Abstract
Background
The Creighton Model FertilityCare System (CrMS) teaches women to identify days when intercourse is likely to result in pregnancy. We sought to assess the impact of the CrMS on time to clinically identified pregnancy (TTP), via per-cycle pregnancy rates (fecundability).
Methods
We conducted a parallel randomised trial at the University of Utah School of Medicine, 2003–2006. Women ages 18–35, in a relationship of proven fertility, who desired to conceive, were block-randomised, stratified for age, with allocation concealment by opaque sequentially numbered sealed envelopes. The control group received the advice to have intercourse 2–3 times per week, and the intervention group received CrMS instruction. All women were asked to begin trying to conceive starting the second cycle in the study and were followed actively up to 7 cycles, without blinding of research personnel. We calculated descriptive statistics and fecundability, and estimated Cox models for TTP. Registration: Clinicaltrials.gov NCT00161395
Results
There were 143 women randomised: 71 to the control group (all analysed) and 72 to the CrMS group (69 analysed). The adjusted hazard ratio for the influence of CrMS on TTP was 0.86 (95% CI: 0.53, 1.38). Fecundability in cycles with intent to conceive was 31% in controls and 36% with CrMS (p=0.32). By the first cycle, fecundability was 17% in controls, and 4% with CrMS (p=0.02). No adverse events were reported.
Conclusions
We found no significant impact of CrMS on TTP or fecundability, but fewer of the women receiving CrMS conceived by the first cycle.
Introduction
Biological and epidemiological data have demonstrated that human fecundity (capacity to reproduce) is dependent upon intercourse occurring on the day of ovulation or the days immediately preceding it, a period of time traditionally called the fertile window.1–5 Hence, fecundability (the probability of conception, usually in a given cycle) and time to pregnancy are intrinsically linked to the timing of intercourse relative to the fertile window.2, 5, 6
A variety of approaches can be used to estimate the fertile window prospectively or retrospectively, based on different biomarkers.1 Observation of vaginal secretions is one approach particularly suited for couples seeking to optimize the timing of intercourse for conception.7–9
The Creighton Model FertilityCare System (CrMS) is a standardized approach to instructing women to observe, record and interpret vaginal secretions, and the fertile window.10, 11 Days when vaginal secretions appear either stretchy or clear, or have a sensation of lubrication are days when ovulation is approaching and intercourse is most likely to result in pregnancy.3, 8, 12, 13
The goal of this study was to assess the impact of instruction in the CrMS on time to pregnancy and fecundability in couples with previously proven fertility and no history of subfecundity, by means of a randomised trial. We also sought to assess the impact of age, parity, education, and cycle intentions on TTP.
Methods
Eligibility
To be eligible for this study, women had to be between the ages of 18 and 35 years, not pregnant, to be planning to conceive, but not to have not yet started trying. They were required to have a history of at least one prior pregnancy within the past 8 years with their current male partner, to not currently breastfeed, to have no use of hormonal contraception for at least the last two menstrual flows (or 4 flows in the case of recent use of an intra-uterine device or depo-medroxy-progesterone acetate), and no medical history to suggest subfecundity. Women were excluded if they reported a time to pregnancy of 9 months or more for their most recent pregnancy, had more than one menstrual cycle in the past year that was less than 24 days or more than 35 days long, had previously used any method that involved the observation of vaginal secretions from cervical fluid, or had used a fertility monitor. All study visits occurred at the University of Utah, in Salt Lake City.
Recruitment
Women were recruited to the study during a 29-month period of 2003–2005 by several means: 1) directed population-based mailings; 2) posters and flyers at physician offices, community centers, and married student housing; 3) local newspaper advertisements; 4) website; 5) word of mouth from other study participants.
Randomisation
At the baseline visit, women completed screening for eligibility prior to randomisation, and completed questionnaire for demographic characteristics, reproductive history, and health habits. We randomised patients with a block scheme, stratified by age (18–29 years, or 30–35 years of age). The randomised assignment was prepared by an independent statistician and revealed at the time of the study enrollment visit, by opening the next opaque sealed envelope in a numbered sequence.
Intervention group
Each woman in the intervention group received instruction in the CrMS through a standard CrMS introductory information session (in person or on a DVD) within a few days after randomisation, and during individual study visits every two weeks for the first two months in the study, followed by once a month thereafter. Up to the third month, this visit schedule matches the recommended schedule for optimal learning of the CrMS;14 after the third month it actually exceeds the recommended schedule, and was chosen in order to download data from the fertility monitors (which stored detailed data only for the last 42 days). All study visits in the intervention group were conducted by a research assistant who was fully trained as a CrMS instructor (Creighton Model FertilityCare Practitioner).14
A standard CrMS daily diary (or “Creighton Model chart”) was used by women record the date, the bleeding and/or mucus observations, and a record of whether intercourse occurred on that day.15
Control group
The control group also had study visits on exactly the same schedule as the CrMS group, but without CrMS instruction. A different research assistant conducted these visits than for the CrMS group. Women used a daily diary to record bleeding observations and intercourse.
Preconception health advice
Both the intervention and control groups received preconception health advice that outlined current health recommendations for women in the periconception or early pregnancy period. This included taking supplements with folic acid and other advice for a healthy pregnancy.16
Blinded fertility monitor
For this study, both the intervention and the control group used a blinded version of the ClearBlue® Easy Fertility Monitor (at the time, called the ClearPlan® Easy Fertility Monitor).17 This monitor did not display any results of the testing other than the fact that the test was completed satisfactorily.
Instructions for conceiving
The CrMS has a standard instruction to all new users (both those avoiding pregnancy and those trying to conceive) to avoid genital intercourse or contact for the first full cycle or month of use (whichever is shorter), to facilitate learning the characteristics of vaginal discharge from cervical mucus without the presence of any residue from semen (which can mimic fertile cervical mucus).14 In order to evaluate the CrMS as it is normally taught, women in the intervention group received this instruction, with this rationale. In an effort to make the instructions between groups as comparable as feasible, women in the control group were also advised to avoid pregnancy during the first cycle in the study, and were given the rationale that we wished to obtain baseline data from their daily diary and the fertility monitor for one cycle prior to trying to conceive.
Women in both study groups were asked to check a box on the top of their daily diary at the beginning of each cycle that indicated their pregnancy intentions for the upcoming cycle: to avoid, to conceive, or unsure. Women in the intervention (CrMS) group were informed that the “most fertile” days were days when clear, stretchy, and/or lubricative (slippery) vaginal secretions are observed from cervical mucus. Women in the control group were given the following advice about conceiving: “Most experts believe that having intercourse 2 or 3 times per week is sufficient to assure that a couple will conceive.”18
Assessment of outcome
The primary outcome for this study was time to pregnancy, based on number of cycles with intention to conceive until the clinical recognition of pregnancy. A directly related outcome was fecundability. Pregnancy was suspected by women based on missed menstruation or other symptoms, and confirmed at a study visit by urine human chorionic gonadotropin (hCG) testing capable of detecting a concentration of 25 mIU/ml or higher (Clearblue® pregnancy tests). Women continued in follow-up until they experienced a clinical pregnancy, they contributed seven full menstrual cycles or nine calendar months after enrollment, they began medical treatment that would influence fertility, they decided to stop trying to conceive, they withdrew from the study, or they were lost to follow-up. Women who conceived during the study follow-up were contacted later to determine the outcome of pregnancy. Study personnel who determined outcomes were not blinded to the group assignment of participants.
Compensation
When the study started, women were compensated $25 for each cycle of participation with reasonably complete data. After we observed that some women may have been delaying conception to receive another cycle of reimbursement, we changed the reimbursement scheme so that women entering the study received $140 whenever they became pregnant, or when they completed follow-up, whichever came first, except that women who became pregnant in the first cycle received only $20.
Sample size and power
Based primarily on budget considerations, our targeted sample size was a minimum of 120 women in the study (60 in each arm of the trial). If the women receiving instruction in the CrMS were to experience the cumulative probability of pregnancy which was reported in a previous CrMS study (66% after 2 cycles),19 and the women in the control group had a cumulative probability of pregnancy reported in another time to pregnancy study without instruction to identify the fertile window (43% after 2 cycles),18 we calculated that this sample size would be adequately powered (>80% power, alpha = 0.05, one-sided chi-square test) to detect a difference in cumulative pregnancy rates, assuming a loss to follow-up of 5%.
Analysis
Women were asked to start their daily diaries at the time of randomisation. First cycles that were less than 14 days long were counted as cycle 0. Women who never started their daily diaries but conceived very soon after randomisation were considered to have conceived during cycle 0.
We used descriptive statistics to assess demographic, past reproductive and other characteristics of participants; and chi-square analysis, and t-tests to assess for differences between the control and CrMS groups. We calculated crude fecundability (proportion of conceptions) for menstrual cycles in the study, stratified by treatment group and cycle intention. We calculated cumulative proportions conceiving in each treatment group over time, for all cycles (including cycle 0), and for cycles with intention to conceive, using life table analysis to adjust for participants exiting the study prior to full follow-up. We used Cox proportional hazards regression with discrete time and the DISCRETE method for tied survival times to examine the impact of the CrMS on time to pregnancy, while also assessing for the impact of pre-specified demographic and reproductive characteristics and cycle intentions on the likelihood of conceiving. One factor was added to the models post hoc: compensation (lump-sum versus per-cycle). All analyses were done for all study cycles, and repeated restricted to study cycles 2–7.
Human subjects protection
The original study protocol and subsequent modifications were approved the University of Utah Institutional Review Board. We obtained written informed consent from all participants. The trial was registered at clinicaltrials.gov, NCT00161395.
Results
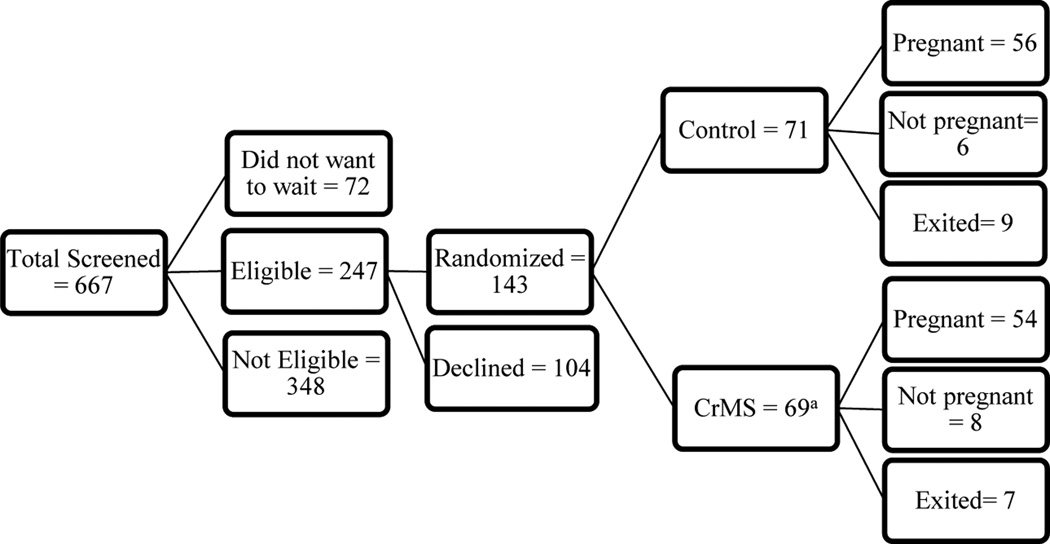
The results of screening for eligibility, randomisation, and follow-up are displayed in Figure 1. Of 667 women screened for the study, a total of 143 women were randomised: 71 to the control group, and 72 to the CrMS group. Of the 143 women randomised, 91 were within the age stratum 18–29 years, and 52 were in the age stratum 30–35 years. Subsequently, it was determined that among the women assigned to the CrMS group, one woman was already pregnant prior to randomisation, and two women were still taking hormonal contraception; these women are excluded from further analysis.
Figure 1.
Flow chart of women screened, eligible, randomized, and status at completion of the study.
a 3 participants randomized to the CrM group were found subsequently to be ineligible; 2 because they were continuing to use hormonal contraception, and 1 because she was pregnant prior to randomization.
Eight women recorded first cycles that were less than 14 days (cycle 0), there were no pregnancies in these cycles. Four women in the control group and three women in the CrMS group never started daily diaries but conceived soon after randomisation, i.e., also during cycle 0. By the first cycle, fecundability was 17% (12/71) in the control group, and 4% (3/69) in the CrMS group (p=0.02).
Within the seven cycles in the study, 56 (79%) of the control group and 54 (78%) of the CrMS group conceived; these pregnancies resulted in 49 (88%) and 46 (85%) live births, respectively. Among control women, 6 women completed seven cycles without pregnancy (3 of whom conceived within the next 5 months). Similarly, among CrMS women, 8 women completed seven cycles without pregnancy (3 of whom conceived within the next 5 months). Finally, 9 women in the control group and 7 women in the CrMS group exited the study prior to the full 7 cycles or 9 months of follow-up without conceiving; reasons for exiting included deciding not to conceive (3 control, 5 CrM), deciding not to continue in the study (3 control and 1 CrM), medical treatment affecting fertility (hormonal contraception or fertility medication; 2 control and 1 CrM), or loss to follow-up (1 control).
As shown in Table 1, the control and CrMS groups were completely comparable with regard to age, proportion of those with only one prior pregnancy, history of miscarriage, education, income, past use of oral contraceptives, alcohol use, and smoking (p≥0.2 for all comparisons). No adverse events were reported during the trial in either group.
Table 1.
Demographic characteristics of participants at screening, stratified by assigned treatment groupa
| Characteristic n (%) |
Total n = 140 |
Control n = 71 |
CrMS n = 69 |
|---|---|---|---|
| Age (mean ± S.D.) | 28.2 ± 3.2 | 28.1 ± 3.2 | 28.3 ± 3.1 |
| More than one prior pregnancy | 78 (55.7) | 40 (56.3) | 38 (55.1) |
| History of miscarriage | 43 (30.7) | 22 (31.9) | 21 (29.6) |
| College graduate | 74 (52.9) | 34 (47.9) | 40 (58.0) |
| Family income ≥ $40,000 per yeara | 85 (61.6) | 46 (64.8) | 39 (58.2) |
| Prior use of oral contraceptives | 134 (95.7) | 69 (97.2) | 65 (94.2) |
| Any prior history of alcohol use | 19 (13.6) | 12 (16.9) | 7 (10.1) |
| Any prior history of smoking | 21 (15.0) | 12 (16.9) | 9 (13.0) |
| Received lump-sum compensation | 100 (71.4) | 50 (70.4) | 50 (72.5) |
Response was missing for Family income for 2 participants; p>0.2 for all comparisons.
Among women who received per-cycle compensation, the crude cumulative proportion conceiving was 73% (29/40); among women who received lump-sum compensation, the crude cumulative proportion conceiving was 83% (83/100); p=0.07.
As shown in Table 2, fecundability for all cycles in the study was 23% in the control group and 21% in the CrMS group (p=0.62); among cycles with intention to conceive, fecundability was 31% in the control group and 36% in the CrMS group (p=0.32). Excluding the first cycle in the study, 80% of cycles had intention to conceive and 12% intention to avoid in the control group; compared to 71% and 18% of cycles in the CrMS group, respectively, and fecundability for all cycles was 27% in the control group, and 28% in the CrMS group (p=0.80).
Table 2.
Proportions of cycles with intent to avoid, conceive, or unsure, and associated crude fecundability, by assigned treatment group, including or excluding the first cyclea
| Including the first cycle | Excluding the first cycle | |||||||
|---|---|---|---|---|---|---|---|---|
| Number (%) of all cycles |
Percentage of cycles resulting in pregnancy (CI 95%) |
Number (%) of all cycles |
Percentage of cycles resulting in pregnancy (CI 95%) |
|||||
| Control | CrMS | Control | CrMS | |||||
| Cycle intention | ||||||||
| To conceive | 137 (60) | 132 (54) | 31 (23, 38) |
36 (28, 45) |
133 (80) | 131 (71) | 31 (23, 39) |
37 (28, 45) |
| To avoid | 78 (34) | 94 (38) | 9 (3, 15) |
2 (0, 5) |
20 (12) | 34 (18) | 0 | 6 (0, 14) |
| Unsure | 14 (6) | 19 (8) | 21 (0, 43) |
5 (0, 15) |
13 (8) | 19 (10) | 23 (0, 46) |
5 (0, 15) |
| All cycles | 229 (100) | 245 (100) | 23 (17, 28) |
21 (16, 26) |
166 (100) | 184 (100) | 27 (20, 33) |
28 (21, 34) |
4 women in the control group and 3 women in the CrMS group had pregnancies in cycle zero, which are not included in this table.
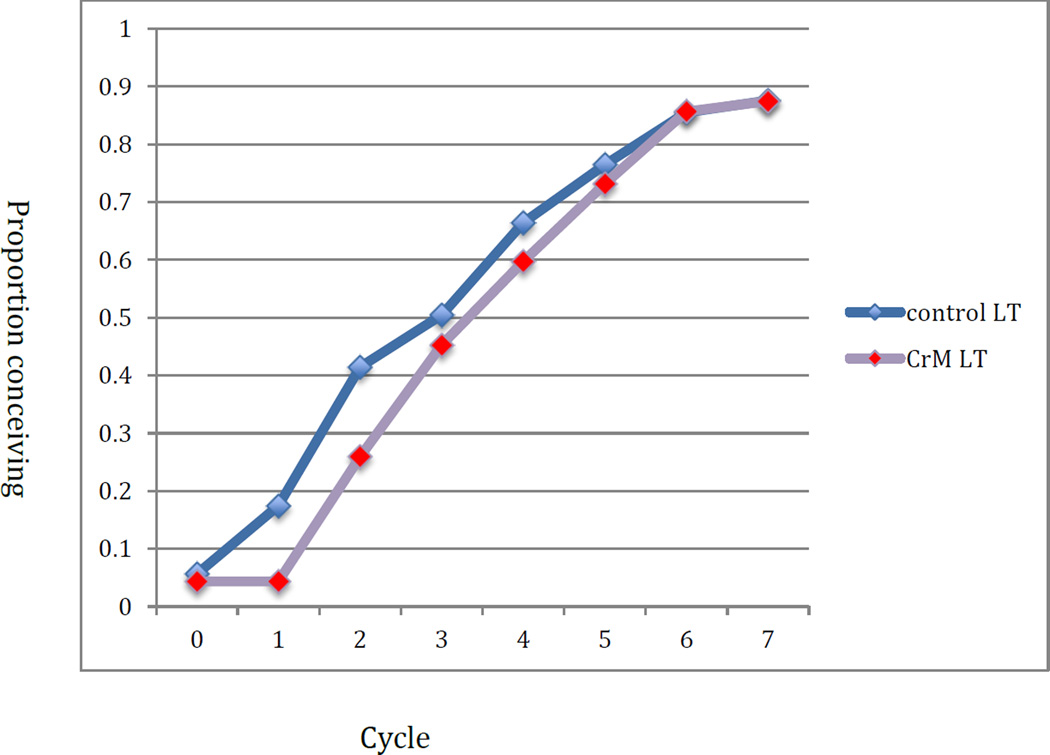
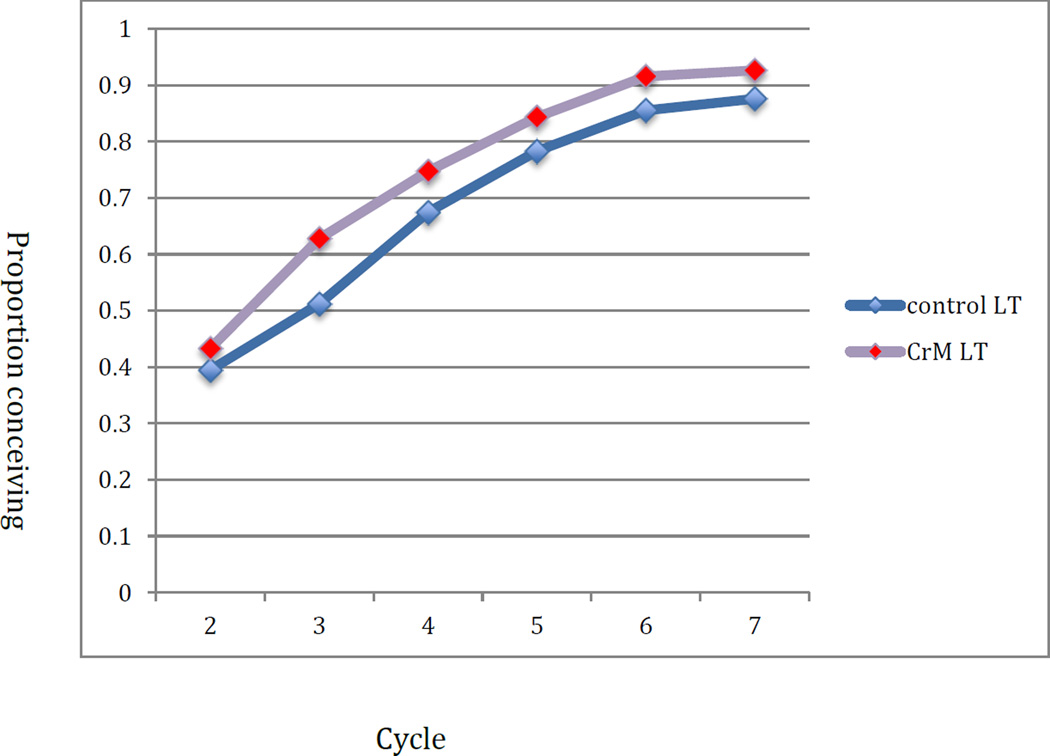
The mean TTP among those conceiving was 2.9 cycles in the control group and 3.5 in the CrMS group (p=0.10); excluding cycles 0 and 1, it was 2.6 cycles in the control group and 2.7 cycles in the CrMS group (p=0.65). Figure 2 displays the cumulative proportion of women conceiving in all cycles regardless of per-cycle intention and including cycle zero, adjusted by life table analysis for those exiting the study. The cumulative probabilities of pregnancy were 50% (95% CI: 39%, 62%) and 45% (95% CI: 34%, 57%) by cycle 3, and 88% (95% CI: 80%, 95%) and 87% (95% CI: 80%, 95%) by cycle 7 in the control and CrMS groups, respectively. Figure 3 displays the results for cycles with the intention to conceive, restricted to cycles 2–7. The cumulative probabilities of pregnancy were 51% (95% CI: 40%, 63%) and 63% (95% CI: 51%, 74%) by cycle 3, and 88% (95% CI: 80%, 95%) and 93% (95% CI: 86%, 99%) by cycle 7 in the control and CrMS groups, respectively.
Figure 2.
Cumulative proportion of women conceiving by assigned treatment group: all cycles, including cycle zero, and all cycle intentions (lifetable analysis)
Figure 3.
Cumulative proportion of women conceiving by assigned treatment group: cycles 2–7 with intent to conceive (lifetable analysis)
In Table 3, the results of two Cox proportional hazards models are displayed, with and without intention to conceive (per cycle) in the model. There was no significant effect for treatment group. Women’s age was negatively associated with the likelihood of pregnancy, while multiparity, and college education were positively associated. Having received lump-sum compensation also trended towards high likelihood of pregnancy, but without statistical significance. Intention to conceive per cycle had the strongest impact on the likelihood of pregnancy, with a hazard ratio of 10.7 (95% CI: 4.7, 24.35).
Table 3.
Characteristics associated with shorter time to pregnancya
| Characteristic | Hazard Ratio [CI 95%] | Hazard Ratio [CI 95%] |
|---|---|---|
| CrMS Group | 0.69 [0.44, 1.08] | 0.86 [0.53, 1.38] |
| Age (years) | 0.88 [0.81,0.96] | 0.89 [0.81, 0.98] |
| More than one prior pregnancy | 1.82 [1.14,2.91] | 1.59 [0.97, 2.6] |
| College graduate | 2.05 [1.22,3.46] | 1.86 [1.08, 3.21] |
| Lump-sum compensation |
1.57 [0.93,2.64] | 1.5 [0.86, 2.6] |
| Cycle intent to conceiveb |
10.7 [4.7, 24.35] |
Two Cox proportional hazards regression models: with and without the time-varying covariate of cycle intent, estimated by DISCRETE partial likelihood
Intent to conceive compared to intent to avoid, unsure, or missing
Discussion
In this randomised trial, we found no overall impact of instruction in the CrMS on time to pregnancy or fecundability among 140 women/couples of previously proven fertility. However, the instruction to avoid pregnancy (control group) or abstain from genital contact (CrMS group) was associated with a significant difference between the groups by the first cycle in the study: 12 (17%) women conceiving in the control group, and 3 (4%) conceiving in the CrMS group. This resulted in the control group having a “jump start” on cumulative pregnancy (Figure 2). When the first cycle is removed from analysis and only cycles with intent to conceive are analysed, the CrMS group had a greater adjusted cumulative probability of pregnancy, with an absolute difference of 12% at cycle 3 (Figure 3). However, in the Cox models, there was no significant impact of study group on time to pregnancy.
We believe that the rationale given to the control group for avoiding pregnancy in the first cycle (to assure proper data collection with the daily diary and the fertility monitor before pregnancy occurs) was less compelling than the rationale given to the CrMS group to abstain from genital contact during the first cycle (to learn to distinguish different types of cervical mucus discharge prior to introducing seminal fluid), and that this resulted in more women in the CrMS group following the instruction to wait until the second cycle to begin to try to conceive.
In a prior 1992 study of 50 couples using the CrMS with a similar female age distribution, which we used for our sample size calculation, 76% conceived in the first cycle of documented intercourse in the fertile window, and 90% by the third cycle.19 Women had already used CrMS for an average of 5.7 cycles to avoid pregnancy, and only women who ultimately conceived were included in the study. Also, a prior multinational study of the Billings Ovulation Method, which teaches women to identify the fertile window in a very similar way, reported a 67% fecundability when intercourse occurred on the peak mucus day, but this result was based on only 9 cycles.20 Even a small level of under-reporting of intercourse in the fertile window during non-conception cycles could substantially bias apparent fecundability upward and TTP downward.
Our previous study examining the day-specific probabilities of conception among CrMS users in the United States found that the maximum probability of conception per cycle was 38%.3 Likewise, a German prospective study that taught women to identify the most fertile days with cervical mucus reported a first cycle pregnancy probability of 38%.21 Our current results are consistent with these estimates, but somewhat higher than those from other prospective observational studies. For example, cumulative probabilities of clinical pregnancy have been reported of 74%, 81%, and 68% at 6 cycles, respectively.18, 21, 22 The restriction of our study to women of proven fertility is a likely reason for the discrepancy.
We were surprised to find no significant impact of the CrMS on time to pregnancy. The impact of targeting intercourse to the fertile window is modeled to be lower in couples with more frequent intercourse and/or higher baseline fecundity, which may help account for the lack of significant difference found in this trial.6 It is possible that women in the control group used some kind of calendar calculation to estimate the fertile window for intercourse, though a correct understanding of the fertile window is far from universal even among educated women.23 There is also evidence for a modest increase in intercourse behavior during the fertile window without any conscious recognition of the fertile window, presumably due to biological factors.24 Nevertheless, another randomised trial using the ClearBlue® fertility monitor to identify the fertile window found a significantly higher rate of conception in the first two cycles in the study among 653 women who had been trying to conceive for up to 2 years.25 Similarly, a recent observational study found that consistent cervical mucus checking was associated with a fecundability ratio of 2.1 (95% C.I. 1.16, 3.81) in 448 women ages 30–44 without any history of subfecundity.26 We plan future analytic work to describe the patterns of intercourse in the two study groups to examine whether instruction in the CrMS altered patterns of intercourse in comparison to the control group.
Even after exclusion of the first cycle in the study (during which women had been asked to avoid pregnancy), women reported an intention to avoid pregnancy in 10% of cycles in the control group and 18% of cycles in the CrMS group. Prospective studies of time to pregnancy may lose precision if they do not assess intentions and/or behaviors on a cycle-by-cycle basis. The multivariable analysis confirmed the very strong impact of cycle intentions on the probability of conception.
The lump-sum compensation scheme was associated with a higher cumulative proportion conceiving (83%) than the per-cycle compensation scheme (73%). We believe it would be wise for investigators conducting prospective studies of time to pregnancy to consider the possible impact of compensation.
The strengths of this study include the randomised design, thorough follow-up, the prospective assessment of pregnancy intentions for each cycle, and the prospective daily diary. Weaknesses include the instruction to avoid pregnancy in the first cycle, an initial per-cycle reimbursement scheme, and small sample size. We believe our sample size calculations were based on overly optimistic assessments of the possible impact of CrMS instruction in couples of proven fertility. Having different research assistants for the two groups avoided cross-contamination of the intervention into the control group, but also opened the possibility for an unmeasured differential impact based on personality and interaction of the research assistants.
In summary, this study found no impact of instruction on fertility awareness via the CrMS on time to pregnancy in couples with proven fertility. We cannot exclude the possibility of an impact if instruction in fertility awareness would be provided without instruction to avoid pregnancy for the first cycle, or if the study were conducted with a larger sample size. This study highlights methodologic issues to consider in future prospective studies of time to pregnancy: many couples are not willing to wait one cycle to begin to conceive, intentions for conception may vary by cycle in couples during their attempts to conceive, and the structure of reimbursement may impact time to pregnancy.
Acknowledgments
This work was supported by 1K23 HD0147901-01A1 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, and by the Health Studies Fund, Department of Family and Preventive Medicine, University of Utah. Modified fertility monitors and data extraction from the monitors were provided by Swiss Precision Diagnostics. We wish to acknowledge the valuable contributions of Thomas W. Hilgers in reviewing study design, implementation, and results. Harry Hatasaka, Mary Bishop Stone (deceased), Becky Crockett, Sebrena Banecker, Sara Feltz, Colette Child, and Richard Holubkov made valuable contributions in preparing and conducting the study. Yao Li and Jared Hansen assisted with data analysis. James Trussell served as a consultant for the study.
References
- 1.Lynch CD, Jackson LW, Buck Louis GM. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatric and Perinatal Epidemiology. 2006;20(Suppl 1):3–12. doi: 10.1111/j.1365-3016.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunson DB, Baird DD, Wilcox AJ, Weinberg CR. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Human Reproduction. 1999;14:1835–1839. doi: 10.1093/humrep/14.7.1835. [DOI] [PubMed] [Google Scholar]
- 3.Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstetrics and Gynecology. 2003;101:1285–1293. doi: 10.1016/s0029-7844(03)00358-2. [DOI] [PubMed] [Google Scholar]
- 4.Keulers MJ, Hamilton CJCM, Franx A, Evers JLH, Bots RSGM. The length of the fertile window is associated with the chance of spontaneously conceiving an ongoing pregnancy in subfertile couples. Human Reproduction. 2007;22:1652–1656. doi: 10.1093/humrep/dem051. [DOI] [PubMed] [Google Scholar]
- 5.Colombo B, Masarotto G. Daily fecundability: first results from a new data base. Demographic Research. 2000;3 [PubMed] [Google Scholar]
- 6.Stanford JB, Dunson DB. Effects of sexual intercourse patterns in time to pregnancy studies. American Journal of Epidemiology. 2007;165:1088–1095. doi: 10.1093/aje/kwk111. [DOI] [PubMed] [Google Scholar]
- 7.Stanford JB, White GL, Hatasaka H. Timing intercourse to achieve pregnancy: current evidence. Obstetrics and Gynecology. 2002;100:1333–1341. doi: 10.1016/s0029-7844(02)02382-7. [DOI] [PubMed] [Google Scholar]
- 8.Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Human Reproduction. 2004;19:889–892. doi: 10.1093/humrep/deh173. [DOI] [PubMed] [Google Scholar]
- 9.Zinaman MJ. Using cervical mucus and other easily observed biomarkers to identify ovulation in prospective pregnancy trials. Paediatric and Perinatal Epidemiology. 2006;20(Suppl 1):26–29. doi: 10.1111/j.1365-3016.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilgers TW, Prebil AM. The ovulation method--vulvar observations as an index of fertility/infertility. Obstetrics and Gynecology. 1979;53:12–22. [PubMed] [Google Scholar]
- 11.Hilgers TW. The medical and surgical practice of NaProTechnology. Omaha: Pope Paul VI Institute Press; 2004. Standardization of teaching; pp. 57–82. [Google Scholar]
- 12.Fehring RJ. Accuracy of the peak day of cervical mucus as a biological marker of fertility. Contraception. 2002;66:231–235. doi: 10.1016/s0010-7824(02)00355-4. [DOI] [PubMed] [Google Scholar]
- 13.Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. British Journal of Obstetrics and Gynaecology. 2001;108:822–829. doi: 10.1111/j.1471-0528.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Hilgers TW, Daly KD, Hilgers SK, Prebil AM. Creighton Model Fertility Care System: A standardized, case management appproach to teaching, Book 1. 2nd. Omaha, NE: Pope Paul VI Institute Press; 2002. [Google Scholar]
- 15.Hilgers TW. Introduction to the Creighton Model System. In: Hilgers TW, editor. The medical and surgical practice of NaProTechnology. Omaha: Pope Paul VI Institute Press; 2004. pp. 43–56. [Google Scholar]
- 16.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care--United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. Morbidity and Mortality Weekly Report Recommondations and Reports. 2006;55:1–23. [PubMed] [Google Scholar]
- 17.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HP, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Human Reproduction. 2000;15:2478–2482. doi: 10.1093/humrep/15.12.2478. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 19.Hilgers TW, Daly KD, Prebil AM, Hilgers SK. Cumulative pregnancy rates in patients with apparently normal fertility and fertility-focused intercourse. Journal of Reproductive Medicine. 1992;37:864–866. [PubMed] [Google Scholar]
- 20.World Health Organisation. A prospective multicentre trial of the ovulation method of natural family planning. III. Characteristics of the menstrual cycle and of the fertile phase. Fertility and Sterility. 1983;40:773–778. [PubMed] [Google Scholar]
- 21.Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Human Reproduction. 2003;18:1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- 22.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. Persistent environmental pollutants and couple fecundity: the LIFE study. Environmental Health Perspectives. 2013;121:231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertility and Sterility. 2014;101:767–774. doi: 10.1016/j.fertnstert.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Human Reproduction. 2004;19:1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JE, Wakelin M, Ellis JE. Increased pregnancy rate with use of the Clearblue Easy Fertility Monitor. Fertility and Sterility. 2007;87:329–334. doi: 10.1016/j.fertnstert.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 26.Evans-Hoeker E, Pritchard DA, Long DL, Herring AH, Stanford JB, Steiner AZ. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertility and Sterility. 2013;100:1033–1038. doi: 10.1016/j.fertnstert.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]