Abstract
Importance
Vitamin D has been associated with a decreased risk of multiple sclerosis (MS) in adulthood; however, some, but not all, previous studies have suggested that in utero vitamin D exposure may be a risk factor for MS later in life.
Objective
To examine whether serum 25-hydroxyvitamin D (25(OH)D) levels in early pregnancy are associated with risk of MS in offspring.
Design
Prospective, nested case-control study
Setting
Finnish Maternity Cohort (FMC)
Participants
We identified 193 individuals with a diagnosis of MS before December 31, 2009 whose mothers are in the FMC and had an available serum sample from the pregnancy with the affected child. We matched 176 cases with 326 controls on region of birth in Finland, date of maternal serum sample collection, date of mother's birth, and date of child's birth.
Exposures
Serum 25(OH)D levels were measured in the maternal samples using a chemiluminescence assay.
Main Outcomes and Measures
Main outcome was the risk of MS associated with 25(OH)D levels. Conditional logistic regression was used, further adjusted for sex of the child, and gestational age and season of sample collection to estimate the relative risks and 95% confidence intervals.
Results
Over 70% of serum samples were collected during the first trimester of pregnancy and average maternal vitamin D levels were in the insufficient vitamin D range, but higher in maternal control than case samples (37.5 vs. 34.6 nmol/L). Maternal vitamin D deficiency (25(OH)D levels <30 nmol/L) during early pregnancy was associated with a nearly 2-fold increased risk of MS in the offspring (relative risk=1.90, 95% confidence interval: 1.20-3.01, p=0.006) as compared to women who were not deficient. Overall, risk of MS tended to decrease with increasing serum 25(OH)D levels, but the linear trend was not statistically significant (p=0.12).
Conclusions and Relevance
Insufficient maternal 25(OH)D during pregnancy may increase the risk of MS in offspring.
Introduction
Inadequate vitamin D nutrition has been identified as a risk factor for developing multiple sclerosis (MS),1 a progressive, neurodegenerative disease of the central nervous system. Two previous prospective studies have found that elevated serum levels of 25-hydroxyvitamin D (25(OH)D), a marker of vitamin D nutrition, in healthy adults are associated with a decreased risk of MS2,3, and another prospective study among adult women found higher dietary intake of vitamin D was associated with lower MS risk.4 Whether this inverse association extends to vitamin D exposure in early life is not clear. Two Swedish prospective studies, one measuring maternal 25(OH)D levels during pregnancy,3 and the other measuring 25(OH)D in dried blood spots collected from newborns, 5 found no association with future MS risk in the child, while a study of gestational dietary vitamin D intake in US women found that higher intake was associated with a decreased risk of MS in the child.6 Additionally, the higher number of spring births observed among MS patients7,8 has been attributed to exposure to lower vitamin D in utero, though other immune effects of sun exposure, seasonal infections, or statistical artifact,1,9 cannot be ruled out. Thus, whether adequate maternal vitamin D levels during pregnancy are associated with risk of MS in the offspring remains unclear.
Therefore, we have conducted a case-control study nested in the Finnish Maternity Cohort (FMC) to examine whether maternal levels of 25(OH)D during early pregnancy are associated with the risk of MS in the offspring.
Methods
Study Population
The FMC was established in 1983 and is comprised of over 800,000 women and more than 1.5 million serum samples10 that were collected during their pregnancies at approximately 10 to 14 weeks gestation for routine pre-natal tests. The samples were collected at municipal Maternity Care Units and shipped to the Finnish National Institute for Health and Welfare in Oulu, Finland,where they were processed and stored at −25°C.11 The FMC includes a sample from ~98% of all pregnancies in Finland since 1983. This study was approved by the data protection authorities at the National Institute for Health and Welfare and by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District and by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health. Since 2002 an informed consent has been asked from the mothers to store the samples for research purposes; use of samples collected prior to 2002 for research purposes is allowed under Finnish law.
MS Case Identification and Control Selection
We identified cases of MS occurring among children born to women in the FMC between January 1, 1983 and December 31, 1991 (children who would be 18-27 years old by December 31, 2009) by searching the Finnish Hospital Discharge Register (HILMO) for the diagnostic codes for MS and related diseases (ICD-10 code G35, G36, H46, ICD-9 and ICD-8 codes 340, 341, 367, 377). HILMO includes both inpatient and outpatient neurological visits. We also searched the registry of the Social Insurance Institution (SSI) to identify cases not in HILMO. The SSI tracks medication reimbursement for disease modifying therapy and other treatments for MS, including glatiramer acetate, interferon-β1a, and interferon- β1b. Medical records of the children with MS were reviewed when available and the diagnosis confirmed by the study neurologists (MS-H, JÅ, KH). For cases that were identified through the SSI, an abstract of the medical record was obtained.
To identify the mothers of the individuals confirmed as having MS, an over-generation linkage step was done via the Population Census Register. The mothers were then linked by their personal identification number to the FMC database, and the pregnancy with the affected child was identified. The child/mother pair was included if there was a serum sample available from this pregnancy.
MS was confirmed and a maternal pregnancy serum sample was available for 193 children (138 cases were confirmed by review of the medical record, and 55 based on prescription of/reimbursement for MS disease modifying therapy). We were able to individually match 176 of these cases to 326 controls on region of birth in Finland (south, southwest, southeast, middle, north), date of maternal sample collection (+/− 60 days), date of mother's birth (+/− 6 months), and date of child's birth (+/− 2 months). There were 17 cases for whom an appropriate matched control could not be found, and an additional 5 controls that were selected, but ultimately not matched.
Serum 25(OH)D Measurement
25(OH)D was measured in the pre-natal serum sample taken from the mother during her pregnancy with the affected child/control using a chemiluminescence microparticle immunoassay (CMIA) using an Architect i2000SR automatic analyser (Abbott Diagnostics). Maternal 25(OH)D levels exhibited the expected seasonal variation: summer, 44.1 nmol/L; winter, 28.9 nmol/L; spring/fall, 33 nmol/L, with the latter seasons being statistically significantly lower than summer levels (p<0.0001 for both). Coefficients of variation derived from repeated quality control samples included in the assay with the study samples were calculated. In samples with “high” 25(OH)D (>100 nmol/L) the CV=3.5%, “medium” 25(OH)D levels (~80 nmol/L) 1.8%, and “low” 25(OH)D levels (<40 nmol/L), 3.0%. In blinded QC pairs where 25(OH)D levels were not known, the CV was 1.1%.
Statistical Analysis
The 25(OH)D levels were modeled in three ways: 1) as a continuous variable, 2) as quintiles based on the distribution of maternal 25(OH)D levels in the controls, and 3) as a priori categories consistent with deficient (<30 nmol/L), insufficient (30-<50 nmol/L), and sufficient (>=50 nmol/L) levels per the Institute of Medicine's guidelines. 12 To account for the matched nested case-control design of the study, conditional logistic regression was used in the main analysis to estimate the rate ratios and 95% confidence intervals, and included the 176 cases with 326 matched controls. In addition to the matching factors, these analyses were further adjusted for sex of the child, and gestational age (in days, continuous) at sample collection and season (summer, winter, spring/fall) of sample collection. In secondary analyses, we also performed an unconditional logistic regression adjusting for all the matching factors in addition to those listed above in all 193 cases and 331 controls and stratified by sex of the child (female: 163 cases, 218 controls; male: 30 cases, 113 controls). A p value <0.05 was considered statistically significant and all analyses were done using SAS v9.3 (Carey, NC).
Results
MS cases and controls were similar with regards to mother's age, and gestational age and season at the time of serum sample collection. (Table) The young average age at MS diagnosis reflects the fact that the source population comprises only individuals born after 1983. Over 70% of serum samples were collected at or before 12 weeks gestation and 99% prior to 28 weeks. There were more females in the case group and the average age at MS diagnosis was 19.8 years. (Table) Maternal 25(OH)D levels ranged from 8.74 nmol/L to 160.5 nmol/L with the average levels in the insufficient range of 25(OH)D, and slightly lower in case mothers than in controls (Table). Only 2 MS cases and 8 controls had maternal 25(OH)D levels >75 nmol/L, and no MS cases and only 1 control had maternal 25(OH)D levels >100 nmol/L. Mean 25(OH)D levels did not differ by trimester of serum collection (1st: 36.6 nmol/L, 2nd: 36.2 nmol/L). No cases and two controls had a mother with an MS diagnosis.
Table.
Characteristics of MS cases and controls, Finnish Maternity Cohort offspring
| Characteristic |
MS Cases (n=193) |
Controls (n=331) |
|---|---|---|
| Female, % | 84 | 66 |
| Mother's age (years)*, mean (SD) | 27.6 (5.3) | 27.7 (5.4) |
| Gestational age (weeks)*, mean (SD) | 11.6 (3.9) | 10.9 (3.3) |
| Season*, % | ||
| Summer | 38.3 | 42.3 |
| Winter | 26.9 | 25.7 |
| Mother's 25(OH)D, nmol/L, mean (SD) | 34.6 (13.7) | 37.5 (16) |
| Age (years) at MS diagnosis, mean (SD) | 19.8 (3.2) | NA |
At time of serum sample collection
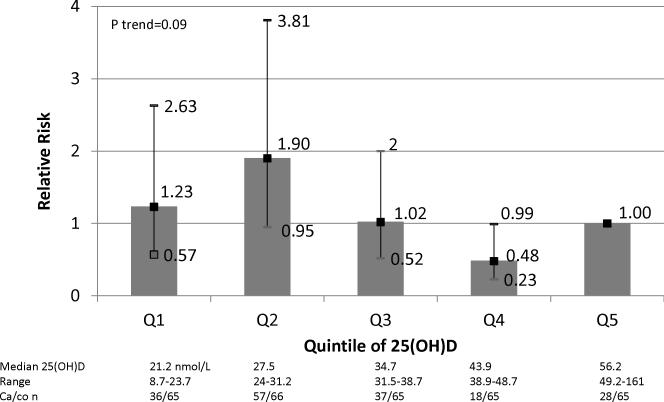
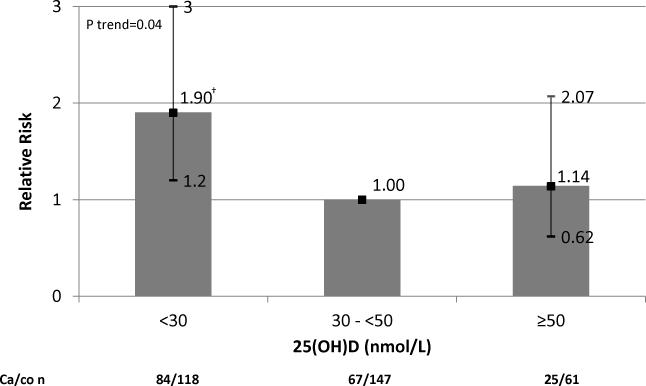
In the matched analysis, adjusted for sex, gestational age, and season at time of sample collection, a 50 nmol/L increase in maternal 25(OH)D level was associated with a non-statistically significant 48% reduced risk of MS in the offspring (RR=0.52, 95%CI: 0.22-1.19, p=0.12). Children of mothers with deficient levels of 25(OH)D during their pregnancy had an increased risk of developing MS as compared to children born to mothers with non-deficient levels. Specifically, maternal 25(OH)D levels <31.5 nmol/L (quintiles 1 and 2) were associated with a 20-90% increased risk of MS among the offspring as compared to maternal 25(OH)D levels in the top quintile (median 25(OH)D=56.2 nmol/L) (p trend=0.09) (Figure 1). Similarly, in multivariate analyses using a priori categories of 25(OH)D levels, clearly deficient maternal 25(OH)D levels during pregnancy were associated with a nearly 2-fold increased risk of MS in the child (<30 nmol/L vs. 30-<50 nmol/L: RR=1.90, 95%CI: 1.20-3.01, p=0.04) (Figure 2).
Figure 1.
Multivariate* relative risk for MS in offspring by quintiles of maternal 25(OH)D during pregnancy.
*Adjusted for sex, gestational age and season at time of sample collection.
Figure 2.
Multivariate* relative risk for MS in offspring by maternal 25(OH)D adequacy during pregnancy.
*Adjusted for sex, gestational age and season at time of sample collection.
†p=0.006
Similar, though slightly attenuated, results were obtained in the unmatched analysis, with a 43% reduced risk of MS associated with every 10 nmol/L increase in maternal 25(OH)D level (RR=0.57, 95%CI: 0.28-1.18, p=0.13), and a 59% increased risk of MS among children born to mothers who were vitamin D deficient during pregnancy (<30 nmol/L vs. 30-<50 nmol/L: RR=1.59, 95%CI: 1.04-2.42, p=0.03). In analyses stratified by sex of the child, this association was only seen in females (<30 nmol/L vs. 30-<50 nmol/L: RR=1.75, 95%CI: 1.09-2.81, p=0.02), though the sample size among male children was small.
Discussion
In this large, prospective, nested case-control study in the FMC, children of women who were vitamin D deficient (25(OH)D levels <30 nmol/L) early in their pregnancy had a 90% increased risk of developing MS as an adult. Two prior studies examining the association between 25(OH)D levels in pregnancy/early life did not find an association with future MS risk in the offspring.3,5 However, there are important differences and limitations to these studies that need to be considered. In the Northern Sweden Maternity Cohort, Salzer et al3 conducted a nested case-control study of primarily first trimester maternal 25(OH)D levels and risk of MS among the offspring; however, there were only 37 cases of MS among the offspring and the association between maternal 25(OH)D level and MS risk was fairly unstable as evidenced by wide confidence intervals of the risk estimate (RR of MS in 25(OH)D > 75 nmol/l vs. <75 nmol/L=1.8, 95% CI: 0.53-5.8) making interpretation, including conclusion of a true null association, difficult. In a more recent Swedish case-control study, Ueda et al5 measured 25(OH)D levels in dried blood spots (DBS) collected for phenylketonuria (PKU) screening from 459 MS cases and 663 controls when they were newborns in 1975 or later. Overall, there was no association between neonatal 25(OH)D levels and risk of MS (odds ratio=1.0, 95% CI: 0.90-1.06). However, there was evidence of 25(OH)D degradation in the older DBS samples, which may have contributed to the null findings, though no associations were seen when restricting to more recent samples in which 25(OH)D degradation did not appear to occur. Another important concern is that with low overall control participation in the study (44% of those eligible), it is possible that the controls do not provide an accurate representation of the 25(OH)D exposure distribution in the general population of newborns. Further, the levels of neonatal 25(OH)D in the Ueda study were primarily in the deficient range (median=25.6 nmol/L, IQR=17-38.4 nmol/L) making it difficult to detect any association with 25(OH)D deficiency and MS risk.13
The strengths of our study include the population-based nature of the FMC (~98% of pregnancies in Finland since 1983 are captured), thus selection bias is minimized, and the extensive national coverage of the HILMO and the SSI registries for MS case identification. There are a few limitations of our study to consider. Maternal 25(OH)D levels during pregnancy are not a direct measure of the 25(OH)D levels that the developing fetus is exposed to. However, several studies have shown that the levels of serum 25(OH)D in neonates directly correlate with maternal 25(OH)D levels during pregnancy or post-partum 14-16, with stronger correlations for the latter. Further, studies of bone growth and development in the fetus 17,18 find that maternal vitamin D deficiency during pregnancy is associated with poorer bone health markers in the fetus. Collectively, these studies suggest that the maternal 25(OH)D levels is an adequate proxy for the 25(OH)D levels that the fetus is exposed to. Another consideration is that the majority of the samples from the FMC (>70%) were collected in the first trimester of pregnancy. Whether the association between maternal vitamin D deficiency and MS risk in the offspring is confined to the first trimester or whether similar associations would be seen with maternal vitamin D deficiency during the second or third trimesters cannot be directly assessed in our study, but it seems unlikely that many deficient women became sufficient later in pregnancy, as even 25(OH)D levels collected during summer months were on average in the insufficient range (44 nmol/L). The average age at MS diagnosis was 19.8 and the oldest age at diagnosis was 27 years. Thus, we cannot rule out the possibility that the association between maternal 25(OH)D levels during pregnancy and MS incidence decreases at older ages. We also did not have information on other MS risk factors the offspring may have such as EBV infection, body mass index in childhood/adolescence, cigarette smoking, vitamin D status, or HLA DRB1*1501 status, and cannot rule out confounding by these factors. Finally, the increased MS risk among individuals born to vitamin D deficient mothers could be explained if these individuals had low circulating 25(OH)D during their childhood and early adult life. A positive correlation between maternal vitamin D status and the vitamin D status in her children would be expected because of probable similarities in behavior (e.g. use of vitamin D supplements or sun protection) and shared genes. Although it has been recently demonstrated in a Mendelian randomization study that individuals carrying alleles associated with lower 25(OH)D levels have an increased MS risk -- a result that supports a causal role of vitamin D in MS 19 -- the genetic contribution in our study is likely to be small, because genetically determined variations in 25(OH)D are modest, and only 50% of the maternal alleles are transmitted to the offspring. On the other hand, behavioral factors are more difficult to quantify, and we cannot estimate to what extent the effects of in utero exposure to vitamin D deficiency could be mediated by 25(OH)D levels later in life.
While the range of maternal 25(OH)D spanned levels of deficient to sufficiency, the majority of women in our study had deficient or insufficient levels (<50 nmol/L), and only 10 mothers had levels above 75nmol/L, one of whom had 25(OH)D levels above 100 nmol/L. Thus, while our results suggest that vitamin D deficiency during pregnancy increases MS risk in the offspring, our study does not provide any information as to whether there is a dose-response effect with increasing levels of 25(OH)D sufficiency. Similar studies in populations with a wider distribution of 25(OH)D are needed.
Correcting maternal vitamin D deficiency in early pregnancy may have a beneficial effect on risk of MS in the offspring.
Acknowledgements
We thank Leslie Unger and Dr. Kathryn Fitzgerald for technical assistance. We thank Dr. Marja-Liisa Sumelahti, MD, PhD, and professor Anne Remes, MD, PhD, for assistance in the MS case ascertainment. This study was funded by the National Institute of Neurological Disorders and Stroke (NIH R01 NS073633, PI: Ascherio). Drs. Munger and Ascherio had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The funding agency had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication. KL Munger has received grant funding from the National Multiple Sclerosis Society. J Åivo has nothing to declare. K Hongell has nothing to declare. M Soilu-Hänninen has obtained research grants from Bayer and Biogen Idec Finland and lecture fees and travel reimbursements from Bayer, Biogen Idec Finland, Genzyme, Merck, Novartis, Sanofi, Orion and Teva. H-M Surcel has nothing to declare. A Ascherio reports grants from National Institutes of Health (NIH) during the conduct of the study; grants from National Multiple Sclerosis Society (NMSS), Department of Defense, Michael J Fox Foundation, Accelerated Cure Project (ACP), and Chronic Fatigue Initiative, and personal fees from Bayer, Almirall, and Serono, outside the submitted work.
A Ascherio reports grants from National Institutes of Health (NIH), National Multiple Sclerosis Society (NMSS), Department of Defense, Michael J Fox Foundation, Accelerated Cure Project (ACP), and Chronic Fatigue Initiative, and honoraria for scientific presentations from Bayer HealthCare, Almirall, and Serono.
Footnotes
Author Contributions
KL Munger contributed to the design of the study, obtaining funding, statistical analysis, and writing the first draft of the manuscript.
J Åivo contributed to data collection and critical editing of the manuscript.
K Hongell contributed to data collection and critical editing of the manuscript.
M Soilu-Hänninen contributed to the design of the study, data collection, and critical editing of the manuscript.
H-M Surcel contributed to the design of the study, obtaining funding, data collection and critical editing of the manuscript.
A Ascherio contributed to the design of the study, obtaining funding, statistical analysis, and critical editing of the manuscript.
Potential Conflicts of Interest
KL Munger has received grant funding from the National Multiple Sclerosis Society.
J Åivo has nothing to declare.
K Hongell has nothing to declare.
M Soilu-Hänninen has obtained research grants from Bayer and Biogen Idec Finland and lecture fees and travel reimbursements from Bayer, Biogen Idec Finland, Genzyme, Merck, Novartis, Sanofi, Orion and Teva.
H-M Surcel has nothing to declare.
References
- 1.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012 Nov 5;8(11):602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 3.Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012 Nov 20;79(21):2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- 4.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 5.Ueda P, Rafatnia F, Baarnhielm M, et al. Neonatal vitamin D status and risk of multiple sclerosis. Ann Neurol. 2014 Jul 1;76(3):338–346. doi: 10.1002/ana.24210. [DOI] [PubMed] [Google Scholar]
- 6.Mirzaei F, Michels KB, Munger K, et al. Gestational Vitamin D and the Risk of Multiple Sclerosis in Offspring. Ann Neurol. 2011;70(1):30–40. doi: 10.1002/ana.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005 Jan 15;330(7483):120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staples J, Ponsonby AL, Lim L. Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis. BMJ. 2010;340(c1640) doi: 10.1136/bmj.c1640. doi:10.1136/bmj.c1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiddes B, Wason J, Kemppinen A, Ban M, Compston A, Sawcer S. Confounding underlies the apparent ‘month of birth’ effect in multiple sclerosis. Ann Neurol. 2013 Jun 6; doi: 10.1002/ana.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedeschi R, Luostarinen T, Marus A, et al. No Risk of Maternal EBV Infection for Childhood Leukemia. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2790–2792. doi: 10.1158/1055-9965.EPI-09-0751. [DOI] [PubMed] [Google Scholar]
- 11.Holl K, Surcel HM, Koskela P, et al. Maternal Epstein-Barr virus and cytomegalovirus infections and risk of testicular cancer in the offspring: a nested case-control study. APMIS. 2008 Sep;116(9):816–822. doi: 10.1111/j.1600-0463.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 12.IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 13.Ascherio A, Munger KL. Not too late to take vitamin D supplements. Ann Neurol. 2014;76(3):321–322. doi: 10.1002/ana.24239. [DOI] [PubMed] [Google Scholar]
- 14.Shor DB, Barzel J, Tauber E, Amital H. The effects of maternal vitamin D on neonatal growth parameters. European journal of pediatrics. 2015 Mar 24;174(9):1169–1174. doi: 10.1007/s00431-015-2517-5. [DOI] [PubMed] [Google Scholar]
- 15.Godang K, Froslie KF, Henriksen T, Qvigstad E, Bollerslev J. Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. Eur J Endocrinol. 2014 Apr;170(4):609–617. doi: 10.1530/EJE-13-0842. [DOI] [PubMed] [Google Scholar]
- 16.Wuertz C, Gilbert P, Baier W, Kunz C. Cross-sectional study of factors that influence the 25-hydroxyvitamin D status in pregnant women and in cord blood in Germany. Br J Nutr. 2013 Nov;110(10):1895–1902. doi: 10.1017/S0007114513001438. [DOI] [PubMed] [Google Scholar]
- 17.Mahon P, Harvey N, Crozier S, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010 Jan;25(1):14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannou C, Javaid MK, Mahon P, et al. The effect of maternal vitamin D concentration on fetal bone. J Clin Endocrinol Metab. 2012 Nov;97(11):E2070–2077. doi: 10.1210/jc.2012-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokry LE, Ross S, Ahmad OS, et al. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLoS Med. 2015 Aug;12(8):e1001866. doi: 10.1371/journal.pmed.1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]