Abstract
Background and aims
Sleep disturbance is a prominent complaint in cocaine and alcohol dependence. This controlled study evaluated differences of polysomnographic (PSG) sleep in cocaine‐ and alcohol‐dependent subjects, and examined whether substance dependence interacts with age to alter slow wave sleep and rapid eye movement (REM) sleep.
Design
Cross‐sectional comparison.
Setting
Los Angeles and San Diego, CA, USA.
Participants
Abstinent cocaine‐dependent subjects (n = 32), abstinent alcohol‐dependent subjects (n = 73) and controls (n = 108); mean age 40.3 years recruited 2005–12.
Measurements
PSG measures of sleep continuity and sleep architecture primary outcomes of Stage 3 sleep and REM sleep. Covariates included age, ethnicity, education, smoking, body mass index and depressive symptoms.
Findings
Compared with controls, both groups of substance dependent subjects showed loss of Stage 3 sleep (P < 0.001). A substance dependence × age interaction was found in which both cocaine‐ and alcohol‐dependent groups showed loss of Stage 3 sleep at an earlier age than controls (P < 0.05 for all), and cocaine‐dependent subjects showed loss of Stage 3 sleep at an earlier age than alcoholics (P < 0.05). Compared with controls, REM sleep was increased in both substance‐dependent groups (P < 0.001), and cocaine and alcohol dependence were associated with earlier age‐related increase in REM sleep (P < 0.05 for all).
Conclusions
Cocaine and alcohol dependence appear to be associated with marked disturbances of sleep architecture, including increased rapid eye movement sleep and accelerated age‐related loss of slow wave, Stage 3 sleep.
Keywords: Aging, alcohol dependence, cocaine dependence, polysomnography, sleep
Introduction
Cocaine dependence, as well as alcohol dependence, constitute enormous global health burdens 1, 2, and cocaine is the second most commonly used illicit drug after cannabis, with a life‐time prevalence of more than 10% in the United States 3. Among cocaine‐dependent people during use and early abstinence, sleep disturbance is a prominent complaint 4. Indeed, more than 70% of cocaine‐dependent people report disordered sleep during early abstinence that persists for weeks or longer during recovery 4, 5, 6. In contrast to the prevalence of sleep complaints, there is limited research using polysomnography (PSG) to evaluate disturbances of sleep continuity and sleep architecture in cocaine dependence 4. Sleep continuity includes total sleep time, sleep latency, sleep efficiency and wake after sleep onset, whereas sleep architectures include amounts of Stages 1–2 sleep and Stages 3–4 sleep (slow wave sleep, SWS), rapid eye movement (REM) sleep and other REM measures (i.e. latency, density and duration). Uncontrolled studies 5, 7, 8, 9, 10, 11, 12 and a limited number of controlled studies 13, 14 have examined PSG parameters during early abstinence in cocaine‐dependent subjects, and reported disturbances of sleep continuity (i.e. prolonged sleep latency; reduced sleep efficiency; and decreased total sleep time) as well as alterations in sleep architecture (i.e. increases in REM sleep, shortened REM latency) 4. SWS also appears to be low during early abstinence (< 1 month), although only a small number of cocaine‐dependent people have been studied to date 4.
Those with alcohol dependence also complain commonly of sleep problems, and these difficulties persist for weeks or months during the course of abstinence 15, 16. Controlled PSG studies have confirmed disturbances of sleep continuity 17, 18, 19, 20. Additionally, alcohol dependence is associated with a loss of Stage 3 slep 21, which persists for more than 2 years of abstinence, due possibly to a homeostatic defect in the regulation of SWS sleep 17, 18. Further, age‐related deterioration of SWS is greater in alcohol dependence, with younger alcohol‐dependent people showing abnormalities of sleep, which are typical of those found in older adults 20. Changes in REM sleep are not prominent in alcohol dependence, although increases in REM sleep and shorter REM latency (i.e. increased REM pressure) during early abstinence predict increased likelihood of relapse in the following 3 months. Hence, abnormalities in sleep architecture and especially REM sleep may be associated with clinical course and prognosis 19, 22. Only one study has compared PSG measures in cocaine versus alcohol dependence; in this small and clinically heterogeneous sample of stimulant abusers with comorbid alcohol dependence compared to alcohol‐dependent people, increases in REM sleep were found in stimulant abusers compared to alcohol dependence 13.
In this controlled study of 213 subjects, we evaluate sleep continuity and architecture in abstinent cocaine‐dependent people (n = 32), abstinent alcohol‐dependent people (n = 73) and control subjects who have no life‐time history of substance dependence or other psychiatric disorder (n = 108); groups were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) criteria 23. We hypothesize that the two substance dependence groups will show a loss of slow wave, Stage 3 sleep and increases in REM sleep compared to controls, and that cocaine‐dependent people will show greater differences in these sleep measures compared to those with alcohol dependence 13. In addition, given evidence that alcohol dependence is associated with age‐accelerated loss of Stage 3 sleep, we hypothesize that cocaine and alcohol dependence will be associated with greater age‐related changes in sleep, including loss of Stage 3 sleep and increases in REM sleep compared to controls.
Methods
Overview of design
Subjects who responded to advertisements underwent a telephone eligibility screening. Eligible subjects were scheduled for an assessment, in which demographic and clinical information including substance use and medical histories was obtained by interview and questionnaires, along with completion of laboratory examination and urine toxicology screening. Following baseline assessment, subjects were admitted to the sleep laboratory with 1 night of adaptation followed by PSG assessment of sleep continuity and sleep architecture.
For the cocaine‐dependent subjects only, study procedures were continued with repeated assessment of sleep along with blood sampling and administration of a single dose of cocaine (40 mg) after completion of this PSG study 24.
Participants
Study procedures were approved by the University of California, Los Angeles (UCLA), Institutional Review Board (IRB), University of California, San Diego (UCSD) IRB and the Research Advisory Panel of California. Non‐treatment‐seeking alcohol‐dependent volunteers were recruited from veteran and community samples in the San Diego and Los Angeles areas, and studied after acute and subacute withdrawal symptoms had resolved; i.e. after at least 2 weeks of abstinence. Non‐treatment‐seeking cocaine‐dependent volunteers were recruited from community samples in the Los Angeles area, and studied after acute withdrawal symptoms had resolved; i.e. after at least 3 days of abstinence. Comparison controls were recruited from community samples in the San Diego and Los Angeles areas. All volunteers were recruited primarily by advertisement in local newspapers, and compensated with grocery vouchers. Assessments were conducted between 2005 and 2012.
Inclusion criteria were: alcohol‐dependent volunteers aged 25–65 years who fulfilled DSM‐IV criteria for alcohol dependence without other current substance dependence 23, cocaine‐dependent volunteers who fulfilled DSM‐IV criteria for cocaine dependence without other current substance or alcohol dependence and comparison controls who fulfilled DSM‐IV criteria for no life‐time history of alcohol dependence, substance dependence or other Axis I psychiatric disorder (i.e. never mentally ill). Nicotine dependence was not ascertained; current smoking history was assessed.
Exclusion criteria were: cardiovascular disease, hypertension, diabetes, peripheral neuropathy, inflammatory disorder, liver disease, neoplasm, pregnancy, recent (< 6 months) use of steroid or immunosuppressant medication, daily psychotropic or hypnotic medication or non‐insomnia sleep disorders such as obstructive sleep apnea or nocturnal myoclonus, as evaluated by medical history interview. No subject was routinely using medications that might compromise interpretation of sleep measures. Laboratory examination excluded volunteers who showed evidence of HIV or hepatitis C, or had an abnormal electrocardiogram. A positive urine toxicology resulted in exclusion, except for non‐treatment‐seeking cocaine‐dependent subjects who were required to have a positive test for cocaine, a negative test for other substances, self‐reported use of more than 1 g of cocaine per week for 6 months and self‐report of being unable to sustain abstinence from cocaine for at least 1 week during the last 6 months.
A total of 127 comparison controls, 80 volunteers with alcohol dependence and 55 volunteers with cocaine dependence, underwent screening and eligibility assessment including PSG screening for sleep apnea and nocturnal myoclonus. Of these participants, we excluded 19 controls, seven alcohol‐dependent subjects and 23 cocaine‐dependent subjects because they did not fulfill eligibility criteria and/or recently used other substances. The remaining sample was comprised of 108 comparison controls, 73 alcohol‐dependent subjects and 32 cocaine‐dependent subjects.
Procedures
The Structured Clinical Interview for DSM‐IV diagnosis was used to determine clinical diagnoses of substance dependence in the two clinical groups and absence of any psychiatric disorder in the controls 25. A semi‐structured interview was used to obtain consumption histories for alcohol, cocaine, other substances and smoking 26. The Pittsburgh Sleep Quality Index (PSQI) and the Hamilton rating scale for depression, 24 items (HAMD‐24) assessed sleep quality 27 and depressive symptoms, respectively 28. Body mass index (BMI) was calculated from height and weight.
All participants slept for 2 consecutive nights, as described previously 17, 24. The first night assessed the presence of sleep apnea or nocturnal myoclonus; only the second night was used analyses. All groups followed identical rest/activity schedules, with lights out at 11:00 p.m., uninterrupted sleep and wake time at 7:00 a.m. To ensure abstinence, subjects were observed hourly by nursing staff during the day, refrained from physical exertion and were not allowed to eat or smoke during the night. Subjects had a standard PSG montage with electrodes placed for electroencephalograph (EEG) (F3, F4, C3, C4, O1, O2), electro‐oculography (EOG) channels (left and right outer canthi with paired mastoid references) and one submental electromyography (EMG) channel (bipolar submentalis). All sleep records were scored visually in accordance with Rechtschaffen & Kales criteria [29] using methods as detailed 13, 17, 30. Sleep efficiency is defined as the percentage of wake time in bed. REM density is measured as the number of REM per minute of time. Sleep research technicians were tested regularly on scoring reliability, and high standards were maintained: sleep latency (r = 0.96), REM latency (r = 0.99), REM density (r = 0.91), amounts of stages 3 (r = 0.85) and total sleep time (r = 0.99) 17.
Outcomes
The primary outcomes were amounts of Stage 3 and REM sleep and the interaction of substance dependence and age on these outcomes, given the known impact of age as well as substance dependence on these measures. We focused on Stage 3 sleep because Stage 4 sleep was not present in a majority of the substance dependence subjects; SWS is a sum of Stages 3 and 4 sleep. Secondary outcomes were measures of sleep continuity, including sleep latency, sleep efficiency and REM latency, density and duration. Pre‐defined covariates included variables related to sleep architecture including age, ethnicity, education, smoking, body mass index (BMI) and depressive symptoms (HAMD‐24).
Sample size
The primary outcomes of Stage 3 and REM sleep, along with previous findings 13, 17, were used to estimate effect sizes for comparisons between controls, alcohol dependence and cocaine dependence; effect sizes were medium (0.48) to large (0.99), assuming an average effect of approximately 0.70. Hence, there was greater than 80% power, assuming the smallest group had n = 33.
Statistical analysis
All measures were examined for distribution assumptions and appropriateness for the planned analyses. The three groups (controls, alcohol‐dependent, cocaine‐dependent) were compared on demographic and clinical measures using analysis of variance (ANOVA) with pairwise comparisons calculated using the least significant difference (LSD) method; χ2 analyses were utilized in the case of categorical variables. For ANOVA of sleep measures, covariates included demographic and clinical variables that differed between the groups. Pairwise comparisons for the sleep variables were performed using the Sidak method to adjust for multiple comparisons. Correlational analyses also explored the relationships between demographic and clinical variables and the primary outcomes of Stage 3 and REM sleep, and were used to inform selection of confounders in regression analyses. Regression analyses were performed to evaluate whether cocaine and alcohol dependence predicted Stage 3 sleep and REM sleep independently of confounders (i.e. those variables that differed between groups and/or those that correlated with primary outcomes). Additional analyses tested whether substance dependence groups interacted with age to alter Stage 3 and REM sleep. To explore significant group × age interactions, the sample was stratified by 7‐year age categories (i.e. maintaining similar numbers of subject in each subgroup), with testing of post‐hoc differences adjusted for multiple comparisons using the Sidak method. All analyses were conducted using SPSS version 22 (IBM, Armonk, NY, USA 2013).
Results
Demographics, substance consumption histories and clinical characteristics
Age, sex and BMI were similar in the three groups (Table 1). Cocaine‐dependent subjects were more likely to be African American. Compared to controls, substance dependence groups had less education, reported more alcohol consumption, were more likely to be smokers and reported more depressive symptoms. Use of cocaine occurred almost exclusively in cocaine‐dependent subjects. Only alcohol‐dependent subjects reported poor sleep quality (PSQI > 5).
Table 1.
Participant demographic and clinical characteristics.
| Controls a (n = 108) | Alcohol‐ dependent b (n = 73) | Cocaine‐ dependent c (n = 32) | F or χ 2 , P | Post‐hoc comparison | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 40.3 ± 11.5 | 41.9 ± 9.5 | 42.8 ± 5.6 | F (2225) = 1.1, P = 0.34 | |
| Sex, male, % | 92.4 | 94.7 | 88.2 | χ2 = 1.4, P = 0.50 | |
| Ethnicity, African American, % | 25.9 | 37.7 | 76.7 | χ2 = 25.6, P < 0.001 | a–c**; b,c** |
| Education, years | 15.5 ± 1.8 | 13.1 ± 3.1 | 11.2 ± 6.8 | F (2208) = 23.9, P < 0.001 | a,b**; a–c**; b,c* |
| Body mass index | 26.1 ± 3.7 | 25.3 ± 3.7 | 25.6 ± 3.7 | F (2165) = 0.9, P = 0.41 | |
| Substance consumption | |||||
| Alcohol, times/day | 1.4 ± 1.2 | 12.7 ± 8.0 | 2.8 ± 2.3 | F (2185) = 111.7, P < 0.001 | a,b**; b,‐c** |
| Alcohol, days/month | 7.0 ± 8.5 | 24.1 ± 7.4 | 11.4 ± 9.5 | F (2189) = 83.8, P < 0.001 | a,b**; b,c**; a–c* |
| Cocaine, times/day | 0 | 0.1 ± 0.4 | 6.1 ± 7.9 | F (2238) = 60.5, P < 0.001 | a–c**; b,c** |
| Cocaine, days/month | 0 | 0.6 ± 1.8 | 20.3 ± 8.6 | F (2,80) = 147.7, P < 0.001 | a–c**; b,c** |
| Smokers, % | 18.8 | 78.9 | 67.9 | χ2 = 14.7, P < 0.05 | a,b**; a–c** |
| Sleep, depression | |||||
| PSQI | 3.6 ± 2.7 | 6.4 ± 3.4 | 4.4 ± 3.1 | F (2,41) = 3.3, P < 0.05 | a,b* |
| HAMD‐24 | 1.1 ± 1.6 | 3.3 ± 3.9 | 5.6 ± 7.2 | F (2182) = 18.4, P < 0.001 | a,b**; a–c**; b,c* |
Data are presented as means ± standard deviation (SD). HAMD‐24 = Hamilton Rating Scale For Depression, 24 items; PSQI = Pittsburgh Sleep Quality Index;
P < 0.05,
P < 0.001.
EEG sleep continuity
EEG sleep continuity measures of sleep latency, sleep efficiency and wake after sleep onset did not differ between the groups, covarying for ethnicity, education, smoking status and HAMD‐24 scores (Table 2). Cocaine‐dependent subjects had longer total sleep time, adjusted for multiple comparisons.
Table 2.
Polysomnographic sleep measures in participants.
| Controls a (n = 108) | Alcohol‐ dependent b (n = 73) | Cocaine‐ dependent c (n = 32) | F d ;, P | Post‐hoc comparison e | Pairwise effect sizes§ a,b; a–c; b,c | |
|---|---|---|---|---|---|---|
| Sleep continuity | ||||||
| Total sleep time, min | 363.1 ± 7.5 | 348.8 ± 8.6 | 393.4 ± 56.0 | F (2160) = 3.8, P < 0.05 | b,c** | 0.21; 0.43; 0.66 |
| Sleep efficiency, % | 80.1 ± 1.5 | 77.9 ± 1.7 | 81.4 ± 3.0 | F (2160) = 0.8, P = 0.44 | 0.16; 0.09; 0.26 | |
| Sleep latency, min | 22.4 ± 3.5 | 30.0 ± 4.0 | 20.1 ± 6.9 | F (2160) = 1.4, P = 0.24 | 0.24; 0.06; 0.31 | |
| Wakefulness after sleep onset, min | 63.3 ± 6.3 | 68.9 ± 7.2 | 74.0 ± 12.5 | F (2160) = 0.1, P = 0.93 | 0.01; 0.10; 0.09 | |
| Sleep architecture | ||||||
| Stage 1, min | 26.6 ± 1.8 | 31.0 ± 2.0 | 30.3 ± 3.5 | F (2158) = 1.3, P = 0.28 | 0.28; 0.22; 0.05 | |
| Stage 1, % | 7.0 ± 1.0 | 11.1 ± 1.1 | 9.3 ± 1.9 | F (2.158) = 2.9, P < 0.05 | a,b** | 0.48; 0.25; 0.21 |
| Stage 2, min | 236.8 ± 5.7 | 220.3 ± 6.5 | 247.9 ± 11.2 | F (2158) = 3.5, P < 0.05 | b,c*** | 0.33; 0.21; 0.55 |
| Stage 2, % | 65.2 ± 1.0 | 63.4 ± 1.1 | 63.1 ± 1.9 | F (2158) = 0.8, P = 0.45 | 0.21; 0.23; 0.03 | |
| Stage 3, min | 22.1 ± 1.8 | 9.9 ± 2.1 | 5.1 ± 3.6 | F (2158) = 11.3, P < 0.001 | a,b***; a–c*** | 0.75; 0.97; 0.29 |
| Stage 3, % | 6.2 ± 0.5 | 2.7 ± 0.6 | 1.1 ± 1.0 | F (2158) = 12.2, P < 0.001 | a,b***; a–c*** | 0.76; 10.04; 0.36 |
| Stage 4, min | 7.4 ± 1.4 | 3.0 ± 1.6 | 1.3 ± 2.7 | F (2158) = 2.5, P = 0.08 | 0.36; 0.46; 0.14 | |
| Stage 4, % | 2.0 ± 0.4 | 0.8 ± 0.4 | 0.2 ± 0.7 | F (2158) = 3.4, P < 0.05 | a,b* | 0.37; 0.53; 0.20 |
| Stage 3 + 4/SWS, min | 29.5 ± 2.8 | 12.9 ± 3.2 | 6.5 ± 5.6 | F (2158) = 8.7, P < 0.001 | a,b***; a–c** | 0.66; 0.86; 0.25 |
| Stage 3 + 4/SWS, % | 8.2 ± 0.8 | 3.5 ± 0.9 | 1.3 ± 1.5 | F (2158) = 9.9, P < 0.001 | a,b***; a–c*** | 0.68; 0.95; 0.33 |
| REM, min | 70.2 ± 3.8 | 84.7 ± 4.4 | 108.3 ± 7.6 | F (2158) = 8.5, P < 0.001 | a,b**; a–c***;b,c** | 0.43; 10.05; 0.69 |
| REM, % | 19.3 ± 0.8 | 23.7 ± 0.9 | 27.4 ± 1.6 | F (2158) = 10.2, P < 0.001 | a,b**; a–c*** | 0.61; 10.07; 0.52 |
| REM measures | ||||||
| Latency, corrected | 76.5 ± 7.3 | 63.5 ± 8.3 | 53.1 ± 14.4 | F (2158) = 1.1, P = 0.35 | 0.20; 0.34; 0.16 | |
| Density | 1.3 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.2 | F (2158) = 5.1, P < 0.05 | a,b**; a–c* | 0.56; 0.55; 0.04 |
| Duration, 1st period | 17.4 ± 1.4 | 20.1 ± 1.5 | 22.9 ± 2.7 | F (2161) = 1.5, P = 0.23 | 0.22; 0.43; 0.23 |
Group omnibus test covarying for African American race, education, smoking status and Hamilton Rating Scale For Depression, 24 items (HAMD‐24);
For post‐hoc comparisons and pairwise effect sizes, labels are controls (a), alcohol dependent (b), and cocaine dependent (c).
multiple comparisons, adjusted using Sidak correction;
P < 0.10,
P < 0.05,
P < 0.001; §Cohen's d, absolute value; SWS = slow wave sleep; REM = rapid eye movement sleep. Data are presented as adjusted means ± standard error (SE).
EEG sleep architecture
Several EEG sleep architecture differed between the groups, covarying for ethnicity, education, smoking status and HAMD‐24 scores (Table 2), with specific group differences identified adjusting for multiple comparisons. Compared to controls, alcohol‐dependent subjects showed increases in the percentage of Stage 1. Compared to alcohol‐dependent subjects, but not controls, there was a trend for cocaine‐dependent subjects to show increases in minutes of Stage 2 sleep. Compared to controls, alcohol‐ and cocaine‐dependent subjects showed a loss of Stage 3 and of SWS sleep, in which cocaine‐dependent subjects evidenced an almost complete absence of absolute and relative amounts of Stage 3 sleep. In contrast, both substance‐dependent groups showed increases in REM sleep, in which cocaine‐dependent subjects showed greater increases in REM sleep minutes and REM density compared to alcoholics. While cocaine‐dependent subjects showed shortened REM latency, the duration of REM during the first period did not differ.
Correlational analyses showed that age and BMI were correlated with Stage 3 and REM sleep (data not shown). Hence, further sensitivity analyses explored whether dichotomized grouping variables (cocaine‐dependent versus others, alcohol‐dependent versus others) predicted Stage 3 and REM sleep independently of age and BMI, as well as variables that differed between the groups (i.e. ethnicity, education, smoking and depressive symptoms). Regression analyses showed that cocaine and alcohol dependence remained significant and independent predictors of loss of Stage 3 sleep and increases in REM sleep (Table 3).
Table 3.
Demographic and clinical predictors of Stage 3 sleep, slow wave sleep and REM sleep.
| Standardized beta coefficients | ||||||
|---|---|---|---|---|---|---|
| Predictor | Stage 3 min | Stage 3% | SWS min | SWS % | REM min | REM % |
| Cocainea | −0.413*** | −0.461*** | −0.346** | −0.402*** | 0.488*** | 0.456*** |
| Alcoholb | −0.366*** | −0.401*** | −0.315** | −0.352*** | 0.268** | 0.323*** |
| BMI | −0.225** | −0.230** | −0.212** | −0.218** | 0.121 | 0.106 |
| Age | −0.163** | −0.092 | −0.234** | −0.168** | −0.175** | −0.048 |
| African American | −0.127 | −0.092 | −0.152* | −0.125 | −0.125 | −0.111 |
| Cigarette use | −0.060 | −0.033 | −0.021 | 0.008 | 0.091 | 0.102 |
| Education | 0.036 | 0.022 | 0.072 | 0.061 | −0.043 | −0.036 |
| HAMD‐24 | 0.043 | 0.041 | 0.084 | 0.090 | −0.146* | −0.201** |
| Overall R 2 | 0.343*** | 0.327*** | 0.312*** | 0.294*** | 0.244*** | 0.223*** |
Cocaine group versus others,
alcohol group versus others; BMI = body mass index, HAMD‐24 = Hamilton Rating Scale For Depression, 24 items; REM = rapid eye movement, SWS = slow wave sleep;
P < 0.10,
P < 0.05,
P < 0.001.
EEG sleep architecture and age
Given that Stage 3 sleep deteriorates with older age 31 and that prior studies have suggested that alcoholics show an accelerated loss of Stage 3 sleep with age 20, additional analyses tested whether substance dependence interacted with age in the loss of Stage 3 sleep. A significant group × age interaction was found for the percentage of Stage 3 sleep (F = 2.28, P < 0.05) (Fig. 1). To evaluate this interaction further, stratification of the sample by 7‐year age categories showed that cocaine‐dependent subjects had a significantly lower percentage of Stage 3 sleep, compared to controls, at two age strata (25–32 and 41–48 years) with trends at the other two strata, and also showed loss of Stage 3 sleep at an earlier age than found in alcohol dependence. The alcohol‐dependent group had significantly lower Stage 3 sleep, compared to controls only at two age strata (i.e. 25–32 and 41–48 years).
Figure 1.
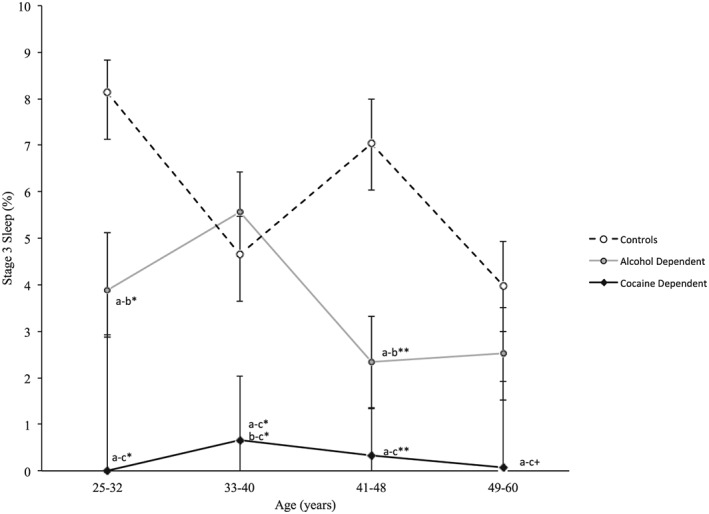
Percentage of Stage 3 sleep across age strata for the controls (a), and alcohol‐ (b) and cocaine‐dependent (c) groups. A significant group × age interaction for percentage of Stage 3 sleep was found (F = 2.28, P < 0.05). Data are presented as means ± standard error (SE). Adjusting for multiple comparisons, Sidak corrected between‐group differences were identified: +P < 0.10, *P < 0.05, **P < 0.001. Effect sizes (d) a,b, a–c, b,c: age group, 25–32 years: 1.52, 2.37, 0.89; age group, 33–40 years: 0.20, 0.86, 1.06; age group, 41–48 years: 1.12, 1.60, 0.48; age group, 49–60 years: 0.42, 1.19, 0.77
Older age is also associated with increases in REM sleep 31, and additional exploratory analyses examined whether substance dependence interacted with age in the increase of REM sleep. A significant group × age interaction was found for percentage of REM sleep (F = 2.27, P < 0.05; Fig. 2). Compared to controls, both alcohol‐ and cocaine‐dependent subjects had a higher percentage of REM sleep at age strata 41–48 years, with a trend for alcohol‐dependent subjects to also have a higher percentage of REM sleep at age strata 33–40 years.
Figure 2.
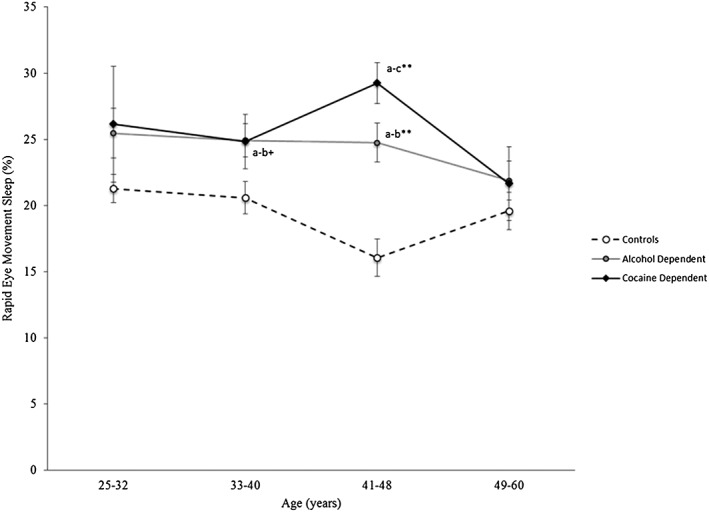
Percentage of rapid eye movement (REM) sleep across age strata for the controls (a), and alcohol‐ (b) and cocaine‐dependent (c) groups. A significant group × age interaction for percentage of REM sleep was found (F = 2.27, P < 0.05). Data are presented as means ± standard error (SE). Adjusting for multiple comparisons, Sidak corrected between‐group differences were found: +P < 0.10, *P < 0.05, **P < 0.001. Effect sizes (d) a,b, a–c, b,c: age group, 25–32 years: 0.61, 0.83, 0.23; age group, 33–40 years: 0.65, 0.59, 0.02; age group, 41–48 years: 1.16, 1.64, 1.05; age group, 49–60 years: 0.20, 0.15, 0.05
Discussion
This study provides an evaluation of objective PSG sleep in substance‐dependent patients during early abstinence compared to never mentally ill controls who were similar in age and sex. In addition, this study examines, for the first time, differences in objective measures of sleep between cocaine‐ and alcohol‐dependent subjects. These novel results show that substance dependence is associated with robust alterations in sleep architecture, including decreased amounts of Stage 3 sleep and SWS, and increased amounts of REM sleep compared to controls. Moreover, cocaine‐dependent subjects show a profound loss of SWS, in which Stages 3 and 4 are almost completely absent and reduced substantially compared to both alcohol‐dependent subjects and controls. Finally, it appears that substance dependence accelerates age‐related loss of Stage 3 sleep. Amounts of Stage 3 in young adult cocaine‐dependent subjects were comparable to low levels found in controls 30 years their senior, with similar but less robust decreases found in alcohol‐dependent subjects. Importantly, these effects were identified from a controlled comparison, using clinically homogeneous groups of cocaine‐ or alcohol‐dependent subjects, in which group differences were not confounded by other patient characteristics. Given that increased REM pressure has prognostic value for relapse in alcohol dependence, measures of sleep architecture have the potential to identify those substance‐dependent patients for increased monitoring following treatment. Alternatively, targeting sleep disturbance by pharmacologically augmenting SWS, for example, and/or reciprocally decreasing REM sleep, has the potential to improve treatment outcomes in alcohol‐ and cocaine‐dependent people 22, 32.
The profound loss of SWS in cocaine dependence, as well as in alcohol‐dependent subjects, is consistent with prior findings that suggest a defect in the homeostatic regulation of sleep in these subjects 17. For example, in those with alcohol dependence, we have found that experimental sleep loss fails to induce increases in SWS and increases in low frequency or delta spectral activity compared to robust increases in controls 17. Despite recent daily use of cocaine in the month prior to early abstinence, which would probably lead to impairments in sleep quality and naturalistic sleep loss, cocaine‐dependent subjects also showed low levels of SWS, suggesting that this population might also have a defect in the homeostatic drive for sleep. Importantly, even with sustained abstinence, evidence suggests that loss of SWS persists in alcohol dependence 18, 30, and possibly cocaine dependence 4, 5.
Loss of sleep depth has implications for morbid outcomes associated with cocaine and alcohol dependence, including risk of cardiovascular disease, hypertension and infectious disease, because sleep depth is thought to contribute to the maintenance of health and the homeostatic regulation of the autonomic, neuroendocrine and immune systems 33. For example, sympathetic activation and nocturnal blood pressure is lowest during SWS, and a failure to show a nocturnal decrease in markers of sympathetic activity is associated with increases in daytime blood pressure 34, 35. In addition, nocturnal sleep supports adaptive immunity, especially during the early portion of the night when SWS is dominant 33, 36. Moreover, we have found that cocaine administration abrogates Toll‐like receptor 4 (TLR‐4) activation, critical in priming anti‐viral immunity 24; such loss of anti‐viral immune responses occurs predominantly during the night in cocaine‐dependent subjects in association with decreases in Stage 3 sleep.
Amounts of REM were also increased in substance‐dependent subjects during abstinence, with cocaine dependence being associated with the greatest increase in REM sleep. Prior controlled studies have found increases in REM sleep in alcohol dependence 20, 21, 37, with similar effects reported in cocaine dependence 13. The neurobiological mechanisms that underlie the increases of REM sleep in substance dependence are not known, although activation of inflammatory mechanisms may play a role, as reviewed previously 33. Increases in mononuclear cell production of interleukin (IL)‐6 and tumour necrosis factor (TNF)‐α correlates with REM sleep amounts 38, 39, 40, and blockade of TNF partially normalizes REM sleep, in which the decrease in REM correlates with the circulating level of the biologically active TNF receptor antagonist 37.
In contrast with prior evidence 4, 16, 20, limited support was obtained to suggest disturbances of sleep continuity in cocaine‐ and alcohol‐dependent subjects during early abstinence. While total sleep time was increased in cocaine‐dependent subjects, sleep continuity did not differ. Notably, absence of sleep continuity disturbances contrasts with prior reports of impairments in sleep quality 4, 5, 6, which may be due to increased perception of sleep problems. Finally, this study evaluated substance‐dependent subjects during early abstinence after acute withdrawal symptoms had resolved. While alterations in sleep continuity vary across the abstinence period, disturbances in sleep architecture, including loss of SWS, do not change substantially within a month of abstinence in cocaine and alcohol dependence 6, 41, 42.
Several limitations require consideration. While the groups were comparable for age, gender and BMI, the cocaine‐dependent subjects were mainly African American. We have reported differences in sleep architecture previously, including loss of SWS in association with African American ethnicity 17, especially in African American alcoholic men. However, when analyses controlled for African American ethnicity, group differences in sleep architecture were still found. Secondly, groups differed on depressive symptoms, although none of the subjects fulfilled diagnostic criteria for current depression, and depressive symptom scores showed only mild severity. Although depressive symptom severity was associated with REM sleep, no relationship was found with SWS. Thirdly, alcohol consumption was higher in the cocaine‐dependent subjects compared to the controls. Fourthly, these findings may not be demonstrated in women, as the majority of subjects were men. Finally, the substance dependence groups were clinically homogeneous, and the differences may not be generalizable to polysubstance abusers who have, for example, both alcohol and cocaine dependence.
In summary, this study shows that sleep architecture is disturbed markedly in cocaine‐ and alcohol‐dependent subjects. Further studies are needed to determine the natural history, clinical and prognostic significance, and the neurobiological mechanisms that might contribute to these changes. Nevertheless, this report represents an important step in characterizing the severity of sleep disturbance in understudied subjects with cocaine or alcohol dependence.
Declaration of interests
None.
Acknowledgements
This study was supported by grants R01 AA 13239 from the National Institute of Alcohol and Alcohol Abuse and RO1 DA16541 from the National Institute of Drug Abuse, and other grant support from the National Institutes of Health to MRI, including R01‐AG034588; R01‐CA119159; R01‐HL079955; R01 DA032922‐01; R01 HL095799; P30‐AG028748; P30‐AG017265; UL RR 033176; the Cousins Center for Psychoneuroimmunology; and UCLA Claude D. Pepper Older Americans Independence Center. The National Institutes of Health had no role in the design and conduct of the study.
Irwin, M. R. , Bjurstrom, M. F. , and Olmstead, R. (2016) Polysomnographic measures of sleep in cocaine dependence and alcohol dependence: Implications for age‐related loss of slow wave, stage 3 sleep. Addiction, 111: 1084–1092. doi: 10.1111/add.13300.
References
- 1. Degenhardt L., Whiteford H. A., Ferrari A. J., Baxter A. J., Charlson F. J., Hall W. D., et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet 2013:; 382: 1564–74. [DOI] [PubMed] [Google Scholar]
- 2. Parry C. D., Patra J., Rehm J. Alcohol consumption and non‐communicable diseases: epidemiology and policy implications. Addiction 2011:; 106: 1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Survey of Drug Use and Health . Results from the 2012 National Survey on Drug Use and Health (NSDUH): Summary of National Findings (United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality). Available at: http://doi.org/10.3886/ICPSR34933.v2. Rockville, MD: 2013.
- 4. Schierenbeck T., Riemann D., Berger M., Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev 2008; 12: 381–9. [DOI] [PubMed] [Google Scholar]
- 5. Angarita G. A., Canavan S. V., Forselius E., Bessette A., Pittman B., Morgan P. T. Abstinence‐related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depend 2014;; 134: 343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan P. T., Malison R. T. Cocaine and sleep: early abstinence. Scientific World Journal 2007; 7: 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan P. T., Pace‐Schott E. F., Sahul Z. H., Coric V., Stickgold R., Malison R. T. Sleep architecture, cocaine and visual learning. Addiction 2008:; 103: 1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan P. T., Pace‐Schott E. F., Sahul Z. H., Coric V., Stickgold R., Malison R. T. Sleep, sleep‐dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend 2006:; 82: 238–49. [DOI] [PubMed] [Google Scholar]
- 9. Pace‐Schott E. F., Stickgold R., Muzur A., Wigren P. E., Ward A. S., Hart C. L., et al. Sleep quality deteriorates over a binge–abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005; 179: 873–83. [DOI] [PubMed] [Google Scholar]
- 10. Johanson C. E., Roehrs T., Schuh K., Warbasse L. The effects of cocaine on mood and sleep in cocaine‐dependent males. Exp Clin Psychopharmacol 1999; 7: 338–46. [DOI] [PubMed] [Google Scholar]
- 11. Watson R., Bakos L., Compton P., Gawin F. Cocaine use and withdrawal: the effect on sleep and mood. Am J Drug Alcohol Abuse 1992; 18: 21–8. [DOI] [PubMed] [Google Scholar]
- 12. Kowatch R. A., Schnoll S. S., Knisely J. S., Green D., Elswick R. K. Electroencephalographic sleep and mood during cocaine withdrawal. J Addict Dis 1992; 11: 21–45. [DOI] [PubMed] [Google Scholar]
- 13. Thompson P. M., Gillin J. C., Golshan S., Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short‐term abstinence. Biol Psychiatry 1995; 38: 831–6. [DOI] [PubMed] [Google Scholar]
- 14. Morgan P. T., Paliwal P., Malison R. T., Sinha R. Sex differences in sleep and sleep‐dependent learning in abstinent cocaine users. Pharmacol Biochem Behav 2009; 93: 54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brower K. J., Krentzman A., Robinson E. A. Persistent insomnia, abstinence, and moderate drinking in alcohol‐dependent individuals. Am J Addict 2011; 20: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brower K. J. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol 2015; 49: 417–27. [DOI] [PubMed] [Google Scholar]
- 17. Irwin M., Gillin J. C., Dang J., Weissman J., Phillips E., Ehlers C. L. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry 2002; 51: 632–41. [DOI] [PubMed] [Google Scholar]
- 18. Drummond S. P., Gillin J. C., Smith T. L., DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res 1998; 22: 1796–802. [PubMed] [Google Scholar]
- 19. Gillin J. C., Smith T. L., Irwin M., Butters N., Demodena A., Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3‐month follow‐up. Arch Gen Psychiatry 1994; 51: 189–97. [DOI] [PubMed] [Google Scholar]
- 20. Gillin J. C., Smith T. L., Irwin M., Kripke D. F., Schuckit M. EEG sleep studies in ‘pure' primary alcoholism during subacute withdrawal: relationships to normal controls, age, and other clinical variables. Biol Psychiatry 1990; 27: 477–88. [DOI] [PubMed] [Google Scholar]
- 21. Colrain I. M., Turlington S., Baker F. C. Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep 2009; 32: 1341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brower K. J. Insomnia, alcoholism and relapse. Sleep Med Rev 2003; 7: 523–39. [DOI] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders DSM‐IV‐TR, 4th edn Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 24. Irwin M. R., Olmos L., Wang M., Valladares E. M., Motivala S. J., Fong T., et al. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J Pharmacol Exp Ther 2007; 320: 507–15. [DOI] [PubMed] [Google Scholar]
- 25. First, M. B. , Spitzer, R. L. , Gibbon, M. , Williams, J. B. Structured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition, version 2.0. New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- 26. Bucholz K., Cadoret R., Cloninger C., Dinwiddie S., Hesselbrock V., Nurnberger J. J., et al. A new, semi‐structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 1994; 55: 149–58. [DOI] [PubMed] [Google Scholar]
- 27. Cole J. C., Motivala S. J., Buysse D. J., Oxman M. N., Levin M. J., Irwin M. R. Validation of a 3‐factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep 2006; 29: 112–6. [DOI] [PubMed] [Google Scholar]
- 28. Endicott J., Cohen J., Nee J., Fleiss J., Sarantakos S. Hamilton Depression Rating Scale. Arch Gen Psychiatry 1981; 38: 98–103. [DOI] [PubMed] [Google Scholar]
- 29. Rechtschaffen, A. , Kales, A . A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda: National Institute of Neurological Disorders and Stroke (NINDS); 1968. [Google Scholar]
- 30. Gillin J. C., Smith T. L., Irwin M., Kripke D. F., Brown S., Schuckit M. Short REM latency in primary alcoholic patients with secondary depression. Am J Psychiatry 1990; 147: 106–9. [DOI] [PubMed] [Google Scholar]
- 31. Ohayon M. M., Carskadon M. A., Guilleminault C., Vitiello M. V. Meta‐analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004; 27: 1255–73. [DOI] [PubMed] [Google Scholar]
- 32. Brower K. J., Aldrich M. S., Hall J. M. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res 1998; 22: 1864–71. [PubMed] [Google Scholar]
- 33. Irwin M. R. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015; 66: 2.1–2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irwin M. R., Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension 2005; 45: 252–7. [DOI] [PubMed] [Google Scholar]
- 35. Irwin M., Thompson J., Miller C., Gillin J. C., Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin‐2 levels in humans: clinical implications. J Clin Endocrinol Metab 1999; 84: 1979–85. [DOI] [PubMed] [Google Scholar]
- 36. Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch 2012; 463: 121–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irwin M. R., Olmstead R., Valladares E. M., Breen E. C., Ehlers C. L. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry 2009; 66: 191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Irwin M., Rinetti G., Redwine L., Motivala S., Dang J., Ehlers C. Nocturnal proinflammatory cytokine‐associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun 2004; 18: 349–60. [DOI] [PubMed] [Google Scholar]
- 39. Redwine L., Dang J., Hall M., Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med 2003; 65: 75–85. [DOI] [PubMed] [Google Scholar]
- 40. Irwin M., Rinetti G., Redwine L., Motivala S., Ehlers C. L. Proinflammatory cytokines and disordered sleep in alcohol dependence. Psychosom Med 2003; 65: A3–4. [Google Scholar]
- 41. Valladares E. M., Irwin M. R. Polysomnographic sleep dysregulation in cocaine dependence. Scientific World Journal 2007; 7: 213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matuskey D., Pittman B., Forselius E., Malison R. T., Morgan P. T. A multistudy analysis of the effects of early cocaine abstinence on sleep. Drug Alcohol Depend 2011; 115: 62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


