Abstract
Background
Cerebellar ataxia is an exclusion criterion for clinical diagnosis of progressive supranuclear palsy (PSP), but a variant with predominant cerebellar ataxia has been reported. The aims of this study were to estimate the frequency of PSP with predominant cerebellar ataxia in an autopsy series from the United States, and to compare clinical, pathologic, and genetic differences between PSP with and without predominant cerebellar ataxia.
Methods
We selected 100 consecutive patients with pathologically-confirmed PSP who had been evaluated at Mayo Clinic (referred to as the Mayo Clinic patient series) from our brain bank database (N = 1085). We next enriched in cases likely to have cerebellar ataxia by searching the remaining 985 cases for (1) an antemortem diagnosis of multiple system atrophy (MSA), or (2) neuropathological evidence of prominent degeneration of the cerebellum or cerebellar afferent nuclei. Subsequently, clinical, pathologic and genetic features were compared between the two groups.
Results
One patient in the Mayo Clinic patient series (1%) met criteria for PSP with predominant cerebellar ataxia and had both cerebellar and mild midbrain atrophy on MRI. Four patients were identified with the targeted search. Four of the five patients were clinically misdiagnosed as MSA. The severity of tau-related pathology and cerebellar degeneration were not different between the two groups. No differences were detected in tau genotypes.
Conclusions
While our data cannot provide definitive information about how to make an accurate clinical diagnosis, it should serve to raise awareness of PSP with predominant cerebellar ataxia in the differential diagnosis of MSA.
Keywords: Cerebellar ataxia, Clinicopathological study, Progressive supranuclear palsy, Multiple system atrophy, MAPT genotype
Introduction
Progressive supranuclear palsy (PSP) is an atypical parkinsonian disorder associated with supranuclear gaze palsy, dysarthria, dysphagia, balance problems, falls and cognitive impairment.1-6 This clinical heterogeneity and lack of specific biomarkers make PSP difficult to diagnose before the patient's death.7-9 Our previous study has revealed that patients with PSP are sometimes misdiagnosed as multiple system atrophy (MSA) because of the presence of cerebellar ataxia.10 Although the first report of PSP described 4 of 9 patients with cerebellar ataxia,2 cerebellar ataxia is one of the exclusion criteria in clinical criteria for PSP.5 Recent studies, however, have reported pathologically-PSP patients with predominant cerebellar ataxia as their initial and principal symptoms (PSP-C).11-14 The frequency of PSP-C varies considerably between cohorts of European descent (0% (0/30) in Austria 15 and 0% (0/100) in a multicenter study in Europe and Canada 16) and in Japanese (14% (3/22) in Japan).11 There are no studies addressing the frequency of PSP-C in the United States. Distinctive pathological features of PSP-C are suggested to include excessive Purkinje cell loss,17 more severe tau pathology in the cerebellar dentate nucleus, and greater frequency of tau-positive inclusions in Purkinje cells.11 The aims of this study were to estimate the frequency of PSP-C in a large autopsy series of PSP from the United States, and to assess clinical, pathologic, and genetic features of PSP-C compared with PSP.
Subjects and Methods
Patient selection
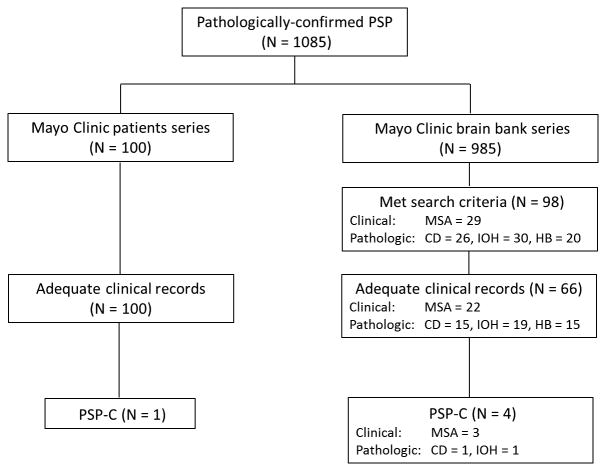
To estimate the frequency of PSP-C, we selected 100 consecutive patients with pathologically-confirmed PSP that had been evaluated at Mayo Clinic between 1998 and 2014 (referred to as the Mayo Clinic patient series) from a brain bank of 1085 pathologically-confirmed PSP patients. In order to enrich for PSP patients that would have ataxia or other cerebellar signs, we searched the database for (1) those with an antemortem diagnosis of MSA, or (2) neuropathologic findings of cerebellar degeneration, inferior olivary hypertrophy, or prominent hindbrain tau pathology (referred to as the Mayo Clinic brain bank series).18 The study design is shown schematically in figure 1.
Figure 1.
Flow chart of study design. Abbreviations: PSP, progressive supranuclear palsy; PSP-C, progressive supranuclear palsy with predominant cerebellar ataxia; MSA, multiple system atrophy; Cbl, cerebellar degeneration; IOH, inferior olivary hypertrophy; HB, hindbrain predominant PSP.
Pathological assessment and diagnosis
All brain autopsies were obtained after consent of the legal next-of-kin or one with legal power of attorney and all underwent a standardized neuropathological assessment. The Mayo Clinic brain bank operates under protocols approved by the Mayo Clinic IRB. One neuropathologist (D.W.D.) made all neuropathological diagnoses. The neuropathologic protocol includes assessment of Alzheimer-type pathology,19 including assignment of Braak neurofibrillary tangle (NFT) stage 20 and Thal amyloid phase 21 with thioflavin S fluorescent microscopy.22 Immunohistochemistry for tau (CP13, 1:1000, from Dr. Peter Davies, Feinstein Institute, North Shore Hospital, NY) was used to establish neuropathological diagnosis of PSP.5 Evaluation of the densities of pretangles/NFT, coiled bodies and tau-positive threads in the cerebellum, dentate nucleus, inferior olivary nucleus, pontine base, subthalamic nucleus, substantia nigra, and globus pallidus was performed semiquantitatively using a four-point scale (0, absent; 1, mild; 2, moderate; 3, severe pathology).23 To assess the frequency of tau-positive Purkinje cells, the number of CP13-positive Purkinje cells was counted in slides of cerebellar vermis. Diagnosis of cerebellar degeneration and inferior olivary hypertrophy were made as part of the standard neuropathologic evaluation. The diagnosis of cerebellar degeneration was made by loss of Purkinje cells and granule cells with accompanying Bergmann gliosis. The diagnosis of inferior olivary hypertrophy was made by detecting swollen and fenestrated neurons and bizarre astrocytes in hematoxylin and eosin (H&E)-stained slides of medulla.24
Quantitative analysis of cerebellar Purkinje cells
To systematically assess Purkinje cell loss in the cerebellar vermis, we measured the linear densities of Purkinje cells (cells/mm) as described in other studies of human cerebellum.25, 26 H&E-stained slides of cerebellar vermis were scanned on the Aperio ScanScope XT slide scanner (Aperio, Vista, CA) and converted to high-resolution digital images. A single observer (S.K.) randomly chose regions of the vermis where the folia were cut perpendicular to the long axis on the folia. The observer drew 10 lines per slide and the number Purkinje cells along linear segments was counted. Purkinje cell layer length was measured using Aperio ImageScope tool (Aperio) (Fig. 2A). Linear density was calculated as the total number of Purkinje cells divided by the line length.
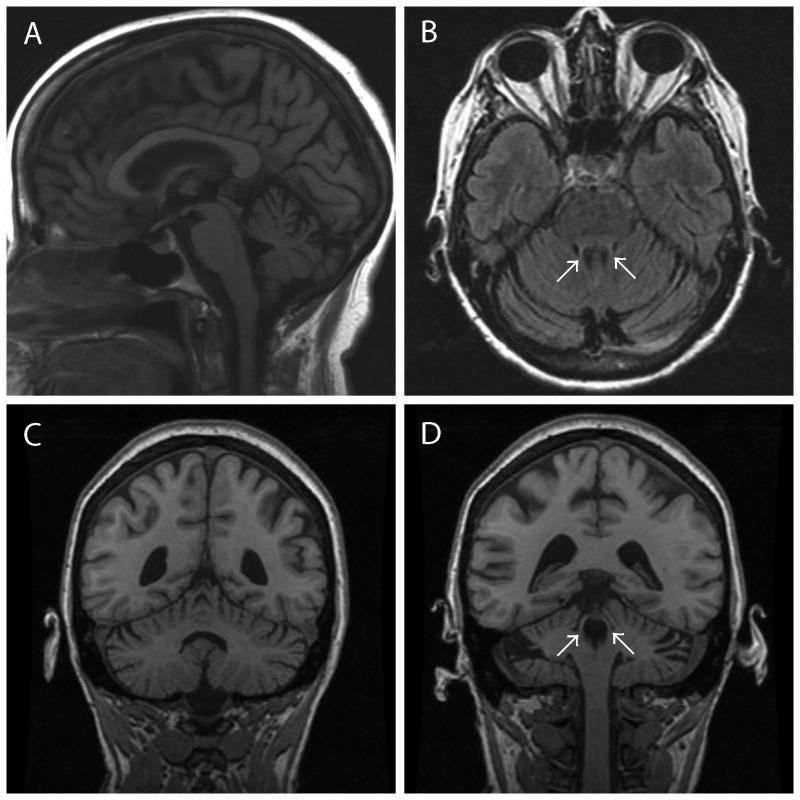
Figure 2.
Representative MRI findings of PSP-C patient 1 at age 62. A Sagittal section on FLAIR reveals obvious cerebellar atrophy and mild atrophy of midbrain (A). An axial section on FLAIR shows thin superior cerebellar peduncles and cerebellar atrophy (B). Coronal sections on T1-weighted images disclose atrophy of cerebellar vermis (C) and superior cerebellar peduncles (D). Arrows indicate the superior cerebellar peduncles (B, D).
Clinical Assessment
A diagnosis of PSP-C was rendered in any patient with a pathological diagnosis of PSP in which the clinical records revealed cerebellar ataxia including ataxic gait, limb ataxia, ataxic speech, and nystagmus as the initial and principal symptom over the course of the disease.11, 12 For further clinicopathologic analysis, we defined “PSP with ataxia” as patients with any degree of cerebellar ataxia, including those that met criteria for PSP-C. We considered patients to have cerebellar ataxia based on the descriptions of neurologists or movement disorder specialists who examined the patients. Clinical data abstraction was blinded to pathological information. Clinical data were abstracted from available medical records collected throughout the course of disease and entered into a database and included: sex, age at symptomatic onset (time of first reported symptom), age at death, initial and final clinical diagnoses, signs and symptoms recorded during the disease course and their timing, neurological findings as documented by a neurologist or movement disorder specialist.27 For each patient, a particular clinical symptom or sign was considered present if specifically stated as present in the clinical records. If clinical symptoms or signs were not described, then for the purpose of analysis, they were considered to be absent. The information on symptoms was gathered from a combination of medical records and pathology records summarizing clinical history.
Genetic Assessments
DNA from frozen brain tissue was obtained with standard protocols. For genotyping, genomic DNA was extracted from frozen brain by standard procedures. Genotyping for MAPT H1/H2 (SNP rs1052554 A/G, A = H1, G = H2) and APOE alleles (SNP rs429358 C/T and rs7412 C/T) was performed using a Taqman SNP genotyping assay (Applied Biosystems, Carlsbad, CA, USA). Genotype calls were obtained with SDS v2.2.2 software (Applied Biosystems).28
Statistical Analysis
All statistical analyses and generation of the graphs were performed in SigmaPlot 12.3 (Systat software, San Jose, CA). Chi-square test or Fisher's exact test were performed for group comparisons of categorical data as appropriate. The t-test or Mann-Whitney U test was used for analyses of continuous variables as appropriate. P-values <0.05 were considered statistically significant.
Results
Demographic and clinical features of PSP patients
In the Mayo Clinic patient series, all 100 patients had adequate medical documentation. One patient met criteria for PSP-C; hence, the frequency of PSP-C in this series was 1%. Among the remaining 985 pathologically-confirmed PSP patients, we identified 98 patients who met search criteria, and 66 of them had adequate medical records (Fig. 1). Four patients met criteria for PSP-C: 2 for clinical diagnosis of MSA, 1 for clinical diagnosis of MSA and cerebellar degeneration, and 1 for pathologic diagnosis of inferior olivary hypertrophy. Therefore, in total, we identified five patients that met criteria for PSP-C (Fig. 1). Clinical and pathologic information for the five PSP-C patients are summarized in the supplementary table. Clinical and pathologic information comparing PSP-C and PSP (data derived from the other 99 patients from Mayo Clinic patient series) are shown in table 1. Early falls and vertical gaze palsy, which are characteristic clinical features of PSP, were less frequent in PSP-C. Autonomic failure, fulfilling Gilman's criteria for clinical diagnosis of probable MSA29 was not noted in any of the PSP-C patients.
Table 1. Clinical and pathologic features comparing between PSP-C vs PSP.
| Features | PSP-C (5) | PSP (99) | P value |
|---|---|---|---|
| Demographic | |||
| Sex, % male | 2 (40%) | 61 (62%) | 0.690a |
| Age at onset | 67.8 ± 5.4 | 66.9 ± 7.7 | 0.789b |
| Age at death | 77.4 ± 4.7 | 74.2 ± 8.3 | 0.406b |
| Disease Duration | 7.0 (5, 9) | 8.0 (8, 8) | 0.171c |
| Symptoms | |||
| Falls | 4 (80%) | 90 (91%) | 0.976 a |
| Early falls | 0 (0%) | 62 (63%) | 0.020 a |
| Memory cognitive complaints | 4 (80%) | 69 (70%) | 0.992 a |
| Speech/voice complaints | 5 (100%) | 83 (84%) | 0.732 a |
| Swallowing difficulties/choking | 3 (60%) | 64 (65%) | 0.789 a |
| Neurological signs | |||
| Axial/neck rigidity | 3 (60%) | 91 (93%) | 0.113 a |
| Resting tremor | 0 (0%) | 18 (18%) | 0.658 a |
| Vertical supranuclear gaze palsy | 1 (20%) | 82 (83%) | 0.004 a |
| Gait ataxia | 5 (100%) | 23 (24%) | 0.001 a |
| Limb ataxia | 5 (100%) | 15 (15%) | <0.001 a |
| Nystagmus | 0 (0%) | 10 (10%) | 0.976 a |
| Upper motor neuron signs | 4 (80%) | 39 (39%) | 0.182 a |
| Pathologic features | |||
| Brain weight (g) | 1148 ± 88 | 1184 ± 146 | 0.573 b |
| Braak NFT stage | IV (0, IV) | II (I, III) | 0.532 c |
| Thal amyloid phase | 0 (0, 3) | 0 (0, 3) | 0.777 c |
| Ratio of Purkinje tau positive | 3/5 (60%) | 61/97 (62%) | 0.731 a |
| Number of pretangles in Purkinje cell layer | 1.0 (0, 2) | 1.0 (0, 3) | 0.490 c |
| Pretangles linear density (cells/mm) | 3.3 (3, 4) | 3.6 (3, 4) | 0.357 c |
| Semi-quantitative analysis | |||
| Coiled bodies in cerebellar white matter | 2.0 (2, 2) | 2.0 (1, 3) | 0.704 c |
| Tau threads in cerebellar white matter | 2.0 (2, 2) | 2.0 (1, 2) | 0.674 c |
| Coiled bodies in dentate nucleus | 1.0 (0, 1) | 1.0 (0, 1) | 0.948 c |
| Tau threads in dentate nucleus | 2.0 (1, 2) | 2.0 (1, 2) | 0.918 c |
| Pretangles/NFT in dentate nucleus | 2.0 (2, 2) | 3.0 (2, 3) | 0.128 c |
| Pretangles/NFT in pontine base | 3.0(3, 3) | 3.0 (2, 3) | 0.509 c |
| Pretangles/NFT in inferior olivary nucleus | 2.0 (2, 2) | 2.0 (1, 2) | 0.305 c |
| Genetic analysis | |||
| MAPT: H1H1 genotype | 5/5 (100%) | 73/82 (89%) | 0.434 a |
| MAPT: H1H2 genotype | 0/5 (0%) | 9/82 (11%) | 0.434 a |
| APOE: ε4 genotype: | 2/5 (40%) | 20/87 (23%) | 0.386 a |
Data are displayed as mean ± SD or median (25th, 75th range) as appropriate. Abbreviations: PSP, progressive supranuclear palsy; PSP-C, PSP, progressive supranuclear palsy with predominant cerebellar ataxia; NFT, neurofibrillary tangles.
Fisher exact test,
t-test,
Mann-Whitney rank sum test
Patients reports of PSP-C
Patient 1
A 71-year-old Caucasian woman had a 9-year history of ataxic gait. Her family history was negative for neurodegenerative disease. At age 62, she started having unsteady gait and was initially diagnosed with alcoholic ataxia due to a 20-year history of alcohol abuse and MRI finding of cerebellar atrophy (Fig. 2). In retrospect, the MRI also had atrophy of superior cerebellar peduncle and mild atrophy of the midbrain (Fig. 2), which are characteristic findings in PSP.30, 31 In contrast, there was no atrophy of middle cerebellar peduncle, “hot cross bun” sign or hummingbird sign. Her symptoms progressed over the years, and her gait ataxia worsened. She also developed appendicular ataxia. Genetic screens for mutations causing spinocerebellar atrophy 1, 2, 3, 6, 7, 8, 10, 17, dentato-rubro-pallido-Luysian atrophy, and Friedreich's ataxia were negative. She developed appendicular rigidity, vertical supranuclear gaze palsy, spastic dysarthria, pyramidal signs, urinary incontinence, dizziness, and photophobia She also had cognitive impairment consistent with frontal lobe dysfunction and executive difficulties. Truncal rigidity and tremors were not described. Based on her cerebellar ataxia, parkinsonian symptoms, autonomic failure, and pyramidal signs, her final clinical diagnosis was MSA-C.
Patient 2
An 81-year-old Caucasian woman had a 7-year history of cerebellar ataxia. Her family history was positive for a sister with Friedreich's ataxia. She developed slurring of speech at age 74, and was diagnosed with ataxic dysarthria; she also had pancerebellar ataxia affecting upper and lower limbs, as well as gait. The results of DNA examination (similar test battery as in patient 1) were negative. MRI of the brain revealed cerebellar atrophy and signal changes in white matter of the brainstem. At age 78, she developed swallowing difficulty and mild memory decline. Neurological examination at age 78 revealed slow saccades without vertical supranuclear gaze palsy and asymmetric lower extremity ataxia worse on the left. Rapid alternating hand movements were very slow, more so on the left than right. She needed a cane to ambulate due to her unsteady gait, and she could not perform tandem gait. She was diagnosed with MSA-C.
Patient 3
A 74-year-old Caucasian man had an 8-year history of cerebellar ataxia. His family history was negative for neurodegenerative disorders. He presented with progressive gait unsteadiness and poor coordination of his limbs at age 66, when he was first noted to have evidence of ataxia. He also had intermittent syncope and episodes of rapid eye movement sleep behavior disorder. Neurological examination at age 71 showed normal cognitive function and eye movements without nystagmus. He had an ataxic dysarthria. He had increased muscle tone, with rigidity in his upper and lower extremities, as well as bradykinesia. Truncal rigidity was not described. His reflexes were brisk, and his plantar reflexes were up-going. He was not ambulatory due to severe ataxia of his lower extremities. The upper extremities had minimal ataxia. He was diagnosed with MSA-C.
Patient 4
A 79-year-old Caucasian man had a 6-year history of ataxic gait and cognitive impairment. He had a family history of Alzheimer's disease in his sister and his aunt. He developed balance problems and an ataxic gait at age 73. On examination he could not stand on one leg or perform tandem gait. He required support to ambulate. Over time he fell frequently, usually forward, and he developed short-term memory loss and personality change, being described as aggressive and angry. On neurological examination at age 74, eye movements were intact, and no parkinsonian symptoms were observed. Given a medical history of type II diabetes mellitus, his ataxia was considered to be largely due to a peripheral neuropathy. At age 75, neurological examination revealed axial rigidity, a few beats of slow nystagmus in a horizontal direction, limitation of upward gaze, truncal ataxia, and finger-to-nose dysmetria. The possibility of a paraneoplastic cerebellar ataxia and progressive supranuclear palsy were considered. An MRI of the brain showed mild generalized brain atrophy and ventricular enlargement. Atrophy of midbrain and superior cerebellar peduncles were not described. By age 76 his speech became slurred and swallowing was affected. At the age of 76, he was diagnosed with PSP.
Patient 5
An 82-year-old Caucasian woman had an 18-year history of cerebellar ataxia. Her family history was negative for neurodegenerative disease. She presented initially with slurred speech and gait unsteadiness at age 64. Examination revealed gait ataxia. Her gait difficulty progressed slowly, and by age 71 she was using a wheelchair. Although the etiology of her symptoms was unknown, she was given a tentative diagnosis of vitamin B12 deficiency at age 73. She later developed dysphagia, urinary incontinence, cognitive decline, and visual hallucinations. Neurological examination at age 81 revealed ataxic dysarthria, slow saccadic pursuit, limb dysmetria on finger-to-nose testing, and cognitive impairment, including impairment of concentration and attention. Supranuclear gaze palsy, truncal rigidity, and tremors were not described. An MRI of the brain revealed midline cerebellar atrophy. Atrophy of midbrain and superior cerebellar peduncle were not described. She was diagnosed with MSA-C.
Pathological Assessment
Diffuse cytoplasmic phospho-tau immunoreactivity (pretangles), tau-positive thread-like processes, and oligodendroglial tau-positive inclusions (so-called “coiled bodies”) were found in cerebellar dentate nucleus (Fig. 2B) or cerebellar white matter (Fig. 2C) in both PSP-C (N = 5) and PSP (N = 99 in the Mayo Clinic patient series). Purkinje cells with cytoplasmic phospho-tau immunoreactivity (pretangles), but without obvious NFT formation (Fig. 2D), were found in 60% of PSP-C patients (3/5) and in 62% of PSP patients (61/97) in cerebellum (Table 1). The linear density of Purkinje cells was not significantly different between the two groups (Table 1). We further assessed the severity of tau-related pathology in cerebellum, cerebellar afferent system, and other vulnerable regions in PSP, including subthalamic nucleus, substantia nigra, and globus pallidus; however, there were no significant differences between the two groups (Table 1). Patient 4 had inferior olivary hypertrophy as well as severe neuronal loss in the inferior olivary nucleus. Severity of tau-related pathology in inferior olivary nucleus was not significantly greater in this patient compared to other PSP-C patients.
We also compared cerebellar pathology in PSP patients with and without cerebellar ataxia. Among 99 PSP patients in the Mayo Clinic patient series, 38 patients had some degree of cerebellar ataxia. We compared 43 PSP with cerebellar ataxia (5 PSP-C + 38 PSP) and 61 PSP without cerebellar ataxia (Table 2). The brain weight was less in PSP with cerebellar ataxia than in PSP without cerebellar ataxia, but in a multiple logistic regression analysis adjusting for age at death, the difference in brain weight was not significant. There were no differences in tau-related pathology or Purkinje cell loss between the two groups (Table 2). In summary, we did not find any significant correlation between the frequency of cerebellar ataxia and the severity of cerebellar pathology.
Table 2. Pathologic features comparing between PSP with and without cerebellar ataxia.
| Ataxia (43) | No ataxia (61) | P value | |
|---|---|---|---|
| Demographic | |||
| Gender, % male | 23/43 (52%) | 40/61 (65%) | 0.255 a |
| Age at death | 74.8 ± 9.0 | 73.9 ± 7.4 | 0.585 b |
| Pathologic features | |||
| Brain weight (g) | 1120 (1060, 1195) | 1200 (1110, 1320) | 0.011 c |
| Braak NFT stage | III (II, III) | II (I, III) | 0.069 c |
| Thal amyloid phase | 1.0 (0, 3) | 0 (0, 3) | 0.200 c |
| Ratio of Purkinje tau positive | 27/42 (64%) | 36/60 (60%) | 0.817 a |
| Number of pretangles in Purkinje cell layer | 1.0 (0, 2) | 1.0 (0, 3) | 0.910 c |
| Pretangles linear density (cells/mm) | 3.6 (3, 4) | 3.7 (3, 4) | 0.421 c |
| Semi-quantitative analysis | |||
| Coiled bodies in cerebellar white matter | 2.0 (1, 3) | 2.0 (1, 3) | 0.860 c |
| Tau threads in cerebellar white matter | 2.0 (1, 3) | 1.0 (1, 2) | 0.496 c |
| Coiled bodies in dentate nucleus | 1.0 (0, 1) | 1.0 (0, 1) | 0.732 c |
| Tau threads in dentate nucleus | 2.0 (1, 2) | 2.0 (1, 2) | 0.332 c |
| Pretangles/NFT in dentate nucleus | 2.0 (2, 3) | 3.0 (2, 3) | 0.335 c |
| Pretangles/NFT in pontine base | 3.0 (2, 3) | 3.0 (2, 3) | 0.477 c |
| Pretangles/NFT in inferior olivary nucleus | 2.0 (1, 2) | 2.0 (1, 2) | 0.192 c |
| Genetic analysis | |||
| MAPT: H1H1 genotype | 36/38 (95%) | 42/49 (86%) | 0.170 d |
| MAPT: H1H2 genotype | 2/38 (5%) | 7/49 (14%) | 0.170 d |
| APOE: ε4 genotype | 10/39 (26%) | 12/53 (23%) | 0.739 a |
Data are displayed as mean ± SD or median (25th, 75th range) as appropriate. Abbreviations: PSP, progressive supranuclear palsy; PSP-C, PSP, progressive supranuclear palsy with predominant cerebellar ataxia; NFT, neurofibrillary tangles;
Chi-square test,
t-test,
Mann-Whitney rank sum test,
Fisher exact test
We identified 26 PSP patients with significant cerebellar degeneration, usually most apparent in the anterior vermis, as part of the standard neuropathologic evaluation from 985 PSP patients in the Mayo Clinic brain bank series. To determine if there was a correlation between cerebellar degeneration and tau pathology, we measured cerebellar pathology in these 26 patients. The severity of coiled bodies and tau-positive threads in cerebellar white matter and dentate nucleus were not different from PSP patients without cerebellar degeneration (data not shown). We selected 5 patients with most severe cerebellar atrophy and assessed Purkinje cell pathology. The linear density of Purkinje cells was lower than PSP (median = 1.5), but the number of tau-positive Purkinje cells was not different. These results suggest that even in PSP patients with significant cerebellar degeneration (26/985 in our database), it is not likely related to tau pathology.
Genetic Assessments
Genetic analysis revealed no significant difference for either MAPT H1/H1 genotype or APOE ε4 frequency between PSP-C and PSP (Table 1). Similarly, there were no significant differences for either MAPT H1/H1 genotype or APOE e4 frequency between PSP with and without cerebellar ataxia (Table 2).
Discussion
This autopsy series of patients with neuropathologically-confirmed PSP suggests that PSP-C is a rare clinical variant in the United States; we identified only five patients with PSP-C in 1085 pathologically-confirmed PSP patients. Based on the Mayo Clinic patient series, the frequency of PSP‐C is 1% in the United States, a frequency that is closer to that reported from Western countries 15, 16 than from Japan.11 The differences in frequency might be related to ethnic and genetic factors as is the case with cerebellar ataxia in MSA – MSA-C is more common in Japan than European countries or the United States.32, 33 Other disorders, that have cerebellar variants, such as adrenoleukodystrophy, also appear to be more frequent in Japan.34 Indeed, ethnic differences in frequency of the MAPT haplotype are well known.35 The H2 haplotype is almost exclusively found in Caucasians, while it is almost completely absent in Japanese.35 Further epidemiological and genetic investigations are needed to determine risk factors for PSP-C.
Because the clinical features of PSP-C overlapped with MSA-C, PSP-C patients were often misdiagnosed as MSA-C.12, 27 Recently, Shimohata and colleagues proposed diagnostic criteria for PSP-C.36 Required items include (A) slowly progressive course, (B) onset > 40 years, (C) supranuclear gaze palsy, (D) truncal and limb ataxia within 2 years after symptom onset, and (E) postural instability with falls within 2 years after symptom onset. Exclusion criteria include marked dysautonomia and “hot cross bun” sign on brain MRI. Probable PSP-C requires A+B+C+D+E, and possible PSP-C requires A+B+D+E. According to these criteria, Patient 1 was consistent with probable PSP-C, and three other patients (Patient 2, 4, and 5) were consistent with possible PSP-C, due to absence of supranuclear gaze palsy. Patient 3 did not meet the criteria because postural instability with falls was absent. None of the five PSP-C patients had marked dysautonomia sufficient to meet Gilman's criteria for clinical diagnosis of probable MSA, and none had evidence of hot cross bun sign on brain MRI. The preliminary criteria for PSP-C seem to fit patients in the United States as well as those in Japan. It is noteworthy that 39% (39/100) of the PSP patients in the Mayo Clinic patient series developed some degree of cerebellar ataxia. The frequency of cerebellar ataxia in PSP, including the original patient reported by Steele, et al.,2 is between 6% and 44%,2, 11, 37, 38 suggesting that cerebellar ataxia is not uncommon in PSP and perhaps should not be an exclusion criteria for clinical diagnosis.
Although pathological features of PSP-C have been studied in only a few patients, our pathological analysis did not reveal any difference between PSP-C and PSP. In addition, we did not find differences if we widened the spectrum to include PSP with any degree of cerebellar ataxia. The presence of tau-positive Purkinje cells was considered to be a pathological feature in PSP-C,11 but the frequency and number of tau-positive Purkinje cells were not different between PSP-C and PSP. In fact, 60% of patients with PSP without cerebellar ataxia also had a few tau-positive Purkinje cells similar to that reported in another study.39 Taken together, the results suggest that a small number of tau-positive Purkinje cells is unlikely to be related to cerebellar ataxia. Although Purkinje cell loss is related to both limb and gait ataxia in patients with MSA,40 we did not find significant Purkinje cell loss in PSP-C or in PSP patients. We also examined cerebellar tau pathology in PSP patients with cerebellar degeneration regardless of clinical presentation. Of the 26 PSP patients that had cerebellar vermal degeneration, there was no correlation to severity of cerebellar tau pathology. Cerebellar degeneration in PSP, like that in other chronic neurologic disorders, may be caused by concomitant nutritional, cerebrovascular or alcoholic disorders.41 This result also confirms that cerebellar ataxia in PSP is unrelated to cerebellar tau pathology.
Some studies have reported that tau pathology in the dentate nucleus is more severe in PSP-C than PSP,11, 13 but we did not find a difference between the two groups. The dentate nucleus is one of the most vulnerable regions in PSP, and if dentate nucleus pathology was the underlying cause of cerebellar ataxia, then the majority of PSP patients should have significant ataxia.
Finally, we assessed the cerebellar afferent system (i.e. inferior olivary nucleus and nuclei of the pontine base) and other vulnerable regions (i.e. subthalamic nucleus, substantia nigra, and globus pallidus); however, the severity of tau-related pathology was not different between PSP-C and PSP. One patient (patient 4) had severe neuronal loss in inferior olivary nucleus, but it did not seem to be a contributing factor to cerebellar ataxia. We identified four additional patients with severe neuronal loss in inferior olivary nucleus among 30 PSP patients with inferior olivary hypertrophy, but none had cerebellar ataxia. Although a clinical feature of inferior olivary hypertrophy is thought to be palatal myoclonus,42 this obscure clinical finding was not reported in any of the PSP patients with inferior olivary hypertrophy. Taken together, we did not find any pathological differences between PSP-C and PSP.
There are some clear limitations of our study. First, it is a retrospective analysis, so symptoms associated with cerebellar ataxia might be underestimated. It is also difficult to judge cerebellar ataxia in some cases. For example, patient 4 in our PSP-C cohort developed ataxia, but the possibility of sensory ataxia could not be ruled out. Videos of neurologic examination would be helpful, but unfortunately, these were not available in this study. Secondly, we screened PSP-C patients using selective inclusion criteria we hypothesized would increase the likelihood of detecting patients with ataxia, hence we may have missed some PSP-C patients had we reviewed medical records on all PSP patients in the brain bank. The selective search criteria were established based on our experience that PSP can be misdiagnosed as MSA.10 Thirdly, it cannot be ruled out that the responsible lesions are present in other areas than those we assessed, although we did not find any difference in tau-related pathology between PSP-C and PSP.
In our previous study, we evaluated clinical diagnostic accuracy and causes of misdiagnosis of clinically diagnosed MSA. Among 134 cases of MSA, 15 cases had PSP at autopsy and the main reason for clinical misdiagnosis was presence of cerebellar ataxia.10 These findings were consistent with PSP-C, and in this study we determined the frequency of PSP-C in our brain bank. PSP-C is rare in the United States and Europe and possible less so in Japan. Clinical features of PSP-C were similar to those of MSA-C, but PSP-C lacked significant autonomic failure fulfilling Gilman's criteria.29 In some cases, brain MRI may help differentiate PSP-C from MSA-C. In addition to cerebellar atrophy expected in MSA, characteristic features of PSP, such as atrophy of midbrain and superior cerebellar peduncles,30, 31 can be seen in PSP-C. While this study cannot provide definitive information about how to make an accurate clinical diagnosis, the data should serve to raise awareness of PSP-C and spur further clinical, neuroimaging, and neuropathological research into this variant. Finally, physicians should be aware of this clinical variant when evaluating patients with atypical parkinsonism and ataxia.
Supplementary Material
Supplementary Table: Clinical and pathological features of PSP-C patients
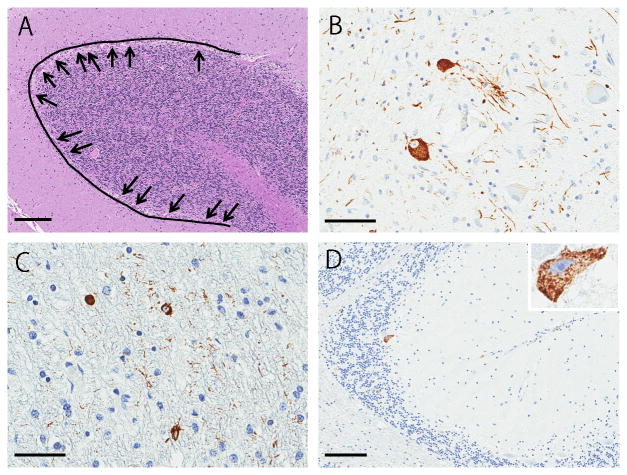
Figure 3.
Histopathological images in cerebellum of PSP-C patients. An example photograph for analysis of Purkinje cell loss (A). We use cerebellar vermis section stained with hematoxylin-eosin and drew 3 mm-length lines in the cerebellar Purkinje cell layers. Purkinje cells are indicated with arrows (A, arrows). Immunohistochemistry for CP13 reveals pretangles and tau-positive threads in dentate nucleus (B), coiled bodies in cerebellar white matter (C), and pretangles in Purkinje cell layer (D, arrow, inset). A: hematoxylin-eosin stain; B-D: CP13 immunostain. Bars = 200 μm in A and D, 100 μm in B and C.
Acknowledgments
We would like to thank the patients and their families who donated brains to help further our knowledge of neurodegeneration. The authors would also like to acknowledge Linda G. Rousseau, Virginia R. Phillips, and Monica Castanedes-Casey for tissue ascertainment and processing.
Financial Disclosures of all authors: Dr. Koga reports no disclosures.
Dr. Josephs receives research support from the NIH (R01-DC010367, R01-DC012519 & R01-AG037491) and the Alzheimer's Association. Dr. Josephs is an editorial board member of Acta Neuropathologica, Journal of Neurology and Parkinsonism and Related Disorders.
Dr. Ogaki reports no disclosures.
Dr. Labbé receives a FRSQ postdoctoral fellowship.
Dr. Uitti receives research support by the NIH (P50-NS072187 and R01-NS057567), from Advanced Neuromodulation Systems, Inc./St. Jude Medical, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. Dr. Uitti is an editorial board member of Neurology.
Dr. Graff-Radford reports no disclosures.
Dr. van Gerpen receives research funds from the Mayo Clinic CR program and NIH (P50-NS072187). This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Dr. Cheshire is consultant for American Academy of Neurology, Neuro SAE examination writer, 2013; and receives support from NIH, Autonomic Rare Diseases Clinical Research Consortium. He is editorial board member of Autonomic Neuroscience.
Dr. Aoki reports no disclosures.
Dr. Rademakers receives research support from the NIH (P50-NS072187, R01- NS076471 and R01-NS080882).
Dr. Wszolek receives research support from the NIH (P50-NS072187). Dr. Wszolek serves as Co-Editor-in-Chief of Parkinsonism and Related Disorders, Associate Editor of the European Journal of Neurology, and on the editorial boards of Neurologia i Neurochirurgia Polska, the Medical Journal of the Rzeszow University, and Clinical and Experimental Medical Letters; holds and has contractual rights for receipt of future royalty payments from patents re: A novel polynucleotide involved in heritable Parkinson's disease; receives royalties from publishing Parkinsonism and Related Disorders (Elsevier, 2013, 2014) and the European Journal of Neurology (Wiley- Blackwell, 2013, 2014).
Dr. Ross received support from NIH (R01-NS078086 and P50-NS072187) and the Michael J. Fox Foundation. He is editorial board member of PLoS ONE, American Journal of Neurodegenerative disease, Molecular Neurodegeneration and Parkinsonism and Related Disorders.
Dr. Dickson receives support from the NIH (P50-AG016574; P50-NS072187; P01-AG003949) and CurePSP: Foundation for PSP | CBD and Related Disorders. Dr. Dickson is an editorial board member of Acta Neuropathologica, Annals of Neurology, Brain, Brain Pathology, and Neuropathology, and he is editor in chief of American Journal of Neurodegenerative Disease, and International Journal of Clinical and Experimental Pathology.
Footnotes
Author Contributions: Shunsuke Koga: Execution of the project; execution of the statistical analysis; Writing of the first draft
Keith A. Josephs: Review and Critique; contribution of patients
Kotaro Ogaki: Review and Critique; interpretation of data
Catherine Labbé: Review and Critique; interpretation of data
Ryan J. Uitti: Review and Critique; contribution of patients
Neill Graff-Radford Review and Critique; contribution of patients
Jay A. Van Gerpen: Review and Critique; contribution of patients
William P. Cheshire: Review and Critique; contribution of patients
Naoya Aoki Review and Critique
Rosa Rademakers: Review and Critique; interpretation of data
Zbigniew K. Wszolek: Review and Critique; contribution of patients
Owen A. Ross: Review and Critique; interpretation of data
Dennis W Dickson: Conception and organization of the project; interpretation of data; Review and Critique
References
- 1.Williams DR, de Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128(Pt 6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8(3):270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1996;60(6):615–620. doi: 10.1136/jnnp.60.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Josephs KA. Key emerging issues in progressive supranuclear palsy and corticobasal degeneration. J Neurol. 2015 doi: 10.1007/s00415-015-7682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18(9):1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 8.Lang AE. Clinical heterogeneity in progressive supranuclear palsy: challenges to diagnosis, pathogenesis and future therapies. Mov Disord. 2014;29(14):1707–1709. doi: 10.1002/mds.26105. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto R, Tsuchiya K, Mimura M. Clinical heterogeneity in progressive supranuclear palsy: problems of clinical diagnostic criteria of NINDS-SPSP in a retrospective study of seven Japanese autopsy cases. Neuropathology. 2010;30(1):24–35. doi: 10.1111/j.1440-1789.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 10.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology. 2015;85(5):404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanazawa M, Shimohata T, Toyoshima Y, et al. Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Mov Disord. 2009;24(9):1312–1318. doi: 10.1002/mds.22583. [DOI] [PubMed] [Google Scholar]
- 12.Kanazawa M, Tada M, Onodera O, Takahashi H, Nishizawa M, Shimohata T. Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia. Parkinsonism Relat Disord. 2013;19(12):1149–1151. doi: 10.1016/j.parkreldis.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki Y, Mori K, Ito M, Tatsumi S, Mimuro M, Yoshida M. An autopsied case of progressive supranuclear palsy presenting with cerebellar ataxia and severe cerebellar involvement. Neuropathology. 2013;33(5):561–567. doi: 10.1111/neup.12012. [DOI] [PubMed] [Google Scholar]
- 14.Barsottini OG, Felicio AC, Aquino CC, Pedroso JL. Progressive supranuclear palsy: new concepts. Arq Neuropsiquiatr. 2010;68(6):938–946. doi: 10.1590/s0004-282x2010000600020. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger K. Cerebellar involvement in progressive supranuclear palsy. Mov Disord. 2010;25(8):1104–1105. doi: 10.1002/mds.23045. [DOI] [PubMed] [Google Scholar]
- 16.Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29(14):1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 17.Utsumi H, Abe K, Yoshii O, Mori H, Suda K, Mizuno Y. A 71-year-old woman with progressive gait disturbance and dementia. No To Shinkei. 1995;47(3):295–307. [PubMed] [Google Scholar]
- 18.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23(4):394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 19.Wider C, Ross OA, Nishioka K, et al. An evaluation of the impact of MAPT, SNCA and APOE on the burden of Alzheimer's and Lewy body pathology. J Neurol Neurosurg Psychiatry. 2012;83(4):424–429. doi: 10.1136/jnnp-2011-301413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 21.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 22.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer's disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116(1):17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsuse O, Dickson DW. Inferior olivary hypertrophy is uncommon in progressive supranuclear palsy. Acta Neuropathol. 2004;108(2):143–146. doi: 10.1007/s00401-004-0878-3. [DOI] [PubMed] [Google Scholar]
- 25.Symanski C, Shill HA, Dugger B, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29(4):496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama A, Ohno K, Hirano A, et al. Cerebellar expression of copper chaperone for superoxide, cytosolic cu/zn-superoxide dismutase, 4-hydroxy-2-nonenal, acrolein and heat shock protein 32 in patients with menkes kinky hair disease: immunohistochemical study. Yonago Acta Med. 2014;57(1):23–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology. 2015 doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128(3):411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003;60(11):1766–1769. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- 31.Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2005;64(4):675–679. doi: 10.1212/01.WNL.0000151854.85743.C7. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(Pt 12):2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa T, Tada M, Kakita A, et al. The phenotype spectrum of Japanese multiple system atrophy. Journal of neurology, neurosurgery, and psychiatry. 2010;81(11):1253–1255. doi: 10.1136/jnnp.2009.182576. [DOI] [PubMed] [Google Scholar]
- 34.Ogaki K, Koga S, Aoki N, et al. Adult-onset cerebello-brainstem dominant form of X-linked adrenoleukodystrophy presenting as multiple system atrophy: case report and literature review. Neuropathology. 2015 doi: 10.1111/neup.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans W, Fung HC, Steele J, et al. The tau H2 haplotype is almost exclusively Caucasian in origin. Neurosci Lett. 2004;369(3):183–185. doi: 10.1016/j.neulet.2004.05.119. [DOI] [PubMed] [Google Scholar]
- 36.Shimohata T, Kanazawa M, Takahashi H, Nishizawa M. Clinicopathological features and diagnostic criteria for progressive supranuclear palsy with predominant cerebellar ataxia [abstract] Mov Disord. 2015;30(Suppl 1):847. [Google Scholar]
- 37.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. Journal of neurology, neurosurgery, and psychiatry. 1995;58(2):167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birdi S, Rajput AH, Fenton M, et al. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord. 2002;17(6):1255–1264. doi: 10.1002/mds.10211. [DOI] [PubMed] [Google Scholar]
- 39.Piao YS, Hayashi S, Wakabayashi K, et al. Cerebellar cortical tau pathology in progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;103(5):469–474. doi: 10.1007/s00401-001-0488-2. [DOI] [PubMed] [Google Scholar]
- 40.Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12(2):133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- 41.Laureno R. Nutritional cerebellar degeneration, with comments on its relationship to Wernicke disease and alcoholism. Handb Clin Neurol. 2012;103:175–187. doi: 10.1016/B978-0-444-51892-7.00010-3. [DOI] [PubMed] [Google Scholar]
- 42.Koeppen AH, Barron KD, Dentinger MP. Olivary hypertrophy: histochemical demonstration of hydrolytic enzymes. Neurology. 1980;30(5):471–480. doi: 10.1212/wnl.30.5.471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table: Clinical and pathological features of PSP-C patients