 1.4 |
| Baylor College of Medicine | 3,819 | 1,664 | 44 | 1,387 | 270 | 20 | 138 | 77 | 56 | 66 | 1.7 |
| Boston University | 5,113 | 2,821 | 55 | 2,114 | 350 | 17 | 210 | 74 | 35 | 65 | 1.3 |
| Northwestern University | 3,065 | 1,508 | 49 | 1,393 | 264 | 19 | 142 | 62 | 44 | 52 | 1.7 |
| University of Alabama, Birmingham | 3,027 | 1,784 | 59 | 1,526 | 307 | 20 | 122 | 60 | 49 | 48 | 1.6 |
| Harbor-UCLA Medical Center | 3,905 | 2,229 | 57 | 2,007 | 375 | 19 | 166 | 114 | 69 | 78 | 2.0 |
| University of California, San Diego | 3,758 | 2,269 | 60 | 1,952 | 352 | 18 | 181 | 75 | 41 | 71 | 1.9 |
| University of Florida | 3,420 | 2,338 | 68 | 2,225 | 512 | 23 | 261 | 96 | 37 | 70 | 2.0 |
| University of Minnesota | 6,421 | 3,160 | 49 | 2,999 | 689 | 23 | 317 | 85 | 27 | 82 | 1.3 |
| University of Pittsburgh | 5,058 | 2,634 | 52 | 2,209 | 394 | 18 | 184 | 86 | 47 | 78 | 1.5 |
| University of Washington | 2,657 | 972 | 37 | 1,232 | 296 | 24 | 140 | 45 | 32 | 44 | 1.7 |
| Yale University | 7,109 | 3,875 | 55 | 3,271 | 590 | 18 | 267 | 99 | 39 | 84 | 1.2 |
| Overall | 51,085 | 27,341 | 54 | 23,889 | 4,700** | 20 | 2,261 | 931 | 41 | 788 | 1.5 |
*Percent randomized/number telephone screens.
**Percent eligible by testosterone level.
Enrollment in the individual trials also varied. A higher percentage of men qualified for the Sexual Function and Vitality Trials at the telephone screen, Table 3. About 45% of men (24%–55%) qualified for the Sexual Function Trial and 49% (32%–63%) for the Vitality Trial. Fewer men, 28% (13%–44%), qualified for the Physical Function Trial.
Table 3.
Telephone Screening Yields Overall and by Site for Individual Testosterone Trials
| Site | No. of Screened | Eligible for SV1 | ||
|---|---|---|---|---|
| Physical Function Trial | Sexual Function Trial | Vitality Function Trial | ||
| Albert Einstein University | 3,733 | 702 (18.8%) | 1,860 (49.8%) | 2,022 (54.2%) |
| Baylor College of Medicine | 3,819 | 840 (22%) | 1,206 (31.6%) | 1,382 (36.2%) |
| Boston University | 5,113 | 1,209 (23.6%) | 2,383 (46.6%) | 2,684 (52.5%) |
| Northwestern University | 3,065 | 817 (26.7%) | 1,050 (34.3%) | 1,253 (40.9%) |
| University of Alabama, Birmingham | 3,027 | 671 (22.2%) | 1,606 (53.1%) | 1,749 (57.8%) |
| Harbor-UCLA Medical Center | 3,905 | 1,659 (42.5%) | 1,832 (46.9%) | 1,892 (48.5%) |
| University of California, San Diego | 3,758 | 1,178 (31.3%) | 2,059 (54.8%) | 2,197 (58.5%) |
| University of Florida | 3,420 | 1,515 (44.3%) | 1,884 (55.1%) | 2,166 (63.3%) |
| University of Minnesota | 6,421 | 840 (13.1%) | 2,874 (44.8%) | 3,096 (48.2%) |
| University of Pittsburgh | 5,058 | 1,389 (27.5%) | 2,363 (46.7%) | 2,517 (49.8%) |
| University of Washington | 2,657 | 827 (31.1%) | 644 (24.2%) | 849 (32.0%) |
| Yale University | 7,109 | 2,543 (35.8%) | 2,999 (42.2%) | 3,326 (45.5%) |
| Overall | 51,085 | 14,190 (27.8%) | 22,760 (44.6%) | 25,043 (49%) |
Several changes were made to the eligibility criteria because the initials yields were lower than expected. The effects of these changes are summarized in Table 4. Changing the T level cutoff raised the yield at SV1 from 12.7% to 21% but slightly decreased yield at SV2. Changing the BMI criterion at Month 3 of enrollment had little effect. The change in the prostate cancer risk threshold at Month 10 had little effect at SV1 and no effect at SV2. Changing the gait speed criterion at Month 13 resulted in a doubling of eligibility at SV2 for the Physical Function Trial.
Table 4.
Effect of Changes to Enrollment Criteria in the Testosterone Trials on Percent Eligible at SV1 and SV2Percent Eligible Before and After Enrollment Criteria Change
| SV1 Eligible | SV2 Eligible at SV2 | |
|---|---|---|
| Testosterone entry criteria (Month: 1) | ||
| Before (T level <250ng/dL at each of 2 visits) | 12.7% | 78.8% |
| After (T level <275ng/dL at each of 2 visits) | 21% | 69.7% |
| Body mass index (BMI) (Month: 3) | ||
| Before (>35kg/m2) | 94.8% | |
| After (>37kg/m2) | 95.2% | |
| Risk of any prostate cancer (Month: 10) | ||
| Before (>30%) | 67.8% | |
| After (>35%) | 70.5% | |
| Physical function: gait speed (Month: 13) | ||
| Before (<1.0 m/s) | 29.2% | |
| After (<1.2 m/s) | 59.2% | |
Note: Percent eligible before and after enrollment criteria change.
Characteristics of Men Enrolled
A total of 470 men (171% of goal) were enrolled in the Sexual Function Trial; 474 men (113% of goal) in the Vitality Trial and 390 men (101% of goal) in the Physical Function Trial. The Sexual Function Trial was overenrolled because it was the first trial to complete enrollment, and we decided keep it open, but only to men who also qualified for the Vitality or Physical Function Trials. Among the 788 men enrolled, 99 (12.5%) participated in all three main trials, 348 (44.2%) in two trials and 341 (43.3%) in one trial. The overlap is shown by the Venn diagram in Figure 2.
Figure 2.
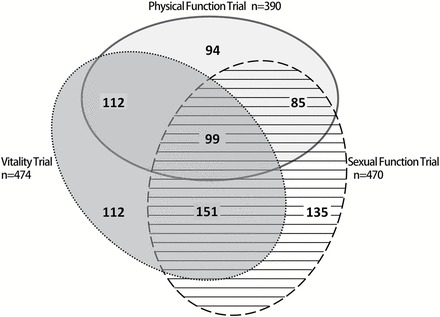
Venn diagram showing overlap in the enrollment of men into the main testosterone trials: physical function, sexual function, and vitality.
Characteristics of the men enrolled in individual trials are summarized in Table 5. Overall, men in the Physical Function Trial tended to be older. Few non-White men were randomized. Most men were obese; 63% had a BMI ≥30kg/m2. There was little difference in total T across the trials.
Table 5.
Characteristics of Men at Screening for the Testosterone Trials: Overall and by Subsequent Enrollment in Physical Function, Sexual Function, and Vitality Trials: Mean (Standard Deviation) or Percent
| Overall | Physical | Sexual | Vitality | |
|---|---|---|---|---|
| Age (years) | 72.2 (5.7) | 73.3 (6.2) | 71.6 (5.3) | 71.9 (5.8) |
| Range | 65–94 | 65–94 | 65–89 | 65–94 |
| Non-White n (%) | 11.4 | 12.8 | 13.4 | 7.8 |
| BMI (kg/m2) | 31.0 (3.5) | 31.6 (3.4) | 31.0 (3.5) | 31.0 (3.6) |
| Range | 18.6–37.0 | 19.8–37.0 | 18.6–37.0 | 18.6–37.0 |
| BMI (≥30.0kg/m2) (%) | 63 | 70 | 63 | 61 |
| Total testosterone (ng/dL) | 212.0 (40.0) | 209.1 (39.9) | 212.9 (40.3) | 210.2 (41.5) |
| Range | 23.0, 274.0 | 53.5, 274.0 | 53.5, 274.0 | 23.0, 274.0 |
| Gait speed (m/s) | 1.1 (0.2) | 1.0 (0.2) | 1.1 (0.2) | 1.1 (0.2) |
| Range | 0.1–1.8 | 0.1–1.2 | 0.1–1.8 | 0.3–1.7 |
| Derogatis inventory of sexual function–sexual desire domain | 14.0 (7.8) | 14.3 (8.1) | 11.8 (6.6) | 14.0 (7.9) |
| Range | 0–33 | 0–33 | 0–33 | 0–33 |
| FACIT-fatigue (0–51) | 36.9 (8.7) | 37.5 (8.4) | 37.8 (8.9) | 31.5 (6.4) |
| Range | 10–52 | 12–52 | 10–52 | 10–49** |
*Testosterone values are the means from the first and second screening visits. The gait speed, DISF, and FACIT-Fatigue values are from the second screening visit.
**One man was mistakenly enrolled in the vitality trial with an ineligible FACIT-Fatigue score
Discussion
The TTrials successfully randomized 788 men from 12 U.S. communities and met the recruitment goals of each trial. Enrolled men had unequivocally low serum T and evidence of both self-reported and objective evidence of physical dysfunction, sexual dysfunction, and/or low vitality. Of all men screened over the telephone, the recruitment yield was 1.5%; the telephone screen to randomization ratio was 65 to 1. Similar to our original estimates, approximately 30 men had to be screened in person for each man randomized. However, several eligibility criteria required modification because the screening yield initially was much lower than expected, highlighting the need for continuous monitoring of recruitment.
The low yield of screenees to randomization resulted from the study design, not low levels of participation. Our results were similar to the Systolic Hypertension in the Elderly Program where 1.3% of initial contacts were randomized (11). Similar to the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, several eligibility criteria were changed to meet the recruitment goals (12). However, unlike Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, we did not have to extend our recruitment period (12). The Lifestyle Interventions and Independence for Elders study screened 9 individuals to randomize one. Our higher ratio likely reflected the requirement for low T since only 9% of those telephone screened had a low T.
Only 20% of men who reported symptoms of testosterone deficiency and had no major comorbidities that would exclude them had a sufficiently low testosterone concentration at SV1 to proceed to SV2. This confirms the nonspecific nature of self-reported symptoms of testosterone deficiency. Forty percent of these men had a follow-up T level <275ng/dL and objective evidence of mobility limitation, sexual dysfunction, or low vitality, supporting the tiered screening strategy in identifying older men with symptoms and signs of testosterone deficiency and low T.
Our experience may prove useful for others recruiting for complex clinical trials. Multiple strategies for recruitment were needed. Similar to Systolic Hypertension in the Elderly Program (11), most men who were screened learned about the study through direct mail. Mass mailings were successful but response rates were low and return mail postage was costly. Similar to Systolic Hypertension in the Elderly Program (11) response rates were higher from mailings to targeted groups for example, male veterans or hospital volunteers, although eligibility varied by the type of mailing list. Reliance on patient registries, electronic medical records, or referral from individual physicians did not yield many enrollees. The TTrials were registered on ClinicalTrials.gov (13) but <0.3% men were referred to the clinic through this site. Only one site used electronic medical records to target men with low T. Although this method is inexpensive, the yield was not greatly different from other sources.
Recruitment costs were lowest for mass mailings and highest for print media. Our costs per participant screened were similar to estimated costs for the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial study, where cost per referral was $59.66 for direct mail and $92.07 for radio. Our recruitment cost estimates do not include personnel, so total recruitment cost would be much higher. In Lifestyle Interventions and Independence for Elders, the total cost per randomization was $840 (14). Our total costs per randomization would likely be much higher than Lifestyle Interventions and Independence for Elders given our higher screening to enrollment ratio.
Generally, changes made to the screening criteria over the course of recruitment improved eligibility. Changing the T level requirement almost doubled the percent eligible at SV1. However, the percent eligible by T level actually decreased slightly at SV2, likely reflecting the intraindividual variability in T level (15); once the higher SV1 cutoff was implemented, more men had T level >300 at SV2, resulting in a lower eligibility rate. These changes were made to facilitate enrollment but in ways that would not significantly change the desired characteristics of the enrolled population. For example, the mean total T concentration, 212.0ng/dL, is clearly in the hypogonadal range.
Few non-White men (≈11%) were randomized. A greater percentage of otherwise eligible African American men were excluded because of prostate cancer risk (62.6%, vs 27.5% in Caucasian men). Hispanic men may be under-represented because of our inability to provide all evaluation tools in Spanish.
In conclusion, we successfully recruited a large number of men with unequivocally low T levels and both self-reported and objective evidence of mobility limitation, low libido, and/or low vitality. We met our recruitment goals despite a very low recruitment yield. Entry criteria were modified to increase recruitment yield without significantly altering the desired characteristics of the enrolled population. Lessons learned included the success of the direct mail approach, the importance of monitoring recruitment and modifying entry criteria if needed, and the efficiency of the TTrials design that allowed men to enroll in more than one trial if qualified.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Testosterone Trials were supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG030644), supplemented by funds from the National Heart, Lung and Blood Institute, National Institute of Neurological Diseases and Stroke, and National Institute of Child Health and Human Development. The Bone Trial was supported by a grant from the National Institute on Aging (R01 AG037679). The Anemia Trial was supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG034661) to the Partnership for Anemia Clinical and Translational Trials in the Elderly consortium. AbbVie (formerly Solvay and Abbott Laboratories) generously provided funding, AndroGel and placebo gel. AMM was supported by the Department of Veterans Affairs Puget Sound Health Care System. TMG is the recipient of an Academic Leadership Award (K24-AG021507) from the National Institute on Aging. The Yale Field Center was partially supported by the Claude D. Pepper Older Americans Independence Center (P30-AG021342) and Yale CTSA (UL1 TR000142). SMR was supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health. CEL was supported by the National Institute for Diabetes, Digestive and Kidney Diseases, National Institutes of Health (DK079626) to the UAB Diabetes Research and Training Center.
Conflict of interest
SSE reports a conference grant from AbbVie during the conduct of the study; KEE reports personal fees from Merck Sharpe & Dohme, outside the submitted work; GRC has served as a consultant to AbbVie, Clarus Therapeutics, Endo Pharma, Ferring, Lilly, Repros Therapeutics, and he has served as an expert witness for Repros Therapeutics and Solvay. He has received research support from Ardana, Unimed and Abbvie; AMM reports grants from National Institute on Aging, National Institutes of Health, during the conduct of the study; grants and personal fees from AbbVie, personal fees from GlaxoSmithKline, personal fees from Endo, personal fees from Lilly, outside the submitted work; CEL was supported by the National Institute for Diabetes, Digestive and Kidney Diseases, National Institutes of Health (DK079626) to the UAB Diabetes Research and Training Center; MEM reports grants from NIH, during the conduct of the study, personal fees from AbbVie, grants and personal fees from Eli Lilly, grants from ENDO Health Solutions, personal fees from Pfizer, outside the submitted work; CW reports grants from Besins Health International, other from AbbVie, during the conduct of the study, grants from Clarus Therapeutics, outside the submitted work; SA reports grants from University of Florida, during the conduct of the study; SB has received research grants from AbbVie, Regeneron, and Lilly, Inc., and has equity interest in FPT, LLC; SB reports personal fees from Eli Lilly, grants from AbbVie, outside the submitted work; RSS reports grants and consulting from AbbVie, Clarus, Ardana, Besins Health, and Endo Pharma; PJS reports grants from NIH and AbbVie for the conduct of this study and has consulted for Watson Laboratories. Remaining authors report no conflict of interest.
Supplementary Material
References
- 1. Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045–1051. doi:10.1001/jama.2014.1361 [DOI] [PubMed] [Google Scholar]
- 2. Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56:2340–2348. doi:10.1111/j.1532-5415.2008.02015.xJGS2015 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi:298/10/1160-a [pii]10.1001/jama.298. 10.1160-b [DOI] [PubMed] [Google Scholar]
- 4. Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: Seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11:362–375. doi:10.1177/1740774514524032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi:10.1002/cncr.10245 [DOI] [PubMed] [Google Scholar]
- 6. Dumurgier J, Elbaz A, Ducimetière P, Tavernier B, Alpérovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi:10.1136/bmj.b4460bmj.b4460 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mutikainen S, Rantanen T, Alén M, et al. Walking ability and all-cause mortality in older women. Int J Sports Med. 2011;32:216–222. doi:10.1055/s-0030-1268506 [DOI] [PubMed] [Google Scholar]
- 8. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0ng/ml or lower. JAMA. 2005;294:66–70. doi:10.1001/jama.294.1.66 [DOI] [PubMed] [Google Scholar]
- 10. Badía X, García-Losa M, Dal-Ré R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997;31:129–140. [DOI] [PubMed] [Google Scholar]
- 11. Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing and staff experience in a total recruitment program for a clinical trial: the SHEP experience. Systolic Hypertension in the Elderly Program. Cooperative Research Group. Control Clin Trials. 1999;20:133–148. doi:S0197245698000555 [pii] [DOI] [PubMed] [Google Scholar]
- 12. Wright JT, Jr, Cushman WC, Davis BR, et al. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT): clinical center recruitment experience. Control Clin Trials. 2001;22:659–673. doi:S0197245601001763 [pii] [DOI] [PubMed] [Google Scholar]
- 13. National Institute on Aging; National Institute of Neurological Disorders and Stroke; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Heart, Lung and Blood Institute; Abbott; University of Pennsylvania. The testosterone trial in older men. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US); 2008–2015. URL of the record NLM Identifier: NCT00799617. [Google Scholar]
- 14. Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi:10.1093/gerona/glt064glt064 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67:853–862. doi:10.1111/j.1365-2265.2007.02976.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


