Abstract
There is extensive evidence that ischemic/reperfusion mediated mitochondrial dysfunction is a major contributor to ischemic damage. However data also indicates that mild ischemic stress induces mitochondrial dependent activation of ischemic preconditioning. Ischemic preconditioning is a neuroprotective mechanism which is activated upon a brief sub-injurious ischemic exposure and is sufficient to provide protection against a subsequent lethal ischemic insult. Current research demonstrates that mitochondria are not only the inducers of but are also an important target of ischemic preconditioning mediated protection. Numerous proteins and signaling pathways are activated by ischemic preconditioning which protect the mitochondria against ischemic damage. In this review we examine some of the proteins activated by ischemic precondition which counteracts the deleterious effects of ischemia/reperfusion thereby maintaining normal mitochondrial activity and lead to ischemic tolerance.
Keywords: Ischemic tolerance, Sirtuins, Nampt, Nrf2, Cerebral ischemia
Introduction
Mitochondria are the primary source of ATP production in the cell and are required for the continued activity of energetically expensive pumps and energy consuming enzymatic reactions which maintain normal cellular homeostasis. However, exposure to even a brief period of ischemia can impede normal mitochondrial function thereby affecting the maintenance of cellular ATP levels and resulting in the generation of free radical production (Zadori et al. 2012; Ferrari 1995; Flamm et al. 1978). Mitochondrial dysfunction following ischemia is considered to be a primary contributor to ischemia/reperfusion mediated injury. Numerous studies have demonstrated that mitochondrial respiration is severely affected by ischemia/reperfusion which may stem from hyperoxidation of respiratory chain proteins, substrate unavailability and reactive oxygen species (ROS) production (Perez-Pinzon et al. 1997a; Rosenthal et al. 1995, 1997). Activation of pro-apoptotic Bcl-2 family members further exacerbate mitochondria dysfunction through outer mitochondrial membrane permeabilization which allows for the release of NADH and cytochrome c and initiates both caspase dependent and independent cell death pathways (Penna et al. 2013; Halestrap 2006; Christophe and Nicolas 2006). Furthermore mitochondria are the primary site of ROS formation and oxidative damage following ischemia/reperfusion (Chen and Zweier 2014; Olsen et al. 2013; Thompson et al. 2012). Mitochondria therefore are a major contributing factor to ischemia/reperfusion injury and as such are a major therapeutic target in the search for protection against ischemic damage.
Ischemic preconditioning (IPC) is an innate neuroprotective mechanism whereby a stressful but non-injurious ischemic episode followed by a period of reperfusion protects against a subsequent injurious ischemic attack. IPC has been demonstrated by numerous laboratories at the organismal, organ, and cellular levels (Gidday 2006). IPC is characterized by an immediate window of protection which last for a few hours and is followed 24 to 48 h later by a second, more robust window of protection which can last for days to weeks (Gidday 2006; Perez-Pinzon et al. 1997b). The early window of protection is initiated through the posttranslational modification of proteins whereas the second or delayed window of protection requires alteration in gene expression (Gidday 2006; Thompson et al. 2013a).
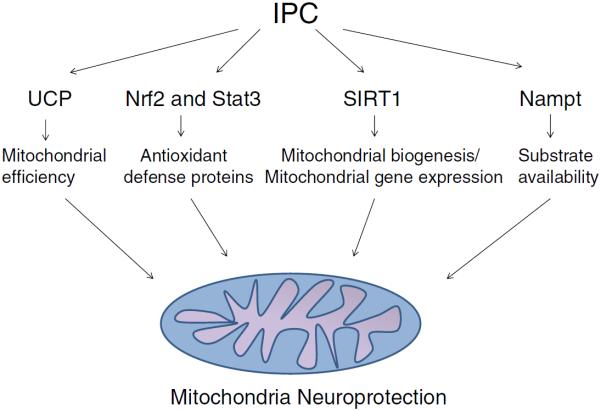
Ischemia activates many trigger mechanisms which initiate a precondition response including neuroactive cytokines (Lin et al. 2009), adenosine receptors (Zhou et al. 2004), ATP-sensitive potassium channels (Perez-Pinzon and Born 1999), and oxidative stress (Otani 2004). These preconditioning triggers in turn activate numerous kinase signaling cascades which orchestrates upregulation of the cellular protective response. One aspect of IPC mediated protection is the prevention of mitochondrial dysfunction following ischemia/reperfusion (Perez-Pinzon et al. 2012). Our laboratory has previously demonstrated that IPC preserved mitochondrial respiration following injurious ischemia during the delayed but not the early window of protection; suggesting that the regulation of mitochondrial physiology is an important component of prolonged ischemic tolerance (Perez-Pinzon et al. 2002; Perez-Pinzon 2004; Dave et al. 2001). Therefore in this review we will examine some of the mechanisms and signaling pathways by which IPC may protect mitochondrial against ischemia/reperfusion injury (Fig. 1).
Fig. 1.
Ischemic preconditioning (IPC) activates numerous signaling pathways which act in concert to protect the mitochondria against the deleterious effects of ischemia/reperfusion
IPC and mitochondrial neuroprotection
Mitochondrial substrate limitation during IPC leads to a rapid reduction in cellular ATP levels. An earlier study demonstrated that the ATP/ADP ratio is depleted within minutes of mitochondrial ATP synthase inhibition in cerebellar granule cells (Budd and Nicholls 1996). This decrease in cellular ATP levels and the parallel increase in ATP metabolites, ADP, AMP, and adenosine, may serve as initiation signals for the induction of ischemia tolerance pathways. For example, binding of adenosine to its A1 receptors activates numerous protective pathways. Confirmatory studies using in vivo and in vitro models demonstrated that adenosine pre-treatment is neuroprotective against an otherwise lethal ischemic insult (Hiraide et al. 2001; Blondeau et al. 2000; Reshef et al. 2000). Altering the levels of ATP metabolites by mild inhibition of the mitochondrial electron transport chain complex I with haloperidol, or complex II with 3-nitropropionate, has been demonstrated to protect the brain from an otherwise lethal hypoxic / ischemia insult (Sugino et al. 1999; Riepe and Ludolph 1997). Similarly an increase in the AMP:ATP ratio following IPC also leads to activation of AMP-activated protein kinase (AMPK). In an earlier study we observed that AMPK is also required for induction of ischemia tolerance and for protein kinase c epsilon (PKCε)-induced ischemic protection (Morris-Blanco et al. 2014; Tokunaga et al. 2004). Finally, the decline in cellular ATP levels following IPC may also directly regulate mitochondrial activity through the opening of mitochondrial ATP-sensitive potassium channels (mito-K+ATP channels). Numerous studies have demonstrated that opening of the mito-K+ATP channels is involved in the induction of ischemia tolerance. For example pharmacological pre-treatment with mito-K+ATP channels openers have been demonstrated to induce cerebral ischemia tolerance (Blondeau et al. 2000; Roth et al. 2006). In contrast, selective inhibition of mito-K+ATP channels using 5-hydroxydecanoate leads to the loss of ischemia tolerance, further confirming a role of mito-K+ATP channels in induction of ischemia tolerance (Liu et al. 2002). In our laboratory, we observed that activation of PKCε following IPC leads to the opening of this channel, by phosphorylation of the mito-K+ATP channel subunit Kir6.2 (Raval et al. 2007). It is suggested that opening of mito-K+ATP channels induces a mild increase in reactive oxygen species (ROS) generation which may trigger ischemia tolerance pathways (Thompson et al. 2012; Raval et al. 2007; Busija et al. 2005). This contention is supported by the observation that cellular ROS level is increased following preconditioning and quenching of ROS during preconditioning by antioxidants abolished preconditioning-mediated neuroprotection (Ravati et al. 2001). Activation of mito-K+ATP channels has also been demonstrated to increase the level of the anti-apoptotic protein, Bcl-2 and decrease the proapoptotic mitochondrial protein Bax in staurosporine-induced neuronal apoptosis, suggesting mito-K+ATP channels may also participate in modulation of apoptotic processes (Ravati et al. 2001). Overall, the above-presented literature thus highlights the importance of mitochondria in induction of ischemia tolerance in the brain.
IPC also participates in suppressing mitochondrial dysfunction following cerebral ischemia. We have observed that IPC leads to increased levels of synaptosomal PKCε which, upon activation during ischemia, increases the efficacy of the mitochondrial electron transport chain by altering phosphorylation status of its different components. This may, in part, be responsible for preservation of mitochondrial function following cerebral ischemia and the prolonged neuroprotection of IPC characterized by the second window of neuroprotection (Dave et al. 2001; Perez-Pinzon et al. 1997c). Activation of mito-K+ATP channels prior to otherwise injurious cerebral ischemia also attenuates cerebral ischemia-induced mitochondrial calcium overload and in turn inhibits opening of the mPTP (Wu et al. 2006). IPC also deactivates the proapoptotic molecule BAD via the hypoxia inducing factor 1 - erythropoietin - phosphoinositide 3-kinase - protein kinase B pathway (Dirnagl and Meisel 2008). Different paradigms of preconditioning are also shown to inhibit ischemia-induced release of cytochrome c from the mitochondria (Liu et al. 2002; Nakatsuka et al. 2000), which initiates caspase activation and apoptotic cell death. This literature demonstrates that preconditioning suppresses post-ischemic mitochondrial dysfunction and thus helps the recovery from ischemia-induced damage. Overall, it appears that mitochondria act as signaling process house for preconditioning-induced ischemia tolerance.
IPC and signaling pathways leading to increase antioxidant capacity roles of Nrf2 and STAT3
Nrf2
An important neuroprotective mechanism of IPC is the amelioration of oxidative stress through upregulation of endogenous antioxidant defense systems. A critical component of the antioxidant defense system is the transcription factor nuclear factor erythoid-2 related factor (Nrf2) which is activated by free radicals and electrophilic stress. Nrf2 is normally bound to its cytosolic repressor protein, Keap1, and degraded under conditions of abundant oxygen tension. However, Keap1 and Nrf2 may be chemically modified through various posttranslational modifications, such as PKC-dependent phosphorylation (Kaspar et al. 2012; Huang et al. 2002), SIRT1-dependent deacetylation (Kawai et al. 2011), and nitric oxide-dependent S-nitrosylation (Um et al. 2011). Most of these chemical modifications enhance Nrf2 disassociation from Keap-1, thus facilitating Nrf2 nuclear translocation and subsequent Nrf2-dependent gene expression. In the nucleus Nrf2 binds to the antioxidant response element (ARE) which allows for the expression of the various target genes involved in global cellular antioxidant response. Prototypical Nrf2 regulated genes include glutathione synthase, heme oxygenase-1, and catalase (Dreger et al. 2009; Dong et al. 2008; Reichard et al. 2007; Chan et al. 2001).
While Nrf2 has been demonstrated to be activated following oxidative stress in various tissues and species, there is debate as to whether transient hypoxic stress can induce neuroprotection via Nrf2. A previous study demonstrated upregulation of Nrf2-targeted gene transcription following IPC in human and rat astrocytes. More importantly, IPC-mediated neuroprotection was mitigated in Nrf2−/− knockout cultures, suggesting a vital role for Nrf2 in IPC neuroprotection (Bell et al. 2011a). Bell et al. (Bell et al. 2011a) demonstrated that both transient ischemia and subtoxic levels of hydrogen peroxide were capable of inducing neuroprotection following lethal oxygen glucose deprivation (OGD, in vitro ischemia model) in mice astrocyte/neuronal mixed cultures (Haskew-Layton et al. 2010). However, mice neuronal-enriched cultures were unable to upregulate Nrf2-dependent gene transcription, suggesting that astrocytes are the primary source of Nrf2. Finally, Nrf2−/− mixed cortical cultures were not protected by exposure to hydrogen peroxide following OGD, supporting a role for Nrf2 in mediating a response to oxidative stress (Bell et al. 2011b).
Some of the debate over Nrf2's neuroprotection may stem from conflicting evidence on how Nrf2 regulates certain chemical modifiers. A recent study suggested that SIRT1, a NAD+ -dependent histone deacetylase, inhibited Nrf2's transcriptional activity (Kawai et al. 2011), while conflicting results have been reported with the use of resveratrol, a polyphenolic antioxidant known to activate SIRT1. Resveratrol was demonstrated to stabilize and restore levels of Nrf2 in the cerebellum in a rodent model of fetal alcohol syndrome (Kumar et al. 2011). Finally, histone deacetylase inhibitors increased Nrf2 activation following focal cerebral ischemia in mice, and resulted in decreased infarct volumes when administered shortly after the induction of focal cerebral ischemia (Wang et al. 2012a). A consensus on the activation of Nrf2 may clarify its neuroprotective role following transient hypoxic or ischemic preconditioning.
STAT3
In addition to Nrf2, STAT3 is another transcription factor which is associated with increasing the cells resiliency to oxidative stress. STATs, or Signal Transducers and Activators of Transcription, have diverse roles in maintaining cellular function. STAT3, specifically, has been shown to be activated following cellular injury through phosphorylation by JAK kinase (Mascareno et al. 2001); this phosphorylation event promotes STAT3 nuclear translocation in the brain, where it can modulate apoptosis (Chin et al. 1997), inflammation (Chen et al. 2013), and ameliorate oxidative stress through Mn Superoxide dismutase (MnSOD) upregulation (Negoro et al. 2001).
Previous studies have highlighted the ability of STAT3 to translocate to the nucleus following a brief exposure to oxygen glucose deprivation (in vitro ischemia model) in mixed rodent cortical cultures. In addition, this study showed that activation of cyclooxygenase 2 (COX-2) by STAT3 resulted in neuroprotection, while inhibition of STAT3 nuclear translocation mitigated the neuroprotective effects of IPC (Kim et al. 2008). Another study noted that STAT3 knockdown in astrocytes increased production of ROS through downregulation of important free radical scavengers (i.e. MnSOD) (Sarafian et al. 2010). As mitochondria are potent producers of ROS in the cell, these studies suggest that STAT3 may interact with mitochondria; indeed, previous work has described a mitochondrial pool of STAT3 (Boengler et al. 2010). In rodent cardiac myocyte mitochondria, STAT3 has been shown to interact with complex I and II of the electron transport chain. Functionally, STAT3-difficient mitochondria displayed increased ROS production from complex I of the electron transport chain (Gagliardi et al. 1988). Loss of STAT3 has also been shown to decrease the enzymatic activity of complex I, while co-immunoprecipitation experiments have shown that STAT3 interacts directly with complex I (Wegrzyn et al. 2009) and the mitochondrial permeability transition pore (MPTP) (Boengler et al. 2010). In regards to the MPTP, loss of STAT3 resulted in increased frequency of opening and activation of the MPTP, causing cytochrome c release and dissolution of mitochondrial membranes (Boengler et al. 2010). The results of these studies suggest that STAT3 mitigates ROS production from complex I, which is a potent site of superoxide generation within mitochondria.
Taken together, Nrf2 and STAT3 are two transcription factors that can activate diverse pathways within a cell to ameliorate oxidative stress. The induction of antioxidant systems and amelioration of mitochondrial ROS production are the primary neuroprotective mechanisms of these factors and appear to be involved in ischemic tolerance.
NAD and ischemic tolerance
Nicotinamide adenine dinucleotide (NAD) is an essential coenzyme used by the TCA cycle and electron transport chain in the maintenance of mitochondrial membrane potential and production of ATP. Cerebral ischemia causes severe reductions in NAD which lead to DNA damage, energy depletion, and neurodegeneration (Zheng et al. 2012; Bi et al. 2012; Wang et al. 2008; Iwashita et al. 2004). Thus, enhancing cellular levels of NAD has been shown to prevent mitochondrial dysfunction and neurodegeneration following ischemic injury (Zheng et al. 2012; Bi et al. 2012). Previous studies have shown that IPC regulates NAD in the hippocampus and cortex (Morris-Blanco et al. 2014; Centeno et al. 1999) which may contribute to the ability of IPC to enhance mitochondrial functioning and neuronal survival.
Studies from the heart and brain have shown that IPC enhances levels of nicotinamide phoshphoribosyltransferase (Nampt) (Morris-Blanco et al. 2014; Yamamoto et al. 2014), the rate-limiting enzyme in the major biosynthetic pathways for the production of NAD (Revollo et al. 2007). Nampt converts nicotinamide to NMN (nicotinamide mononucleotide), which is then converted to NAD by NMNAT (NMN adenyltransferase) (Ying 2008). Nampt contains hypoxia response elements in its promoter (Segawa et al. 2006) and is important for mediating protection during ischemic events. For example, Nampt overexpression was shown to prevent neurodegeneration following MCAO, whereas Nampt inhibition exacerbated ischemic infarction (Wang et al. 2012a, b). Furthermore, a study performed in primary neuronal cultures revealed that Nampt maintains mitochondrial function and neuroprotection against oxygen-glucose deprivation (in vitro ischemia model) through its ability to produce NAD (Bi et al. 2012).
The importance of Nampt in IPC-mediated protection has been shown in studies of the heart where Nampt inhibition attenuated cell survival at both early and late phases of ischemia/reperfusion injury (Yamamoto et al. 2014; Nadtochiy et al. 2011). However, the mechanism by which IPC regulates Nampt and NAD has not been fully defined. Recent studies performed by our laboratory and others have indicated that in the cortex, IPC activates AMPK (Morris-Blanco et al. 2014; Jiang et al. 2014) an enzyme involved in increasing Nampt mRNA and whole-cell NAD levels (Fulco et al. 2008). Interestingly, Nampt overexpression has been shown to protect the brain against ischemic injury in an AMPK-dependent manner (Wang et al. 2011), demonstrating an important functional network between Nampt and AMPK.
In contrast to Nampt, the contribution of NMNAT to IPC mediated ischemic tolerance is hitherto undefined. Numerous studies have demonstrated that NMNAT is protective against axonal degeneration which is an early hallmark of many neuronal disorders such as ischemia/reperfusion. Recent research suggests that NMNAT neuroprotection may be independent if its NAD synthesis activity. For example Sasaki et al. (Sasaki et al. 2009) demonstrated that NMNAT enzymatic activity but not NAD levels were required to prevent axonal degeneration. In a different study, overexpression of NMNAT increased tau degradation which was dependent upon NMNAT binding to phosphorylated tau but independent of NMNAT enzymatic activity (Ali et al. 2012). Studies by Zhia et al. (Zhai et al. 2008) suggest that NMNAT is a stress activated chaperone which act similar to heat shock protein 70 in the proteasome mediated pathway and functions independently of its enzymatic activity. Therefore it appears that NMNAT may act in parallel to its NAD salvage pathway function to protect against neuronal degeneration following neurotoxic events such as ischemia/reperfusion.
In the brain, the mitochondria contain a large portion of the total cellular NAD (Alano et al. 2007), indicating the importance of mitochondrial pools of NAD to neuronal and astrocyte function. Several studies in the heart and brain have indicated that mitochondrial NAD can be maintained despite substantial depletion of cytoplasmic NAD (Alano et al. 2007; Yang et al. 2007; Du et al. 2003; Di Lisa et al. 2001). In neurons, enhancements in mitochondrial NAD following oxidative stress preserved mitochondrial membrane potential, enhanced respiration, and prevented the release of apoptosis- inducing factor (Du et al. 2003). Our laboratory has recently shown that IPC enhances levels of mitochondrial Nampt and NAD in the cortex by activating PKCε (Morris-Blanco et al. 2014). We showed that PKCε is involved in the translocation or maintenance of a mitochondrial specific pool of Nampt which functions to enhance levels of mitochondrial-localized NAD (Morris-Blanco et al. 2014). These increases in mitochondrial NAD may not only be important for maintaining mitochondrial bioenergetics, but also for the functioning of NAD-dependent mitochondrial-localized enzymes such as poly-ADP ribose polymerases (PARPs) and sirtuins, which are linked to cell survival following ischemic injury (Iwashita et al. 2004; Wang et al. 2011).
SIRT1 and mitochondria
Recently it has been demonstrated that the class III NAD+ dependent deacetyltransferase, sirtuin 1 (SIRT1), is required for preconditioning-mediated ischemic tolerance (Della-Morte et al. 2009; Raval et al. 2006). SIRT1 is a member of the sirtuin family of deacetylase and ADP-ribosylase enzymes which are involved in numerous cellular activities including cellular stress response, genome stability and energy metabolism (Thompson et al. 2012, 2013a; Pantazi et al. 2013; Orozco-Solis and Sassone-Corsi 2014; Chang and Guarente 2014; Morris et al. 2011). The activities of SIRT1 are primarily characterized in the nucleus while those of SIRT3, 4 and 5 are localized to the mitochondria (Morris et al. 2011). The requirement of NAD+ for sirtuin activity places the sirtuins in a position of linking the energy status of the cell to nuclear and mitochondrial signaling. Although the role of SIRT3, 4 and 5 in preconditioning-mediated ischemic tolerance is not known, there is increasing evidence that SIRT1 may play a pivotal role in protecting mitochondria from ischemic damage.
Mitochondrial physiology is finely controlled by a network of transcriptional regulatory proteins which regulate the expression of nuclear encoded mitochondrial proteins. SIRT1 controls gene expression by deacetylating both histone and non-histone proteins including numerous transcription factors such as p53, forkhead box O (FOXO), and NF-KappB (Yeung et al. 2004; Brunet et al. 2004; Cheng et al. 2003; Zhang et al. 2011). Therefore SIRT1 mediated ischemic protection may stem from the regulation of nuclear encoded mitochondrial gene expression. For example SIRT1 has emerged as a major regulator of mitochondrial biogenesis through the activation of peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), an important metabolic transcriptional co-activators of genes involved in mitochondrial metabolism (Rodgers et al. 2008; Nemoto et al. 2005; Knutti and Kralli 2001). These studies are consistent with a SIRT1 dependent regulation of mitochondrial physiology.
Recent studies by our laboratory (Thompson et al. 2013b) and Aquilano et al. (Aquilano et al. 2010) have demonstrated that SIRT1 can also localize to the mitochondria; suggesting a direct regulation of mitochondrial activity by SIRT1. Aquilano et al. (Aquilano et al. 2010) showed that in the mouse brain, liver and muscle SIRT1 was localized to the mitochondrial matrix where it interacted with mitochondrial DNA, PGC-1α and the transcription factor TFAM; suggesting a transcriptional regulatory role of SIRT1 in the mitochondria which may allow coordination of both nuclear and mitochondrial gene expression. Our laboratory has further demonstrated that mitochondrial SIRT1 protein levels and activity are increased following IPC (Thompson et al. 2013b). This increase in mitochondrial SIRT1 protein levels was only observed in neuronal cells and only in somal mitochondria. This specificity for a subpopulation of neuronal mitochondria appears to be related to the dependency of SIRT1 on HSP90, which shows a similar localization pattern, for mitochondrial import. Increases in mitochondrial SIRT1 correlated temporarily with a delayed increase in nuclear SIRT1 activity and with ischemic tolerance suggesting a protective role of mitochondrial SIRT1. In the heart, caloric restriction, a known activator of sirtuins, prime the mitochondria for ischemic stress by altering the acetylation levels of the electron transport chain proteins NADH-ubiquinone oxidoreductase 75-kDa subunit (NDUFS1) and cytochrome bc 1 complex Rieske subunit leading to a reduction in reactive oxygen species formation (ROS) (Shinmura et al. 2011). These results were mimicked by resveratrol treatment, but not by Kaempferol, which increases expression and mitochondrial localization of SIRT3 (Shinmura et al. 2011); suggesting the possibility that NDUFS1 and Rieske may be regulated by other sirtuins such as SIRT1. However, in contrast to this study our laboratory found that the respiration rate of non-synaptic mitochondria isolated from the brain of preconditioned animals, which display increased SIRT1 levels, was unaffected by acute SIRT1 inhibition (Thompson et al. 2013b). Therefore, it is unlikely that mitochondrial SIRT1, alone, is sufficient to regulate global reprogramming of the mitochondria such as has been described for SIRT3 (Hebert et al. 2013) but rather it may work in concert with mitochondrial SIRT3, 4 or 5 to impart mitochondrial ischemic tolerance.
Uncoupling proteins (UCPs)
“Coupled” mitochondrial oxidative phosphorylation is when the electron transport chain (ETC) which harvests energy from the pumping of protons across the inner mitochondrial membrane, giving rise to the proton motive force that ultimately drives ATP synthesis. Mitochondrial “uncoupling” is a process that “short circuits” oxidative phosphorylation by allowing protons to leak back into the mitochondrial matrix and in essence releases potential energy as heat. This is accomplished physiologically via the mitochondrial uncoupling proteins (UCP) family of anion-carrier proteins located on/in the inner mitochondrial membrane (reviewed in (Krauss et al. 2005)). UCPs have been identified as potential targets for ischemic tolerance.
Uncoupling was originally thought to be an artifact of mitochondrial isolation, however the discovery of UCP1 (Nicholls and Locke 1984), UCP2 (Fleury et al. 1997) and UCP3 (Boss et al. 1997), as well as the closely related UCP4 (Mao et al. 1999) and 5 (also known as BMCP1) (Sanchis et al. 1998), has led to intense investigation into the biological functions of this protein family. UCP2 is expressed in the brain and has been the most widely studied thus far. UCP2 has been implicated in regulating the speed of neurotransmission via local heat production, reducing the buffering capacity and ATP synthesis efficiency of mitochondria by decreasing mitochondrial membrane potential, induction of mitochondrial biogenesis, basal mitochondrial ROS production and pain sensation in the spinal cord, among others (an extensive review of the CNS UCPs can be found in (Andrews et al. 2005)). UCP4 and 5 also exhibit CNS expression and have been linked to similar functions such as attenuation of oxidative stress and modulation of synaptic transmission (Ramsden et al. 2012).
The exact role of UCPs and neuroprotection is unclear with evidence suggesting that activation or upregulation is protective while others suggest that inhibition or downregulation is beneficial. For example, Mattiasson et al. (Mattiasson et al. 2003) demonstrated that mice overexpressing human UCP2 are protected from transient focal ischemia, possibly through a reduction in ROS production. In a subsequent study by Deierborg et al. (Deierborg et al. 2008) UCP2 overexpression was shown to protect thalamic neurons from global cerebral ischemia. Likewise, Haines et al. (Haines et al. 2010) demonstrated that transient focal ischemia-induced injury is exacerbated in UCP2−/− mice and in a separate study confirmed previous work that UCP2 overexpression is neuroprotective (Haines and Li 2012). In both cases the authors provide evidence for a potential mechanism involving modulation of the neuroinflammatory response. On the other hand, de Bilbao and colleagues (de Bilbao et al. 2004) showed that the same UCP2−/− mice were actually resistant to ischemic injury in a model of permanent focal ischemia. Here, ischemic tolerance was attributed to enhanced antioxidant defenses in the UCP2−/− mice.
In preconditioning studies, both up and down-regulation of UCP2 have been observed. Preconditioning rat brain with sublethal ischemia both in vivo and in vitro was shown to upregulate UCP2, which was associated with protection (Mattiasson et al. 2003). Work from our laboratory determined that the IPC mimetic resveratrol decreases UCP2 expression which correlated with protection from global cerebral ischemia (Della-Morte et al. 2009). Despite the discrepancy in UCP2 regulation by preconditioning, the extent to which UCP2 is up or down regulated could produce differential effects in terms of functional benefits. Mild uncoupling (increased UCP levels) is thought to be protective by decreasing the mitochondrial membrane potential and in turn ROS production whereas coupling (decrease in UCP levels) may increase the membrane potential and possibly make utilization of energy substrates more efficient.
The disparity of these results in the contribution of UCP proteins in ischemic tolerance may be attributed to several factors. Without inducible systems to circumvent genetic compensation the effects of overexpression or deletion of UCP2 could represent a chronic adaptation to the overabundance or lack of the protein, as the authors of these studies have noted. Additionally, the expression pattern of UCP2 (as well as UCP4 and 5) differs in the mouse and the rat, perhaps accounting for some inconsistency of results across species (Alan et al. 2009). Moreover, varying models of cerebral ischemia have been utilized in these studies and while the pathophysiology is similar, transient vs permanent occlusion in the UCP2−/− mice experiments may underlie the contradictory results (Sicard and Fisher 2009). Similarly, while IPC and IPC mimetics share common pathways for ischemic tolerance, they do not completely overlap and can have separate and distinct molecular outcomes (Morris et al. 2011), which should be considered when comparing IPC and other preconditioning agents.
Further studies are warranted to explicate the role of UCPs in neuroprotection, ischemic tolerance and preconditioning. Improved techniques that allow for temporal and spatial manipulation of UCPs will greatly enhance our ability to tease apart the intricacies of their function and manipulation. Undoubtedly, expansion of our knowledge of these novel proteins will help evaluate their clinical potential in the treatment of neurological diseases.
Conclusions
Therapeutic intervention for the treatment of ischemia has proven extremely elusive and has ended in the failure of numerous clinical trials. However the body is endowed with an innate neuroprotective program against ischemic damage which is activated by mild ischemic stress. The therapeutic potential of IPC has increased interest in understanding the signaling pathways and mechanisms by which IPC mediates neuroprotection; with the ultimate goal being the pharmacological emulation of IPC in a clinical setting. Significant research indicates that mitochondrial dysfunction is a pivotal trigger in inducing cellular death pathways following ischemia. As discussed in this review, it is also becoming clear that mitochondria are both an inducer and a major target of IPC mediated neuroprotection. It appears that IPC preserves mitochondrial function through upregulation of pathways which counteract the damaging effects of ischemia on the mitochondria, such as antioxidant defense (Nrf2 and STAT's), mitochondrial biogenesis and reprogramming (SIRT1 and other sirtuin's), substrate availability (Nampt) and mitochondrial efficiency (UCP's), to name a few. Although significant steps have been made in understanding how IPC protects mitochondria against ischemic injury there is still lots to be learned about the submitochondrial targets which are differentially modulated by IPC allowing for continued normal mitochondrial activity following ischemic exposure.
Acknowledgements
This work was supported by grants from NIH/NINDS NS45676, NS054147 and NS34773 (to M.A.P.P) and NS073779 (to K.R.D.), by a NIH F31 Predoctoral award NS080344-01 (to S.V.N.) and by American Heart Association, Greater Southeast Affiliate Postdoctoral (to J.W.T) and Predoctoral (to K.B.M.) grants.
References
- Alan L, Smolkova K, Kronusova E, Santorova J, Jezek P. Absolute levels of transcripts for mitochondrial uncoupling proteins UCP2, UCP3, UCP4, and UCP5 show different patterns in rat and mice tissues. J Bioenerg Biomembr. 2009;41:71–78. doi: 10.1007/s10863-009-9201-2. [DOI] [PubMed] [Google Scholar]
- Alano CC, Tran A, Tao R, Ying W, Karliner JS, et al. Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res. 2007;85:3378–3385. doi: 10.1002/jnr.21479. [DOI] [PubMed] [Google Scholar]
- Ali YO, Ruan K, Zhai RG. NMNAT suppresses tau-induced neurodegeneration by promoting clearance of hyperphosphorylated tau oligomers in a Drosophila model of tauopathy. Hum Mol Genet. 2012;21:237–250. doi: 10.1093/hmg/ddr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, et al. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Fowler JH, Al-Mubarak B, Horsburgh K, Hardingham GE. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid Med Cell Longev. 2011a;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A 108. 2011:E1–2. doi: 10.1073/pnas.1015229108. author reply E3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Li H, Ye SQ, Ding S. Pre-B-cell colony-enhancing factor exerts a neuronal protection through its enzymatic activity and the reduction of mitochondrial dysfunction in in vitro ischemic models. J Neurochem. 2012;120:334–346. doi: 10.1111/j.1471-4159.2011.07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Plamondon H, Richelme C, Heurteaux C, Lazdunski M. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–474. doi: 10.1016/s0306-4522(00)00304-3. [DOI] [PubMed] [Google Scholar]
- Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, et al. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Busija DW, Katakam P, Rajapakse NC, Kis B, Grover G, et al. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res Bull. 2005;66:85–90. doi: 10.1016/j.brainresbull.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Centeno JM, Orti M, Salom JB, Sick TJ, Perez-Pinzon MA. Nitric oxide is involved in anoxic preconditioning neuroprotection in rat hippocampal slices. Brain Res. 1999;836:62–69. doi: 10.1016/s0006-8993(99)01610-8. [DOI] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Xu D, Lan X, Jia B, Sun L, et al. A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr Mol Med. 2013;13:1474–1484. doi: 10.2174/15665240113139990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe M, Nicolas S. Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm Des. 2006;12:739–757. doi: 10.2174/138161206775474242. [DOI] [PubMed] [Google Scholar]
- Dave KR, Saul I, Busto R, Ginsberg MD, Sick TJ, et al. Ischemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampus. J Cereb Blood Flow Metab. 2001;21:1401–1410. doi: 10.1097/00004647-200112000-00004. [DOI] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, et al. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. J Neurochem. 2004;89:1283–1292. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- Deierborg T, Wieloch T, Diano S, Warden CH, Horvath TL, et al. Overexpression of UCP2 protects thalamic neurons following global ischemia in the mouse. J Cereb Blood Flow Metab. 2008;28:1186–1195. doi: 10.1038/jcbfm.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10:2023–2033. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger H, Westphal K, Weller A, Baumann G, Stangl V, et al. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- Du L, Zhang X, Han YY, Burke NA, Kochanek PM, et al. Intramitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- Ferrari R. Metabolic disturbances during myocardial ischemia and reperfusion. Am J Cardiol. 1995;76:17B–24B. [PubMed] [Google Scholar]
- Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9:445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi JM, Batt M, Khodja RH, Le bas P. Mural thrombus of the aorta. Ann Vasc Surg. 1988;2:201–204. doi: 10.1016/S0890-5096(07)60001-6. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Haines B, Li PA. Overexpression of mitochondrial uncoupling protein 2 inhibits inflammatory cytokines and activates cell survival factors after cerebral ischemia. PLoS One. 2012;7:e31739. doi: 10.1371/journal.pone.0031739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA. Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab. 2010;30:1825–1833. doi: 10.1038/jcbfm.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, et al. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A. 2010;107:17385–17390. doi: 10.1073/pnas.1003996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraide T, Katsura K, Muramatsu H, Asano G, Katayama Y. Adenosine receptor antagonists cancelled the ischemic tolerance phenomenon in gerbil. Brain Res. 2001;910:94–98. doi: 10.1016/s0006-8993(01)02647-6. [DOI] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, et al. A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther. 2004;310:425–436. doi: 10.1124/jpet.104.066944. [DOI] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, et al. Ischemic Preconditioning Provides Neuroprotection by Induction of AMP-Activated Protein Kinase-Dependent Autophagy in a Rat Model of Ischemic Stroke. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8725-6. [DOI] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK. Antioxidant-induced INrf2 (Keap1) tyrosine 85 phosphorylation controls the nuclear export and degradation of the INrf2-Cul3-Rbx1 complex to allow normal Nrf2 activation and repression. J Cell Sci. 2012;125:1027–1038. doi: 10.1242/jcs.097295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Raval AP, Perez-Pinzon MA. Preconditioning mediated by sublethal oxygen-glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J Cereb Blood Flow Metab. 2008;28:1329–1340. doi: 10.1038/jcbfm.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12:360–365. doi: 10.1016/s1043-2760(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh CK, Lavoie HA, Dipette DJ, Singh US. Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol Pharmacol. 2011;80:446–457. doi: 10.1124/mol.111.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Huang CC, Chang KF. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. 2009;66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Mao W, Yu XX, Zhong A, Li W, Brush J, et al. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999;443:326–330. doi: 10.1016/s0014-5793(98)01713-x. [DOI] [PubMed] [Google Scholar]
- Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, et al. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. doi: 10.1161/01.cir.104.3.325. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, Perez-Pinzon MA. Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab. 2014;34:1024–1032. doi: 10.1038/jcbfm.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka H, Ohta S, Tanaka J, Toku K, Kumon Y, et al. Cytochrome c release from mitochondria to the cytosol was suppressed in the ischemia-tolerance-induced hippocampal CA1 region after 5-min forebrain ischemia in gerbils. Neurosci Lett. 2000;278:53–56. doi: 10.1016/s0304-3940(99)00894-0. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Olsen LF, Issinger OG, Guerra B. The Yin and Yang of redox regulation. Redox Rep. 2013;18:245–252. doi: 10.1179/1351000213Y.0000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Solis R, Sassone-Corsi P. Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience. 2014;264:76–87. doi: 10.1016/j.neuroscience.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- Pantazi E, Zaouali MA, Bejaoui M, Folch-Puy E, Ben Abdennebi H, et al. Role of sirtuins in ischemia-reperfusion injury. World J Gastroenterol. 2013;19:7594–7602. doi: 10.3748/wjg.v19.i43.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna C, Perrelli MG, Pagliaro P. Mitochondrial pathways, permeability transition pore, and redox signaling in cardioprotection: therapeutic implications. Antioxid Redox Signal. 2013;18:556–599. doi: 10.1089/ars.2011.4459. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–327. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Born JG. Rapid preconditioning neuroprotection following anoxia in hippocampal slices: role of the K+ATP channel and protein kinase C. Neuroscience. 1999;89:453–459. doi: 10.1016/s0306-4522(98)00560-0. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Antioxidants, mitochondrial hyperoxidation and electrical recovery after anoxia in hippocampal slices. Brain Res. 1997a;754:163–170. doi: 10.1016/s0006-8993(97)00066-8. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Xu GP, Mumford PL, Dietrich WD, Rosenthal M, et al. Rapid ischemic preconditioning protects rats from cerebral anoxia/ischemia. Adv Exp Med Biol. 1997b;428:155–161. doi: 10.1007/978-1-4615-5399-1_22. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997c;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Basit A, Dave KR, Busto R, Veauvy C, et al. Effect of the first window of ischemic preconditioning on mitochondrial dysfunction following global cerebral ischemia. Mitochondrion. 2002;2:181–189. doi: 10.1016/s1567-7249(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden DB, Ho PW, Ho JW, Liu HF, So DH, et al. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012;2:468–478. doi: 10.1002/brb3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-kappaB. J Neurochem. 2001;78:909–919. doi: 10.1046/j.1471-4159.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef A, Sperling O, Zoref-Shani E. Opening of K(ATP) channels is mandatory for acquisition of ischemic tolerance by adenosine. Neuroreport. 2000;11:463–465. doi: 10.1097/00001756-200002280-00007. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- Riepe MW, Ludolph AC. Chemical preconditioning: a cytoprotective strategy. Mol Cell Biochem. 1997;174:249–254. [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Feng ZC, Raffin CN, Harrison M, Sick TJ. Mitochondrial hyperoxidation signals residual intracellular dysfunction after global ischemia in rat neocortex. J Cereb Blood Flow Metab. 1995;15:655–665. doi: 10.1038/jcbfm.1995.81. [DOI] [PubMed] [Google Scholar]
- Rosenthal M, Mumford PL, Sick TJ, Perez-Pinzon MA. Mitochondrial hyperoxidation after cerebral anoxia/ischemia. Epiphenomenon or precursor to residual damage? Adv Exp Med Biol. 1997;428:189–195. [PubMed] [Google Scholar]
- Roth S, Dreixler JC, Shaikh AR, Lee KH, Bindokas V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest Ophthalmol Vis Sci. 2006;47:2114–2124. doi: 10.1167/iovs.05-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis D, Fleury C, Chomiki N, Goubern M, Huang Q, et al. BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. J Biol Chem. 1998;273:34611–34615. doi: 10.1074/jbc.273.51.34611. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Montes C, Imura T, Qi J, Coppola G, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, Fukuhara A, Hosogai N, Morita K, Okuno Y, et al. Visfatin in adipocytes is upregulated by hypoxia through HIF1alpha-dependent mechanism. Biochem Biophys Res Commun. 2006;349:875–882. doi: 10.1016/j.bbrc.2006.07.083. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, et al. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011;109:396–406. doi: 10.1161/CIRCRESAHA.111.243097. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Fisher M. Animal models of focal brain ischemia. Exp Transl Stroke Med. 2009;1:7. doi: 10.1186/2040-7378-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hashimoto N. 3-Nitropropionic acid induces ischemic tolerance in gerbil hippocampus in vivo. Neurosci Lett. 1999;259:9–12. doi: 10.1016/s0304-3940(98)00875-1. [DOI] [PubMed] [Google Scholar]
- Thompson JW, Narayanan SV, Perez-Pinzon MA. Redox signaling pathways involved in neuronal ischemic preconditioning. Curr Neuropharmacol. 2012;10:354–369. doi: 10.2174/157015912804143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Dave KR, Young JI, Perez-Pinzon MA. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics. 2013a;10:789–797. doi: 10.1007/s13311-013-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JW, Dave KR, Saul I, Narayanan SV, Perez-Pinzon MA. Epsilon PKC increases brain mitochondrial SIRT1 protein levels via heat shock protein 90 following ischemic preconditioning in rats. PLoS One. 2013b;8:e75753. doi: 10.1371/journal.pone.0075753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Um HC, Jang JH, Kim DH, Lee C, Surh YJ. Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide. 2011;25:161–168. doi: 10.1016/j.niox.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, et al. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Tian WW, Viollet B, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhu X, Kim Y, Li J, Huang S, et al. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012a;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, et al. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012b;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Shen F, Lin L, Zhang X, Bruce IC, et al. The neuroprotection conferred by activating the mitochondrial ATP-sensitive K+channel is mediated by inhibiting the mitochondrial permeability transition pore. Neurosci Lett. 2006;402:184–189. doi: 10.1016/j.neulet.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, et al. Nicotinamide mononucleotide, an intermediate of NAD+synthesis, protects the heart from ischemia and reperfusion. PLoS One. 2014;9:e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, et al. Nutrient-sensitive mitochondrial NAD+levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Zadori D, Klivenyi P, Szalardy L, Fulop F, Toldi J, et al. Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J Neurol Sci. 2012;322:187–191. doi: 10.1016/j.jns.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, et al. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Gan L, Vosler PS, Gao Y, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Han J, Xia W, Shi S, Liu J, et al. NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett. 2012;512:67–71. doi: 10.1016/j.neulet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Zhou AM, Li WB, Li QJ, Liu HQ, Feng RF, et al. A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neurosci Res. 2004;48:397–404. doi: 10.1016/j.neures.2003.12.010. [DOI] [PubMed] [Google Scholar]