Abnormal decision-making is a central feature of both behavioural and semantic variants of frontotemporal dementia. Chiong et al. present evidence for a double dissociation in which the behavioural variant is associated with diminished loss aversion, while the semantic variant is associated with increased discounting of delayed rewards.
Keywords: frontotemporal dementia, semantic dementia, behavioural neurology, computational psychiatry, impulsivity and inhibition disorders
Abnormal decision-making is a central feature of both behavioural and semantic variants of frontotemporal dementia. Chiong et al. present evidence for a double dissociation in which the behavioural variant is associated with diminished loss aversion, while the semantic variant is associated with increased discounting of delayed rewards.
Abstract
Many neuropsychiatric disorders are marked by abnormal behaviour and decision-making, but prevailing diagnostic criteria for such behaviours are typically qualitative and often ambiguous. Behavioural variant frontotemporal dementia and semantic variant primary progressive aphasia (also called semantic dementia) are two clinical variants of frontotemporal dementia with overlapping but distinct anatomical substrates known to cause profound changes in decision-making. We investigated whether abnormal decision-making in these syndromes could be more precisely characterized in terms of dissociable abnormalities in patients’ subjective evaluations of valence (positive versus negative outcome) and of time (present versus future outcome). We presented 28 patients with behavioural variant frontotemporal dementia, 14 patients with semantic variant primary progressive aphasia, 25 patients with Alzheimer’s disease (as disease controls), and 61 healthy older control subjects with experimental tasks assaying loss aversion and delay discounting. In general linear models controlling for age, gender, education and Mini-Mental State Examination score, patients with behavioural variant frontotemporal dementia were less averse to losses than control subjects (P < 0.001), while patients with semantic variant primary progressive aphasia discounted delayed rewards more steeply than controls (P = 0.019). There was no relationship between loss aversion and delay discounting across the sample, nor in any of the subgroups. These findings suggest that abnormal behaviours in neurodegenerative disease may result from the disruption of either of two dissociable neural processes for evaluating the outcomes of action. More broadly, these findings suggest a role for computational methods to supplement traditional qualitative characterizations in the differential diagnosis of neuropsychiatric disorders.
Introduction
Frontotemporal dementia (FTD) comprises three clinical variants with linked aetiologies that cause progressive degeneration of the frontal and temporal lobes. The most common clinical variant, behavioural variant FTD, is marked by dramatic changes in personality and behaviour. Behavioural variant FTD is associated with greater atrophy in frontal and insular regions than in the temporal regions. A second clinical variant, semantic variant primary progressive aphasia (PPA), also called semantic dementia, is classified as a language disorder but is also recognized to cause similar changes in personality and behaviour. Semantic variant PPA is associated with greater atrophy in temporal regions than in frontal and insular regions.
As with many neuropsychiatric disorders, the abnormal behaviours observed in these subtypes of FTD are largely characterized in a qualitative fashion. Clinicians usually rely upon third-party report rather than quantitative measurement or computational modelling to describe patients’ behaviour. For example, among the recent international consensus diagnostic criteria for behavioural variant FTD are ‘impulsive, rash or careless actions’ (Rascovsky et al., 2011). The ambiguity and subjectivity of these qualitative descriptors may contribute to high rates of community misdiagnosis. In our centre, 50.7% of patients with behavioural variant FTD were initially misdiagnosed in the community as having a primary psychiatric disorder (Woolley et al., 2011), while 60.0% of patients referred by community physicians for suspected behavioural variant FTD did not meet criteria for behavioural variant FTD. Unlike cognitive operations such as memory or executive function, there are no standard clinical tests for decision-making impairments in neuropsychiatric disorders. As a result, clinicians also lack tools for tracking the progression of such impairments, and have no method for prospectively identifying patients at greatest risk of serious harms such as overspending, financial exploitation, and fraud (Chiong et al., 2014).
Here we take a quantitative approach to characterizing these differences using neuroeconomic tools. Neuroeconomics is an emerging interdisciplinary field that combines insights from economics, psychology and neuroscience to examine neural mechanisms underlying decision-making (Zak, 2004; Loewenstein et al., 2008). Ongoing work in neuroeconomics supports the view that human decisions can be understood in terms of individuals’ subjective evaluations of the possible outcomes of their actions; and furthermore, that these evaluations are computed primarily in prefrontal/striatal and limbic brain networks known to be specifically targeted in FTD. Together, these findings suggest that abnormal decision-making in behavioural variant FTD and semantic variant PPA results in part from the targeted disruption of brain systems responsible for computing evaluations of action outcomes.
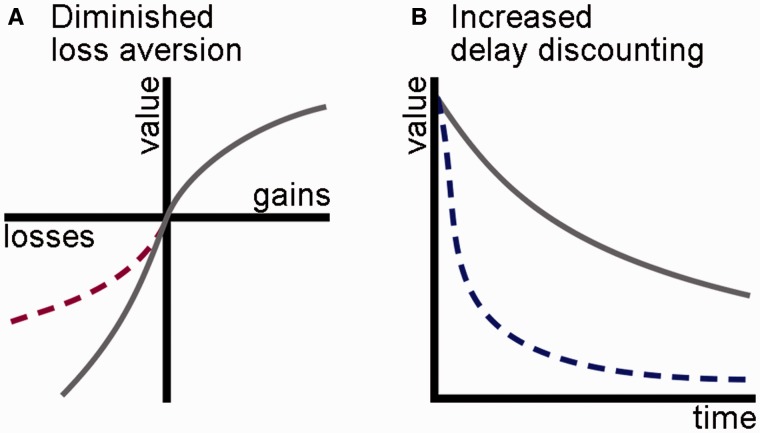
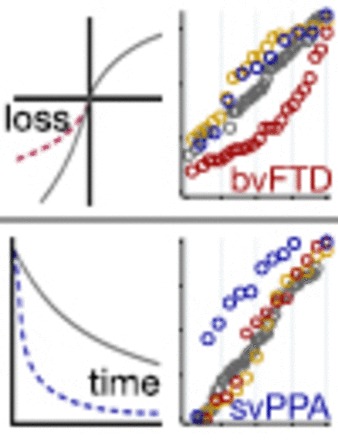
We addressed how abnormalities in two sets of cognitive processes may underlie decision-making impairments observed in these variants of FTD; of note, these two models need not be mutually exclusive, nor exhaustive. The first concerns changes in patients’ evaluation of positive and negative valence, while the second concerns changes in their evaluation of present and future time. On the first model patients’ ‘impulsive, rash or careless actions’ may reflect diminished sensitivity to negative outcomes (Fig. 1A). Healthy subjects are more strongly motivated to avoid potential losses than to achieve potential gains, a phenomenon termed loss aversion (Kahneman and Tversky, 1979; Trepel et al., 2005). As one example, healthy people typically reject a gamble that offers a 50% chance of winning $100 and a 50% chance of losing $100, as a loss of $100 is evaluated more negatively than a gain of $100 is evaluated positively. Instead, on average healthy subjects will only enter such 50-50 mixed gambles if the amount that could be won is at least 1.5-times the amount that could be lost; the threshold win-loss ratio at which an individual subject becomes willing to bet is termed lambda. If this normal aversion to losses is diminished in patients, they would be more likely than healthy subjects to risk monetary losses or other negative consequences.
Figure 1.
Two mechanisms for change in patients’ evaluation of action outcomes. (A) While healthy subjects are more strongly affected by prospective losses than gains (represented by the steeper slope of the grey curve on the negative side of the x-axis), this loss aversion may be diminished in patients (represented by the shallower slope of the dashed red curve). (B) While healthy subjects discount the value of rewards that occur later in time (represented by the downward slope of the grey curve), delay discounting may be increased in patients (represented by the steeper slope of the dashed blue curve).
A second cognitive alteration that could underlie patients’ ‘impulsive, rash or careless actions’ is diminished sensitivity to future outcomes (Fig. 1B). Healthy people subjectively discount rewards that are delayed in time; for example, a healthy person might prefer to receive $100 today over receiving $150 in 1 year (Samuelson, 1936; Ainslie, 1975; Peters and Buchel, 2011). If patients discount more steeply over such delays than healthy people, they would be more likely to forego important long-term goals for the sake of minor immediate rewards. Other clinical populations with impaired judgement such as addicts (Kirby et al., 1999), compulsive gamblers (Dixon et al., 2006), and patients with structural orbitofrontal damage (Sellitto et al., 2010) demonstrate such increases in delay discounting, while in healthy subjects increased delay discounting is associated with important health-related behavioural traits such as increased body-mass index, lack of physical exercise and smoking (Chabris et al., 2008; Sutter et al., 2013).
In these experiments, we used established neuroeconomic tasks to explore differences in loss aversion and delay discounting in patients with behavioural variant FTD and semantic variant PPA relative to healthy older controls as well as patients with Alzheimer’s disease. We hypothesized that impaired sensitivity to valence and impaired sensitivity to time would have dissociable effects on behavioural variant FTD and semantic variant PPA patients’ decision-making.
Materials and methods
Patients and control subjects
Twenty-eight patients with behavioural variant FTD, 14 patients with semantic variant PPA, 25 patients with Alzheimer’s disease (as disease controls), and 61 healthy control subjects were recruited for the study. The semantic variant PPA cohort included patients with both predominantly left-lateralized and predominantly right-lateralized anterior temporal atrophy, and the laterality of semantic variant PPA patients was characterized by brain MRI. All patients were diagnosed by consensus among a multidisciplinary team of neurologists, neuropsychologists and nurses after a comprehensive evaluation including a clinical history, neurological examination and extensive neuropsychological testing according to established research criteria (Gorno-Tempini et al., 2011; McKhann et al., 2011; Rascovsky et al., 2011). Healthy older subjects were verified as normal on the basis of a clinical interview, neurological examination and neuropsychological testing. Patients were recruited in early stages of illness because of the cognitive demands of the decision-making tasks; in addition, control conditions (described below) were used in each task to exclude subjects with uninterpretable data. Due to time constraints and subject fatigue, not all subjects completed both experimental tasks, and some subjects were excluded for poor control condition performance in one task and not the other. Demographic, clinical and neuropsychological data for the patients and control subjects included in the behavioural analyses are summarized in Table 1.
Table 1.
Demographic, clinical and neuropsychological characteristics of study cohorts
| Experiment 1: Loss aversion |
Experiment 2: Delay discounting |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 58) | Alzheimer’s disease (n = 21) | bvFTD (n = 25) | svPPA (n = 10)a | F-test P | Control (n = 54) | Alzheimer (n = 14) | bvFTD (n = 14) | svPPA (n = 13)b | F-test P | |
| Gender (M/F) | 32/26 | 10/11 | 14/11 | 7/3 | 0.72 | 27/27 | 6/8 | 6/8 | 7/6 | 0.91 |
| Age (y) | 68.2 (7.4) | 65.7 (11.1) | 64.0 (7.9) | 64.3 (6.4) | 0.13 | 67.6 (7.1) | 63.0 (9.8) | 62.3 (6.6) | 65.2 (6.1) | 0.041 |
| Education (y) | 17.8 (1.9) | 18.3 (4.1) | 16.9 (3.4) | 17.1 (4.1) | 0.39 | 17.7 (1.9) | 17.4 (2.7) | 16.6 (3.5) | 16.3 (3.7) | 0.24 |
| MMSE (30) | 29.3 (1.0) | 21.7 (5.7) | 25.6 (3.0) | 25.5 (2.5) | <0.0001 | 29.4 (1.0) | 19.8 (7.6) | 26.3 (3.0) | 23.6 (4.0) | <0.0001 |
| Clinical Dementia Rating Total | 0.0 (0.1) | 0.9 (0.4) | 1.3 (0.5) | 0.7 (0.4) | <0.0001 | 0.0 (0.1) | 0.9 (0.4) | 1.2 (0.5) | 0.7 (.5) | <0.0001 |
| Clinical Dementia Rating sum of boxes | 0.0 (0.2) | 5.0 (2.7) | 7.0 (2.6) | 4.0 (2.8) | <0.0001 | 0.0 (0.1) | 5.3 (2.3) | 6.5 (2.7) | 4.2 (2.8) | <0.0001 |
| Memory: Modified Rey-Osterreith Delay (17) | 12.5 (3.1) [43/58] | 4.0 (4.0) [19/21] | 8.7 (4.4) [25/25] | 7.9 (2.6) [10/10] | <0.0001 | 12.5 (3.0) [41/54] | 3.6 (3.0) [12/14] | 8.6 (4.2) [14/14] | 7.6 (3.1) [12/13] | <0.0001 |
| Language: Boston naming test (15) | 14.6 (0.7) [42/58] | 11.6 (2.8) [20/21] | 13.7 (1.1) [25/25] | 5.9 (3.6) [9/10] | <0.0001 | 14.7 (0.7) [45/54] | 10.8 (3.2) [12/14] | 13.6 (1.2) [14/14] | 4.5 (3.5) [11/13] | <0.0001 |
| Executive: Digit span backwards | 5.6 (1.4) [57/58] | 3.7 (1.6) [20/21] | 4.0 (1.1) [25/25] | 5.0 (0.9) [10/10] | <0.0001 | 5.6 (1.4) [54/54] | 2.9 (1.7) [13/14] | 3.9 (1.0) [14/14] | 4.8 (1.3) [12/13] | <0.0001 |
| Executive: Modified trails time (s) | 23.6 (9.1) [57/58] | 80.6 (38.4) [20/21] | 64.4 (40.3) [24/25] | 37.9 (11.9) [10/10] | <0.0001 | 23.3 (9.2) [54/54] | 92.8 (30.2) [12/14] | 56.6 (44.5) [13/14] | 41.0 (11.4) [12/13] | <0.0001 |
| Executive: letter fluency | 17.1 (4.6) [57/58] | 9.4 (5.7) [20/21] | 9.6 (6.1) [25/25] | 9.8 (4.9) [10/10] | <0.0001 | 17.4 (4.5) [54/54] | 8.1 (4.9) [13/14] | 9.1 (5.3) [14/14] | 8.9 (3.5) [12/13] | <0.0001 |
| Emotion: Affect matching (16) | 12.5 (1.8) [37/58] | 12.3 (1.9) [20/21] | 10.2 (3.1) [25/25] | 10.6 (2.1) [10/10] | 0.0007 | 12.7 (1.7) [36/54] | 12.4 (1.6) [11/14] | 10.4 (3.4) [14/14] | 10.2 (2.1) [12/13] | 0.0007 |
Values represent mean (SD). For neuropsychological tests, numbers in square brackets represent the number of subjects for whom data are available.
aFour right-predominant.
bSix right-predominant.
All participants or their legally authorized representatives gave written informed consent according to the Declaration of Helsinki, and the study was approved by the Committee on Human Research at UCSF.
Experiment 1: Loss aversion
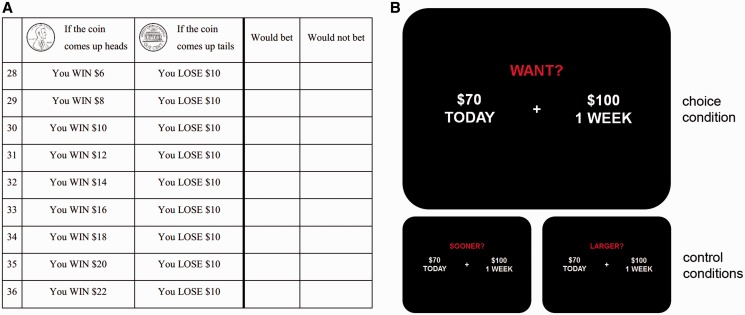
In the loss aversion task, subjects were endowed with $30 and then presented with a series of four choice titrators. Each titrator presented a series of nine potential gambles for which subjects indicated their willingness or unwillingness to bet, corresponding to win-loss ratios ranging from 0.6 to 2.2 at intervals of 0.2 (Fig. 2A). This range was chosen because in previous studies the mean lambda in healthy subjects was typically ∼1.5. In two titrators the potential gains were fixed with varying potential losses determined by the series of win-loss ratios, while in two titrators the potential losses were fixed with varying potential gains; also, in two titrators the gambles were presented in order of ascending win-loss ratio, while in two titrators the gambles were presented in order of descending win-loss ratio. After subjects decided whether or not to bet on each of the 36 possible gambles, a number from 1–36 was drawn from a box and the gamble corresponding to the chosen number was played by flipping a coin. If the subject had decided not to bet on the chosen gamble, he or she would keep the $30 endowment, while if the subject had decided to bet on the chosen gamble, his or her endowment was increased or reduced depending on the outcome of the coin flip.
Figure 2.
Neuroeconomic tasks to assess sensitivity to valence and time. (A) One of four choice titrators used in the loss aversion paradigm. (B) Choice condition of interest, and control conditions, in the delay discounting paradigm.
Lambda was calculated as the win-loss ratio at the midpoint between where the subject switched from being unwilling to being willing to gamble, and was averaged across the four titrators. As a control condition to ensure comprehension of the task, subjects were excluded from the primary analysis if on two or more trials no interpretable switch point could be identified (e.g. if a subject rejected a gamble with a high win-loss ratio while accepting a gamble with a lower win-loss ratio in the same series, or if a subject always gambled or always refrained from gambling). Excluded subjects were included in a follow-up sensitivity analysis (assigning a lambda value of 0.6 for titrators in which subjects always gambled, a lambda value of 2.2 for titrators in which subjects never gambled, and no lambda value if switches were uninterpretable).
Experiment 2: Delay discounting
We modified a delay discounting task that has been previously described (Boettiger et al., 2007; Kayser et al., 2012). In this task, subjects were presented with 128 hypothetical choices between smaller immediate rewards ($3–90) and larger rewards ($5–100) delayed between 1 week and 6 months (Fig. 2B). These two options were randomly assigned to the left and right sides of a computer screen, and subjects pressed a button to indicate a preference for either the left or right option. Stimuli were presented and responses were recorded using E-prime (Psychology Software Tools, Inc., Pittsburgh, PA). The behavioural outcome of interest was the impulsive choice ratio (ICR), representing the proportion of choices in which the subject selected the smaller immediate reward rather than the larger delayed reward. Unlike other measures of delay discounting such as the hyperbolic discount rate or parameters derived from quasi-hyperbolic models, the ICR is a simple proportion of impulsive choices that requires no assumptions about the shape of the discount function (Kayser et al., 2012). Changes in delay discounting due to neurological disease could manifest either as shifts of this function or as distortions of its shape. The ICR is a measure of delay discounting that accommodates both possibilities; in previous studies the ICR has been strongly correlated with the hyperbolic discount rate (Boettiger et al., 2007; Kayser et al., 2012; Lebreton et al., 2013).
Given the semantic and cognitive demands of this computer-based task, we included two control conditions with 10 trials each. In the first control condition, instead of asking which of two choices the subject would prefer, we asked which of two choices would pay sooner than the other. In the second control condition, we asked which of two choices would pay a larger payment. In these trials options were also randomly assigned to the left and right sides of the screen, and control condition trials were randomly interspersed with trials of interest. Only participants correctly answering at least 8 of 10 questions in each control condition were included in the analysis. Excluded subjects were then included in a follow-up sensitivity analysis (using responses entered by excluded subjects in the condition of interest).
Neuroimaging acquisition
Neuroimaging data were collected on a Siemens 3 T Trio scanner. A T1-weighted 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence was acquired with the following parameters: 160 sagittal slices; slice thickness =1 mm; field of view = 256 × 256 mm; matrix = 230 × 256; repetition time = 2300 ms; echo time = 2.98 ms; flip angle = 9°.
Between-group voxel-based morphometry
To identify regional atrophy in the behavioural variant FTD and semantic variant PPA groups, voxel-based morphometry (VBM) preprocessing and analyses were performed using the SPM8 (Wellcome Department of Cognitive Neurology, London) and VBM8 (Christian Gaser, University of Jena) software packages running on MATLAB R2013a (MathWorks, Natick, MA). We compared control subjects to patients with behavioural variant FTD and semantic variant PPA who successfully completed either the loss aversion or the delay discounting task, or both. Image volumes were inspected for quality both visually and using the VBM8 check sample homogeneity function. Intersubject registration was performed with the Diffeomorphic Anatomical Registration Through Exponential Lie algebra (DARTEL) procedure (Ashburner, 2007) by warping each subject’s image to a template created from 50 healthy older control subjects from our centre. Spatially normalized, segmented and modulated grey matter images were smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel. Subjects with behavioural variant FTD and semantic variant PPA were compared with control subjects, covarying out age, gender and total intracranial volume (to account for individual differences in head size). Differences in grey matter volumes at each voxel between each disease group and control subjects were assessed using the general linear model, and statistical maps from each model were corrected for multiple comparisons at P < 0.05 based on family-wise error across the whole brain.
Statistical behavioural analysis
For each of the two experimental neuroeconomic parameters measured (lambda and ICR), we constructed a general linear model with diagnosis and gender as categorical predictors and with age, educational attainment (in years), and Mini-Mental Status Examination score (MMSE, a proxy for disease severity) as interval predictors. For Experiment 1, lambda was modelled with a Gaussian distribution and an identity link; for Experiment 2, ICR (a proportion between 0 and 1) was modelled with a binomial distribution and a logit link. To ensure the robustness of our statistical analyses given the empirical distributions of lambda and ICR, we calculated P-values and bias-corrected percentile confidence intervals with bootstrap analyses using 1000 replications.
In addition, to explore the relationship between the two parameters (loss aversion and delay discounting) at the individual level, Spearman rank correlation of lambda and ICR values was performed for the 86 subjects who successfully completed both tasks. Statistical analyses of behavioural results were conducted in Stata 12.1 (StataCorp, College Station, TX); a two-tailed P-value of <0.05 was considered significant.
Neuropsychological mediation analysis
To explore the relationship between traditional neuropsychological measures and new neuroeconomic tasks, we constructed a series of mediation models to test whether the influence of diagnosis on lambda and ICR is mediated by performance on conventional measures of delayed memory, language, executive function, or emotion recognition (Supplementary Fig. 1). These models are further detailed in the Supplementary material.
Brain structure–behaviour correlations in voxel-based morphometry
Preprocessed grey matter images described above in the between-group VBM analysis were used in two separate structural correlation analyses for loss aversion and delay discounting, implemented in SPM8 with covariates added for age, gender, total intracranial volume, and MMSE score. Associations between predictors of interest and grey matter volumes at each voxel were assessed using the general linear model, and statistical maps from each model were thresholded at voxelwise P < 0.005 and then corrected for multiple comparisons at P < 0.05 based on cluster extent according to Gaussian random field theory.
Results
Control condition performance
In Experiment 1, 120 subjects were presented with the loss aversion task, out of which six were excluded (two controls, two patients with behavioural variant FTD, and two patients with semantic variant PPA) because switch points could not be identified; in all but one case this issue was due to the subject either betting on all possible gambles or betting on no possible gambles. In Experiment 2, 116 subjects were presented with the delay discounting task, out of which 21 were excluded (four controls, 10 patients with behavioural variant FTD, one patient with semantic variant PPA, and six patients with Alzheimer’s disease) due either to inability to complete the task or to poor performance in the control conditions. Altogether, 86 subjects (52 controls, 11 patients with Alzheimer’s disease, 14 patients with behavioural variant FTD, and nine patients with semantic variant PPA) successfully completed both tasks.
Group voxel-based morphometry
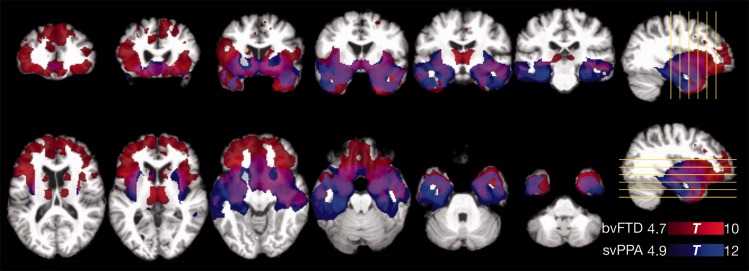
Consistent with the clinical diagnoses, the subject groups demonstrated distinct but overlapping patterns of atrophy in VBM analyses. Comparing the behavioural variant FTD cohort to the control cohort, a predominantly bifrontal and limbic pattern of atrophy was observed, with statistical peaks in the right and left anterior insula, ventral striatum, left anterior cingulate/superior frontal gyrus, and medial thalami. Comparing the semantic variant PPA cohort to the control cohort, a predominantly bitemporal and limbic pattern of atrophy was observed, with statistical peaks in the left and right temporal pole, left and right amygdala/hippocampal head, and left and right inferior temporal lobe. Substantial overlap was observed in the two patterns of atrophy, particularly in the temporal poles, anterior insula, amygdala, ventral striatum and ventromedial prefrontal cortex (Fig. 3).
Figure 3.
VBM of brain atrophy in behavioural variant FTD patients compared to controls (red), and in semantic variant PPA patients compared to controls (blue). Images are displayed according to neurological convention (right = right). bvFTD = behavioural variant FTD; svPPA = semantic variant PPA.
Experiment 1: Diminished loss aversion in behavioural variant FTD, but not in semantic variant PPA or Alzheimer’s disease
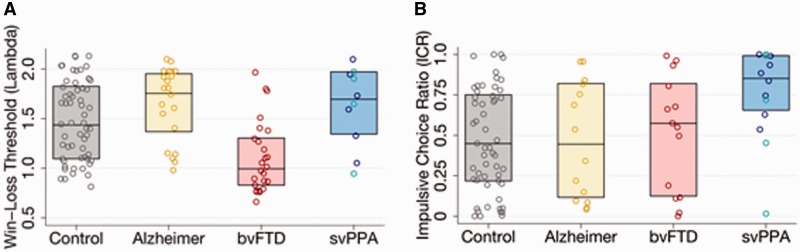
Among healthy older control subjects, the mean lambda was 1.48 [95% confidence interval (CI) = 1.38–1.58], indicating that control subjects generally refrained from 50-50 mixed gambles unless the amount that could be won was at least 1.48-times the amount that could be lost. In patients with behavioural variant FTD the mean lambda was 1.12 (95% CI = 0.99–1.27), while in patients with semantic variant PPA it was 1.63 (95% CI = 1.37–1.85), and in patients with Alzheimer’s disease it was 1.66 (95% CI = 1.50–1.82) (Fig. 4A). In a general linear model controlling for age, gender, education, and MMSE score, lambda values for patients with behavioural variant FTD were significantly lower than those of controls (beta = −0.43, P < 0.001, 95% CI = −0.62–0.24), while lambda values for patients with semantic variant PPA (beta = 0.10, P = 0.45) and Alzheimer’s disease (beta = 0.10, P = 0.38) tended to be higher than those of controls, though these differences were not statistically significant. Educational attainment was negatively associated with lambda (beta = −0.028, P = 0.023, 95% CI = −0.49–0.00), while none of the other covariates were independently associated with lambda in the model. In addition, the difference in lambda values between patients with behavioural variant FTD and patients with semantic variant PPA was also statistically significant (P < 0.001, 95% CI = 0.22–0.79). Finally, in a planned sensitivity analysis including the two controls, two patients with behavioural variant FTD, and two patients with semantic variant PPA previously excluded for inability to identify a switch point, these associations remained significant.
Figure 4.
Neuroeconomic behaviour by diagnosis. (A) Distribution of lambda (win-loss ratio threshold) values in four cohorts. In the semantic variant PPA (svPPA) cohort, subjects with predominantly left-lateralized anterior temporal atrophy are represented in blue, while subjects with predominantly right-lateralized anterior temporal atrophy are represented in turquoise. Boxes represent interquartile range. (B) Distribution of ICR (the proportion of trials selecting the smaller immediate reward over the larger delayed reward) in four cohorts. Boxes represent interquartile range.
Experiment 2: Increased delay discounting in semantic variant PPA, but not in behavioural variant FTD or Alzheimer’s disease
Among healthy older controls, the mean ICR was 0.46 (95% CI = 0.37–0.54), indicating that control subjects chose the smaller immediate reward over the larger delayed reward nearly half the time. In patients with semantic variant PPA the mean ICR was 0.75 (95% CI = 0.59–0.87), while in patients with behavioural variant FTD it was 0.51 (95% CI = 0.32–0.67) and in patients with Alzheimer’s disease it was 0.47 (95% CI = 0.30–0.64) (Fig. 4B). In a general linear model controlling for age, gender, education, and MMSE score, ICR values for patients with semantic variant PPA were significantly higher than those of controls (beta = 1.53, P = 0.019, 95% CI = 0.28–2.85), while ICR values for patients with behavioural variant FTD (beta = 0.30, P = 0.56) and Alzheimer’s disease (beta = 0.61, P = 0.42) did not differ significantly from those of controls. Educational attainment was negatively associated with ICR (beta = −0.12, P = 0.05, 95% CI = −0.23 to −0.003), while none of the other covariates were independently associated with ICR in the model. The difference in ICR values between patients with semantic variant PPA and patients with behavioural variant FTD neared statistical significance (P = 0.080, 95% CI = −0.14–2.61). Finally, in a planned sensitivity analysis including the four controls, 10 patients with behavioural variant FTD, one patient with semantic variant PPA, and six patients with Alzheimer’s disease previously excluded for poor performance on the control conditions these associations remained significant. Among patients with semantic variant PPA included in the primary analysis, there was one outlier whose task behaviour did not fit the overall pattern observed in this diagnostic group (Fig. 4B); further clinical characterization of this patient is provided in the Supplementary material.
Individual-level behavioural relationships between loss aversion and delay discounting
Given the syndromic dissociation found between loss aversion and delay discounting across two variants of FTD, we examined whether there were associations between these two phenomena at the individual level. Among 86 subjects who successfully completed both tasks, there was no relationship between lambda and ICR values (Spearman’s ρ = 0.021, P = 0.85); in subgroup analyses there was also no significant relationship between lambda and ICR values in any of the four cohorts.
Associations with traditional neuropsychological measures
While performance on traditional measures of delayed memory, language, executive function and emotion recognition was significantly associated with diagnosis, we did not find evidence that these cognitive operations mediate the association between behavioural variant FTD diagnosis and diminished loss aversion, or the association between semantic variant PPA diagnosis and increased delay discounting. Further details from these mediation analyses are provided in the Supplementary material.
Brain structure–behaviour voxel-based morphometry
For both the loss aversion and delay discounting tasks, no regional grey matter volumes were significantly associated with task performance after testing for multiple comparisons based on cluster extent (Supplementary Tables 1 and 2).
Discussion
We present evidence for a double dissociation between loss aversion and delay discounting in two variants of FTD. Behavioural variant FTD is associated with diminished loss aversion but does not affect performance on a delay discounting task; while semantic variant PPA is associated with increased delay discounting but does not affect performance on a loss aversion task. At an individual level across the entire sample as well as within each diagnostic group, there is no association between loss aversion and delay discounting. Finally, in a neuropsychological mediation analysis we did not find evidence that these alterations in decision-making are accounted for by standard neuropsychological measures. These findings have implications for the quantitative characterization of behavioural abnormalities in neurodegenerative disease. More broadly, these findings suggest a role for computational methods, alongside more traditional qualitative characterization, in the differential diagnosis of neuropsychiatric disorders.
Impulsive (short-sighted) choice in semantic variant PPA
While semantic variant PPA is often clinically characterized as a language disorder with loss of word and object meaning (especially in patients with predominantly left rather than right anterior temporal degeneration), it has long been recognized that patients with semantic variant PPA undergo behavioural changes similar to those observed in behavioural variant FTD. The extent of the behavioural similarities and differences between these two clinical variants of FTD are still incompletely understood. As one established behavioural difference, behavioural variant FTD has been associated with gluttony and indiscriminate eating, while semantic variant PPA has been associated with restrictive eating patterns centred on a preferred food item or class of food (Snowden et al., 2001).
Early case reports of semantic variant PPA also noted behavioural abnormalities suggesting impairments in time perception and organization, such as ‘clock-watching’ behaviour and rigid adherence to fixed routines (Snowden et al., 1989, 2001). More recent work suggests that patients with semantic variant PPA have specific deficits in projecting into the future (as opposed to remembering the past) (Duval et al., 2012; Irish et al., 2012a, b), which may be associated with an increased risk of suicide associated with this diagnosis (Hsiao et al., 2013; Sabodash et al., 2013). One interpretation that may unify these observations is that patients with semantic variant PPA have deficits in projecting themselves into future states of affairs, which reduces their subjective valuation of future states of affairs and biases their decisions towards present over delayed outcomes. Supporting this interpretation, eliciting future thoughts in healthy subjects reduces delay discounting, and the magnitude of this effect is predicted by neural coupling between the anterior cingulate cortex and hippocampus/amygdala (Peters and Buchel, 2010). In a diffusion tensor imaging study, less impulsive choice was associated with higher fractional anisotropy and lower mean diffusivity in frontal and temporal white matter tracts, with the largest cluster in the left inferior fronto-occipital and inferior longitudinal fasciculi extending through the left inferior and superior temporal gyri, parahippocampal gyrus, and fusiform cortex (Olson et al., 2009).
We note that variability in task performance among normal subjects was high (Fig. 4B). The range of choices in our task was derived from earlier studies that sought to model variability among subjects without neurological disease (Boettiger et al., 2007; Kayser et al., 2012); thus, our version of the task may not have been optimally designed to capture the full range of variability due to neuropathology. In future studies, trials featuring a broader range of choices (for instance, with more extreme cost penalties for choosing the smaller immediate option) may better discriminate among diagnostic groups.
Insensitivity to losses in behavioural variant FTD
From the earliest symptomatic stages of disease, patients with behavioural variant FTD often make disastrous judgements that display remarkable insensitivity to negative future consequences (Chiong et al., 2014). Patients in our behavioural variant FTD cohort were less averse to losses, but were not more impulsive (short-sighted) than controls. Of note, our findings in delay discounting differ from those in another recent study, in which patients with behavioural variant FTD were reported to be more impulsive than controls (Lebreton et al., 2013). An important difference between the studies was our use of two control conditions to ensure semantic comprehension for differences in reward magnitude and differences in reward timing, which may also have restricted our behavioural variant FTD patient cohort to those in the very early stages of disease (mean MMSE 26.3 ± 3.0, as compared with 22.9 ± 5.2 in Lebreton et al., 2013). One interpretation is that the Lebreton et al. (2013) behavioural variant FTD cohort may have included patients with more advanced disease who did not understand the task; however, random task performance would be expected to drive the ICR towards 0.5 rather than towards impulsivity, and in this study behavioural variant FTD patient performance was not much more variable than healthy control performance. An alternative interpretation is that delay discounting is not abnormal in very early stages of behavioural variant FTD but is affected as the disease progresses (potentially as neurodegeneration spreads to involve more brain regions overlapping with semantic variant PPA).
Taken together, Experiments 1 and 2 suggest that impaired judgements in very early behavioural variant FTD reflect a specific insensitivity to negative consequences, rather than insensitivity to future consequences. This finding illustrates how neuroeconomic experimental paradigms can dissect mechanisms underlying clinical observations of patient behaviour by distinguishing among different factors (such as negative or positive valence versus present or future time) present in everyday decisions.
Findings in Alzheimer’s disease and clinical concordance
In this study we recruited patients with Alzheimer’s disease as disease controls to facilitate comparison with these two variants of FTD. Of note, while patients with behavioural variant FTD had diminished loss aversion compared to normal controls, patients with Alzheimer’s disease had a tendency to increased loss aversion compared to normal controls, though this was not statistically significant (lambda 1.66 versus 1.48, P = 0.38). This tendency is consistent with a chart review of behavioural variant FTD and Alzheimer’s disease in which patients with behavioural variant FTD made more real-world financial errors consistent with diminished sensitivity to losses (such as risky investments, shoplifting, and excessive loans, 36% in behavioural variant FTD versus 0% in Alzheimer’s disease), but in which patients with Alzheimer’s disease made more financial errors consistent with exaggerated sensitivity to losses (such as paranoia about theft or hiding valuables, 6% in behavioural variant FTD versus 9% in Alzheimer’s disease) (Chiong et al., 2014). These experimental and clinical findings suggest further examination of exaggerated loss aversion in Alzheimer’s disease.
Implications for computational neuropsychiatry
These experiments also illustrate that qualitative characterizations applied in the diagnosis of neuropsychiatric disorders (such as the ‘impulsive, rash, or careless actions’ of behavioural variant FTD) can be mechanistically ambiguous, encompassing abnormalities in distinct computations that are realized in dissociable neural systems. In the case of FTD, abnormal decision-making observed by clinicians could plausibly result either from diminished sensitivity to negative valence or from diminished sensitivity to rewards delayed in time. In two distinct experimental paradigms, patients with one clinical variant of FTD demonstrate the former abnormality, while patients with a second clinical variant demonstrate the latter. While our study was not designed to establish the clinical utility of these two particular neuroeconomic tasks, our study does demonstrate in principle that modelling disease-related change in patients’ evaluations of different features of action outcomes (such as valence and time) may resolve ambiguities inherent in prevailing symptom-based, qualitative diagnostic schemas that often do not align with underlying mechanisms (Insel et al., 2010).
Limitations
The primary limitation of our study is the small number of patients who were able to successfully complete the decision-making tasks, as the cognitive demands of the tasks limited recruitment to patients in the earliest stages of disease. This limitation was most evident in the case of semantic variant PPA, given both the rarity of the disease and the semantic demands of the decision-making tasks themselves. Anticipating concerns about the ability of patients to understand the decisions that we presented to them, we adopted relatively demanding control conditions for each task (especially for the delay discounting task) that may have been more cognitively demanding than the task of interest, and so may have excluded subjects who would have been able to provide interpretable data. However, in sensitivity analyses including excluded subjects, our findings were unchanged.
Problems due to small sample size and resultant low power are increasingly recognized in neuroscience (Button et al., 2013); however, study power is a function of both sample size and effect size (Ioannidis, 2005). Abnormal economic decision-making is a common and characteristic feature of FTD, and prior clinical work supports the application of a neuroeconomic conceptual framework to model these impairments (Chiong et al., 2014). Thus, while this application of neuroeconomic tasks is novel, the present study is one in which large effects could be anticipated and in which the prior likelihood of rejecting both null hypotheses was high.
Supplementary Material
Acknowledgements
We thank Samuel Gong, Elizabeth Epstein, Ivy Huang and Roxanne Moslehi for assistance with image preprocessing and analysis; Katherine P. Rankin for guidance on the imaging analyses; and our healthy volunteers, patients, and patients’ families for their generous participation in research.
Glossary
Abbreviations
- FTD
frontotemporal dementia
- ICR
impulsive choice ratio
- MMSE
Mini-Mental State Examination
- PPA
primary progressive aphasia
- VBM
voxel-based morphometry
Funding
This work was supported by the National Institutes of Health (NIA K23AG043553, NIA R01AG022983, NCATS KL2TR000143, NIA P01AG019724, NIA P50AG023501, NIA R01AG032306, NIMH R01MH098023) and the Larry Hillblom Foundation.
Supplementary material
Supplementary material is available at Brain online.
References
- Ainslie G. Specious reward—behavioral theory of impulsiveness and impulse control. Psychol Bull 1975;82: 463–96. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: Fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci 2007;27:14383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365–76. [DOI] [PubMed] [Google Scholar]
- Chabris CF, Laibson D, Morris CL, Schuldt JP, Taubinsky D. Individual laboratory-measured discount rates predict field behavior. J Risk Uncertainty 2008;37:237–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W, Hsu M, Wudka D, Miller BL, Rosen HJ. Financial errors in dementia: testing a neuroeconomic conceptual framework. Neurocase 2014;20:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Jacobs EA, Sanders S. Contextual control of delay discounting by pathological gamblers. J Appl Behav Anal 2006;39:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Desgranges B, de La Sayette V, Belliard S, Eustache F, Piolino P. What happens to personal identity when semantic knowledge degrades? A study of the self and autobiographical memory in semantic dementia. Neuropsychologia 2012;50:254–65. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao JJ, Kaiser N, Fong SS, Mendez MF. Suicidal behavior and loss of the future self in semantic dementia. Cogn Behav Neurol 2013;26:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. PLoS Med 2005;2:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010;167:748–51. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 2012a;135:2178–91. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Exploring the content and quality of episodic future simulations in semantic dementia. Neuropsychologia 2012b;50:3488–95. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory - analysis of decision under risk. Econometrica 1979;47:263–91. [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci 2012;32:9402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 1999;128:78–87. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Bertoux M, Boutet C, Lehericy S, Dubois B, Fossati P, et al. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol 2013; 11:e1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annu Rev Psychol 2008;59:647–72. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year–olds: a diffusion tensor imaging study. J Cogn Neurosci 2009; 21: 1406–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron 2010;66:138–48. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci 2011;15:227–39. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabodash V, Mendez MF, Fong S, Hsiao JJ. Suicidal behavior in dementia: a special risk in semantic dementia. Am J Alzheimers Dis Other Demen 2013;28:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson PA. A note on measurement of utility. Rev Econ Stud 1936;4:155–61. [Google Scholar]
- Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci 2010;30:16429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosur Psychiatry 2001;70:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding P, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 1989; 2:167–82. [Google Scholar]
- Sutter M, Kocher MG, Glatzle-Rutzler D, Trautmann ST. Impatience and uncertainty: experimental decisions predict adolescents' field behavior. Am Econ Rev 2013;103:510–31. [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Cogn Brain Res 2005;23:34–50. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry 2011;72:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ. Neuroeconomics. Philos Trans R Soc Lond B Biol Sci 2004;359:1737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.