TO THE EDITOR
Chronic urticaria (CU) is defined as recurrent, transitory, pruritic-raised wheals present on most days of the week for >6 weeks1. The pathogenesis of CU is unknown; but it is thought to be the result of autoimmunity, at least in some patients. Evidence supporting this comes from assays suggesting the presence of functional autoantibodies. IgG autoantibodies to IgE or to the high-affinity IgE receptor (FcεRI) have been directly detected in ~40-50% of CU patients.2 While of interest, identifying these antibodies is laborious and not fully validated, therefore, numerous surrogate assays have been developed. A positive autologous serum skin test (ASST) can be identified in 30-67% of CU patients3 and is an indirect reflection of autoantibodies. However, the ASST is positive in 37% of non-CU patients3, making it of less certain clinical relevance. Alternatively, the sera of 40-50% of CU patients can induce the release of histamine from the basophils of healthy subjects (basophil histamine-releasing assay, HRA).4 Limitations to this test are based on inter-laboratory reproducibility and variation in healthy basophil donor characteristics;2 and as with the ASST, the HRA lacks diagnostic specificity for CU. More recently, flow cytometry has been used to evaluate the ability of CU patients’ serum to activate donor basophils as determined by the upregulation of CD203c (ectonucleotide pyrophosphatase/phosphodiesterase)5 In the validation studies for this assay, the upregulation of CD203c surface expression was evaluated on basophils obtained from a healthy atopic donor after exposure for 10 minutes to serum obtained from 32 CU patients and 11 healthy controls. (see Yasnowsky et al.5 for more detailed description). The data are expressed as the percent of basophils expressing more CD203c than 99% of basophils incubated with buffer only. A result ≥5% is highly specific for CU as this result was never seen using serum obtained from healthy controls5. Serum factors driving increased CD203c-expression in this assay are unknown and as with the HRA are assumed to reflect autoantibodies, however this has never been determined. Furthermore, factors driving histamine-release from basophils may differ from those responsible for CD203c upregulation.
Treating CU is challenging, as many patients are refractory to the standard pharmacological therapy. Omalizumab, a humanized anti-IgE monoclonal antibody, has been approved for the treatment of CU. During phase 3 trials, omalizumab was effective in 52% of CU patients based on Urticaria Activity Score (UAS7) of <6, but only 34% had complete clinical response (UAS7 of 0)1. Given this modest response rate, the requirement to administer this agent for up to 3 months before recognizing treatment failure, and the expense associated with its use, the identification of biomarkers predicting responsiveness would prove valuable. In the omalizumab CU clinical trials, the HRA was positive in only 25-30% of subjects and had no value in predicting therapeutic response1. The greater specificity of the CD203c assay for CU suggests it may have superior predictive value in determining omalizumab responsiveness.
Therefore, we performed a retrospective chart review of 41 consecutive adult patients with antihistamine-refractory CU seen at the University of Virginia outpatient allergy clinic between 2011-2013 in order to investigate use of the basophil CD203c assay as a biomarker of responsiveness to omalizumab in CU. This study was approved by the University of Virginia Health System Institutional Review Board (IRB-HSR #18100). Basophil CD203c-upregulating activity was obtained in all subjects prior to omalizumab administration (National Jewish Health Advance Diagnostic Laboratories, Denver CO). Clinical response was evaluated after a minimum of 3 months of treatment based upon patient’s and physician’s subjective determination. Fisher’s exact test was used to compare basophil CD203c-upregulating activity in responders and non-responders (GraphPad Prism 6.0) and p<0.05 was considered significant.
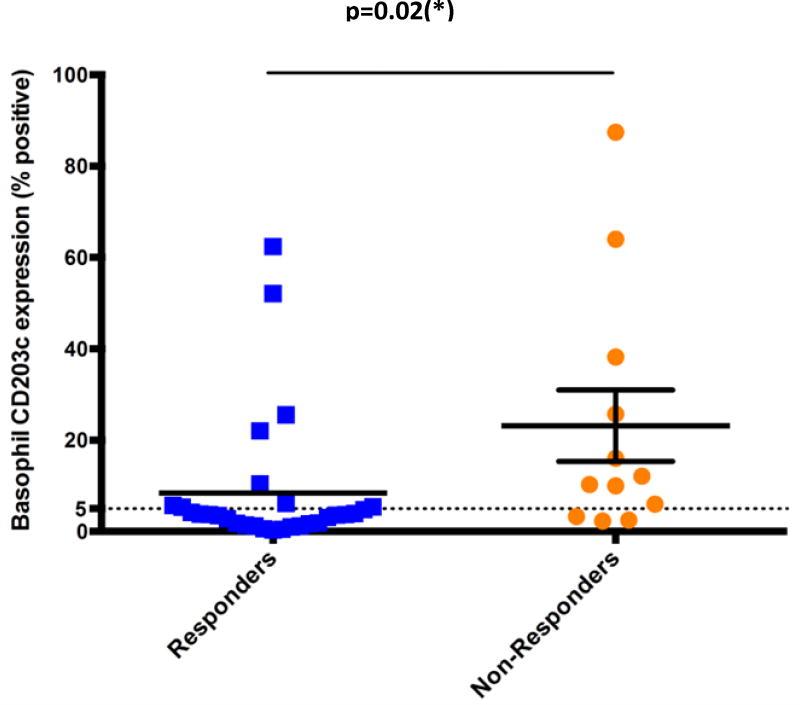
Patients’ characteristics are summarized in Table-I. CD203c-upregulating activity was present in 18/41 subjects (43.9%). Subjects were treated with omalizumab 300 mg SC q4weeks for at least 3 months. Omalizumab was effective in 29/41 (71%) CU patients overall, slightly higher that what has been reported in published studies1, 6,7,9. Of the 18 subjects demonstrating CD203c-upregulating activity, only 9 (50%) had clinical improvement with omalizumab (Figure I). In contrast, of the 23 without CD203c-upregulating activity, 20 (87%) had a clinical response to omalizumab (p=0.02, Fisher’s exact test). Thus having a negative result predicts a much greater likelihood of responding to omalizumab with the odds ratio of a negative test being 6.7 (95% CI; 1.4-31) No correlation of efficacy was found with age, sex, or the presence of thyroid autoantibodies (not shown).
Table 1.
Subjects’ Characteristics and Responder Analysis
| Subjects’ Characteristics (n=41) | |
| Male, n (%) | 8 (19.5%) |
| Age (mean±SD) | 45.9 (SD 17) |
| Hypothyroidism, n (%) | 8 (19.5%) |
| Thyroid auto-antibodies, n (%) | 8 (19.5%) |
| Basophil CD203c-inducing activity, n (%) | 29 (70.7%) |
| Responder Analysis (n=29) | |
| CD203c-inducing Activity | Clinical Responder, n (%) |
| Present (n=18) | 9/18 (50%) |
| Absent (n=23) | 20/23 (87%)* |
| Non-Responder Analysis (n=12) | |
| CD203c-inducing Activity | n (%) |
| Present | 9/12 (75%) |
| Absent | 3/12 (25%) |
p<0.02. Fischer’s exact test was used to compare basophil CD203c-upregulating activity in responders and non-responders (GraphPad Prism 6.0).
Figure 1.
Analysis of the ability of CU patients’ serum to induce basophil CD203c expression, segregated by clinical response to omalizumab. Data are presented as the percent of basophils expressing CD203c after incubation of patient’s serum with the donor’s basophils; 5% or more is considered positive1.
Although not proven, basophil CD203c-upregulating activity is thought to reflect the presence of autoantibodies to IgE and/or FcεRIα suggesting that the presence of these autoantibodies unexpectedly predicted a lower likelihood of clinical response. Clearly, this observation needs to be repeated in a prospective fashion, but if confirmed in those studies, this holds promise as a clinically useful biomarker predicting response to treatment. The mechanism of action of omalizumab in CU is not known and this understanding will ultimately be central to predicting responders. Omalizumab binds to free IgE in the circulation and therefore prevents IgE from binding to mast cells and basophils6. As the binding of IgE is required to stabilize receptors on the cell surface, there is a secondary robust reduction in FcεRI expression on basophils after treatment with omalizumab7. Therefore, one mechanism of action for omalizumab in CU may be through down-regulation of the antigenic target of the autoantibodies.7 However, in one – albeit modestly sized – study this down-regulation of FcεRI expression did not extend to mast cells, the presumed source of disease in CU,5 questioning the role for this mechanism in CU. This interpretation is supported by the current study, as if this were the mechanism, it would be predicted that those with CD203c-upregulating activity would be more likely to have responded.
We are therefore compelled to develop an alternative explanation for its mechanism of action. One obvious consideration is the possibility that CU is in fact an IgE-mediated “allergic” disease, and omalizumab is capturing free IgE that presumably is directed against self-antigens. Interestingly, ~30-50% of CU patients have thyroiditis and in a previous study, 54.2% of CU patients were found to have IgE-anti-TPO present8 suggesting one plausible target for such IgE auto-antibodies. Further investigation is ongoing into the presence of IgE auto-antibodies in CU patients and their utility as a biomarker in predicting efficacy of omalizumab in CU.
Clinical Implication.
Lack of basophil CD203c upregulating activity in serum of patients with chronic urticaria correlated with clinical response to omalizumab in this retrospective study. If confirmed in prospective studies, this holds promise as a clinically useful biomarker of response to treatment.
Acknowledgments
Dr. James Patrie, PhD for his help with the statistical analysis of the data.
References
- 1.Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. The Journal of allergy and clinical immunology. 2013;132:101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. The Journal of investigative dermatology. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 3.Guttman-Yassky E, Bergman R, Maor C, Mamorsky M, Pollack S, Shahar E. The autologous serum skin test in a cohort of chronic idiopathic urticaria patients compared to respiratory allergy patients and healthy individuals. Journal of the European Academy of Dermatology and Venereology : JEADV. 2007;21:35–39. doi: 10.1111/j.1468-3083.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 4.Eckman JA, Hamilton RG, Saini SS. Independent evaluation of a commercial test for "autoimmune" urticaria in normal and chronic urticaria subjects. The Journal of investigative dermatology. 2009;129:1584–1586. doi: 10.1038/jid.2008.416. [DOI] [PubMed] [Google Scholar]
- 5.Yasnowsky KM, Dreskin SC, Efaw B, et al. Chronic urticaria sera increase basophil CD203c expression. The Journal of allergy and clinical immunology. 2006;117:1430–1434. doi: 10.1016/j.jaci.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. The Journal of allergy and clinical immunology. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 7.MacGlashan DW, Jr, Bochner BS, Adelman DC, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. Journal of immunology. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 8.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase--a novel pathomechanism of chronic spontaneous urticaria? PloS one. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;7:57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]