Abstract
Probiotics have shown beneficial effects on health and prevention of diseases in humans. However, a concern for application of probiotics is the loss of viability during storage and gastrointestinal transit. The aim of this study was to develop an encapsulation system to preserve viability of probiotics when they are administrated orally and apply Lactobacillus rhamnosus GG (LGG) as a probiotic model to evaluate the effectiveness of this approach using in vitro and in vivo experiments. LGG was encapsulated in hydrogel beads prepared using pectin, a food grade polysaccharide, glucose, and calcium chloride, and lyophilized by freeze-drying. Encapsulated LGG was cultured in vitro under the condition that mimicked the physiological environment of the human gastrointestinal tract. Compared to non-encapsulated LGG, encapsulation increased tolerance of LGG in the acid condition, protected LGG from protease digestion, and improved shelf time when stored at the ambient condition, in regard of survivability and production of p40, a known LGG-derived protein involved in LGG’s beneficial effects on intestinal homeostasis. To evaluate the effects of encapsulation on p40 production in vivo and prevention of intestinal inflammation by LGG, mice were gavaged with LGG containing beads and treated with dextran sulphate sodium (DSS) to induce intestinal injury and colitis. Compared to non-encapsulated LGG, encapsulated LGG enhanced more p40 production in mice, and exerted higher levels of effects on prevention of DSS-induced colonic injury and colitis and suppression of pro-inflammatory cytokine production. These data indicated that the encapsulation system developed in this study preserves viability of LGG in vitro and in vivo, leading to longer shelf time and enhancing the functions of LGG in the gastrointestinal tract. Thus, this encapsulation approach may have the potential application for improving efficacy of probiotics.
Keywords: colitis, encapsulation, glucose, Lactobacillus rhamnosus GG, pectin, viability
1. Introduction
The symbolic interaction between the intestinal microbiota and the host plays important roles in maintaining health. Research has shown that interruption of this interaction is associated with several pathological conditions, such as inflammatory bowel disease (IBD), consisting of ulcerative colitis and Crohn’s diseases (1–4). Probiotics are live microorganisms which, when consumed in adequate amounts, confer a health benefit on the host. The well-studied probiotics include Lactobacillus, Bifidobacterium, and Saccharomyces. Lactobacillus and Bifidobacterium are commonly used as food supplements in yogurt and other fermented foods, and functional foods that represent the most rapidly developed products in food industries (5–11). Probiotics have been studied extensively in humans and experimental animals. Although there is a lack of large and well-controlled clinical studies to unequivocally prove the clinical efficacy of probiotics, evidence is emerging to support roles of probiotics in maintaining health and preventing and/or treating several intestinal diseases. For example, probiotics show benefits for preventing relapse of pouchitis (12, 13) and ulcerative colitis (5, 6).
Lactobacillus rhamnosus GG (LGG) is a naturally occurring gram-positive bacterium originally isolated from the healthy human intestine (14). There are a growing number of clinical studies suggesting that LGG is effective for the prevention and treatment of diarrhea, and infectious diseases in the respiratory, urogenital, or gastrointestinal tracts (15–17). LGG has also been used as a vaccine adjuvant (18). Currently, more research is ongoing to elucidate the mechanisms of probiotic actions, and to prove the relationship between the consumption of probiotics and a particular healing process.
The route of administration for probiotics is oral administration. Two criteria are required for administered probiotic bacteria to exert their functions. Probiotics must be protected from the stress of an extremely acid environment in the stomach and enzymatic deactivation in the stomach and the small intestine (19, 20). For example, LGG can grow after incubation in simulated gastric contents with the pH range 3.0–7.0 for 4 hours, but LGG is unable to grow at pH < 3.0 (21). It is well known that encapsulation is the most significant and efficient technology for the preservation of probiotics against adverse environmental conditions. Currently, the widely studied core materials include alginate, chitosan, carrageenan, gums, gelatin, whey protein, starch, and compression coating (22–30). These encapsulation systems have demonstrated acid resistance to some extent, however, only a few are able to retain viability of probiotics at pH as low as 2.0 (28). However, several disadvantages of these materials have been reported. For example, alginate microparticles are very porous, thus have less ability for protecting bacteria from their environment (31). Furthermore, it has also been reported that probiotics should be encapsulated in certain size range of alginate beads. Probiotic bacteria in small size alginate beads are susceptible to the stimulated gastric condition (32).
Since each one of the encapsulating materials has its own unique characteristics of capsule formation and the ability to influence the viability of probiotics during storage, processing, and in the gastrointestinal tract, there is a need to develop new encapsulation system to provide better protection for probiotics against adverse conditions in the gastrointestinal tract and improve shelf life of probiotics. To research this goal, we designed and developed an encapsulation system using pectin and glucose as the core materials coated with calcium chloride.
Pectin is a food grade, non-digestible polysaccharide extracted from the cell wall tissues of citrus, apples, and other higher plants. Primarily, pectin contains large amounts of poly (D-galacturonic acid) bonded via a −1, 4-glycosidic linkage. Pectin also contains neutral sugars, such as L-rhamnose, either inserted in or attached to the main chains. Pectin presents as aggregates of macromolecules in acid environments and is resistant to protease and amylase, which are active in the upper gastrointestinal tract, but is digested by a number of microflora in the colon. Because of these unique characteristics, pectin has been successfully used for the construction of several oral delivery systems for controlled drug delivery to the colon (33–37). In our previous studies, a LGG-derived soluble protein, p40, was encapsulated in pectin-derived beads and released at the colon site, resulting in amelioration of intestinal injury and colitis (35–37).
The aim of this study was to develop an encapsulation system to preserve viability of probiotics when they are administrated orally and apply LGG as a probiotic model to evaluate the effectiveness of this approach using in vitro and in vivo experiments. We generated hydrogel beads using pectin, glucose, and calcium chloride, and lyophilized beads by freeze-drying. We demonstrated that this encapsulation system preserved viability of LGG in vitro for improving shelf time, and protects LGG against the low acid condition and enzyme digestion, thereby enhancing the ability of LGG to produce p40 and prevent colitis. Thus, this encapsulation approach may have the potential application for improving efficacy of probiotics.
2. Materials and Methods
2.1. Materials
Citrus pectin LC950 with the degree of esterification (DE) of 32% and the galacturonic acid content of 69.7% was purchased from Danisco (Copenhagen, Denmark). Lecithin was from Zenobia Company, LLC (Bronx, NY, USA). D-glucose, calcium chloride, sodium chloride, sodium hydroxide, potassium chloride, potassium phosphate monobasic, sodium phosphate dibasic, and hydrochloric acid were obtained from Mallinckrodt Baker, Inc. (Paris, KY, USA). Sodium taurocholate, maleic acid, pancreatin, bile bovine, pectinolytic enzymes from A. niger, hexadecyltrimethylammonium bromide, and o-dianisidine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dextran sulphate sodium (DSS, MW 36–50 kDal) was from MP Biomedicals, LLC (Solon, OH, USA). Lactobacillus rhamnosus GG (LGG, ATCC 53103) was purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). Lactobacilli MRS broth and Lactobacilli MRS agar were purchased from Becton Dickinson & Co. (Sparks, MD, USA). HRP-conjugated goat anti-rabbit IgG antibody was from Cell Signaling Technology, Inc. (Danvers, MA). Teramethylbenzidine (TMB) was from BD Biosciences (San Jose, CA). Pure MPO was from Calbiochem (San Diego, CA). RNA isolation kit was from Qiagen (Valencia, CA).
Deionized water was prepared using MilliQ Water Systems OM-154 (Millipore Corporation, Bedford, MA, USA). All enzymes were stored at 4 °C and filtered using a 0.45-μm filter prior to use.
2.2. Encapsulation of LGG
LGG was cultured in Lactobacillus MRS broth at 35°C for 24 hours to reach the stationary phase. Then LGG was washed twice using NaCl (0.9%), and re-suspended in NaCl (0.9%) at 109 CFU/ml.
Pectin microspheres were prepared by dispersing the pectin solution (3.0%, w/v) containing a known amount of glucose and LGG into a calcium chloride solution (5%, w/v) under constant stirring at room temperature. The experiment was carried out by the use of a computer-controlled microsphere producer, equipped with 8 disperse channels, a sample reservoir, and a reaction chamber above on magnetic stirrer. A water pump has one end fitted with a filter (solvent reservoir filter) connecting to the reaction chamber and another end connecting to a deionized water tank and a waste collector which assures quick draining out of reaction solutions and pumping in of washing solutions in a precise time period (36). Microspheres were formed immediately upon pectin drops contacting the CaCl2 solution, and were allowed to continuously stir in the CaCl2 solution for additional 15 minutes. The CaCl2 solution in the reaction chamber was then pumped out and deionized water was pumped into the chamber for washing beads by continuously stirring for 5 minutes. This process was repeated 5 times. At the end, no Ca++ could be detected in the washing solution by AgNO3 titration. Then, beads were separated from water and lyophilized by freeze-drying. Beads with encapsulated LGG were used immediately after preparation, or stored at −20°C and at the ambient temperature for up to 28 days for in vitro and in vivo experiments.
2.3. Characterization of the hydrogel beads
Pectin hydrogel beads were examined for morphology and the encapsulation of bacteria using scanning electron microscopy (SEM), confocal microscopy, and optical microscopy.
For SEM examination, hydrogel beads were fractured in liquid nitrogen. Sample fragments were mounted on aluminum specimen stubs with colloid silver adhesive and coated with a thin layer of gold by dc sputtering. A Quanta 200 FEG SEM (FEI, Hillsboro, OR) was used to collect images. Images were taken in the high vacuum/secondary electron imaging model at 2,500X and 10,000X.
For optical microscopy, images were collected with a Leica MZ FLIII stereoscopic microscope (SMZ1500, Nikon Instruments Inc., Melvilla, NY) equipped with a DFC420C camera and Leica LAS V. 3.8 software (National Institute of Health, MD).
For confocal microscopy, samples of pectin hydrogel beads containing LGG were placed on microscope slides and observed using an IRBE optical microscope with a 10X lens integrated with a model TCS-SP laser scanning confocal microscope (Leica Microsystems, Exton, PA). The parameters for image acquisition were set at 485/500–530 nm (excitation/emission).
2.4. Evaluation of survival and function of encapsulated LGG in vitro
The hydrogel beads encapsulating LGG were incubated in aqueous medium that mimics the physiological conditions of the gastrointestinal tract (38). The basic components of the medium are calcium chloride, potassium phosphate monobasic, potassium chloride, sodium chloride, sodium hydroxide, sodium phosphate dibasic, sodium taurocholate, hydrochloric acid, maleic acid, and lecithin with appropriate concentrations. The simulated small intestinal fluid contained additional trypsin (1 g/L), pancreatin USP (1 g/L) or bile solution (0.1%). The simulated colonic fluid contained an additional mixture of pectinolytic enzymes (120 FDU/mL). In the control experiments, non-encapsulated LGG was used.
At predetermined time intervals, a fraction of the pectin beads was taken for detecting survival of bacteria using the standard plate count method (20, 39). Briefly, beads were broken down in sterile 0.1% peptone solution in a stomacher and serially diluted was performed using 0.1% peptone. An aliquot of each solution was plated on MRS agar and incubated at 37 °C under anaerobic conditions for 16 hours followed by enumeration. The total number of viable cells was reported as colony-forming units (CFUs) per ml.
LGG with and without encapsulation was stored at room temperature up to 1 month. The ability of LGG to grow was detected by culturing encapsulated LGG and non-encapsulated LGG with 1, 2, and 4-week storage time and fresh LGG without any storage in MRS broth at 35°C. LGG growth rate was determined by measuring the time for the bacterial culture to reach OD600=0.6 (stationary phase). To examine the function of LGG in culture, we detected p40 production in culture supernatants collected from LGG culture with OD600= 2. Our previous study showed that the maximal amount of p40 is recovered in the LGG culture supernatant culture with OD600= 2.
Production of the LGG-derived functional protein, p40, was detected by Western blot analysis (35). Bacterial culture supernatants were mixed with Laemmli sample buffer and proteins were separated by SDS-PAGE for Western blot analysis using a rabbit anti-p40 antibody, generated by our group (7).
2.5. Detection of p40 production by encapsulated LGG in mice
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center.
The fecal p40 level was examined using ELISA to evaluate the viability of the encapsulated LGG in mice. Briefly, six to eight-week old wild type C57BL/6 mice were administered LGG containing beads (108 LGG/bead/mouse, once a day), beads without LGG, or non-capsulated LGG (108 in 500 μL of PBS/day) for two days. Mice fed with drinking water only were used as control. Feces were collected before and after treatment. Feces were solubilized in 50 mM Tris, pH 7.5, containing 150 mM NaCl, 1% Triton-X-100 and protease and phosphatase inhibitors, and homogenized using TissueLyser. The supernatants were diluted in 0.1 M sodium carbonate, pH 9.5 (1:10 dilution) and incubated in the 96-well dish with a flat bottom for 24 h at 4°C overnight. The plate was washed with wash buffer (0.05% tween-20 in PBS) and blocked by 10% FBS in PBS for 1 h at room temperature. The plates with the coated proteins were incubated with a rabbit polyclonal anti-p40 antibody (7) (1:500 dilution in PBS) for 2 h at room temperature, followed by a HRP-conjugated goat anti-rabbit IgG antibody (1: 2000 dilution in PBS) for 1 h at room temperature. The substrate solution, teramethylbenzidine (TMB), was used to develop the reaction through incubation for 30 min. This reaction was stopped by 2N H2SO4. The OD at 450 nm wavelength was measured. Purified p40 was used to generate a concentration curve.
2.6. Induction of experimental colitis in mice and encapsulated LGG treatment
The efficacy of the encapsulated LGG on the prevention and treatment of IBD was evaluated using a mouse model of colitis. Six to eight-twelve week old wild type C57BL/6 mice administered 3% DSS in drinking water for 4 days to induce intestinal injury and acute colitis. Mice were gavaged with LGG containing beads (1× 108 LGG encapsulated in bead/mouse, once a day), beads without LGG, or non-capsulated LGG (108 in 500 μL of PBS/day) starting three days prior to DSS treatment, until mice were sacrificed. Mice fed with drinking water only were used as control.
2.7. Analysis of inflammation in the colon
Paraffin-embedded colon tissue sections were stained with hematoxylin and eosin for light microscopic examination to assess colon injury and inflammation. Samples from the entire colon were examined by a pathologist blinded to treatment conditions. Intestinal lesions were evaluated using a scoring system as reported before (37). A modified combined scoring system including degree of inflammation (scale of 0–3) and crypt damage (0–4), percentage of area involved by inflammation (0–4) and crypt damage (0–4), and depth of inflammation (0–3) was applied for colitis induced by DSS. An additive score is between 0 (no colitis) and 18 (maximal colitis).
2.8. MPO assay
Myeloperoxidase (MPO) activity was examined using MPO assay. Colonic tissues were weighed and homogenized in tissue suspension buffer containing 50 mM potassium phosphate (pH 6.0) and 5 mg/ml hexadecyltrimethylammonium bromide at a ratio of 50 mg tissue to 1 ml of buffer. MPO level was detected using the reaction buffer containing 17% o-dianisidine, 5 mM potassium-phosphate, and 0.0005% H2O2, as described before (8). Pure MPO was used to prepare a concentration curve. Tissue suspension buffer was used as negative control. The results were calculated as: Unite of MPO/g colon tissue.
2.9. Real-time PCR analysis
For real-time PCR analysis, total RNA was isolated from homogenized colonic tissues using an RNA isolation kit and was treated with RNase-free DNase. Reverse transcription was performed using the High Capacity cDNA Reverse Transcription kit and the 7300 Real Time PCR System (Applied Biosystems, Foster City, CA). The data were analyzed using Sequence Detection System V1.4.0 software. All primers were purchased from Applied Biosystems, TNF (Mm00443259) and KC (Mm00433859). The relative abundance of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used to normalize levels of the mRNAs of interest. All cDNA samples were analyzed in triplicate.
2.10. Statistical analysis
Statistical significance for multiple comparisons in each study was determined by one-way ANOVA followed by a Newman-Keuls analysis using Prism 5.0 (GraphPad Software, Inc.). T-test for paired samples. A p value < 0.05 was defined as statistically significant. Data are presented as mean±SD. A p value < 0.05 was defined as statistically significant.
3. Results and Discussion
3.1. Characterization of encapsulated LGG in vitro
Several pectin-derived hydrogel beads have been synthesized and tested for oral protein drug delivery with compositional and/or structural variations according to the nature of core materials and end applications (33, 35, 37, 40). In the present research, hydrogel beads were made from pectin polysaccharide of low DE and high galacturonan content by chelating with calcium ions using a home-made microsphere maker. By altering the pressure at the exit of the disperser, hydrogel beads with the volumes ranging from 5 to 500 μl could be obtained. All beads made at the same exit pressure had the same size. As shown in the Figure 1, LGG were evenly dispersed within the beads, and the density of the bacteria in the beads was determined by the initial concentration of LGG suspended in the pectin solution (comparison of A2 to A3, and B2 to B3).
Figure 1.
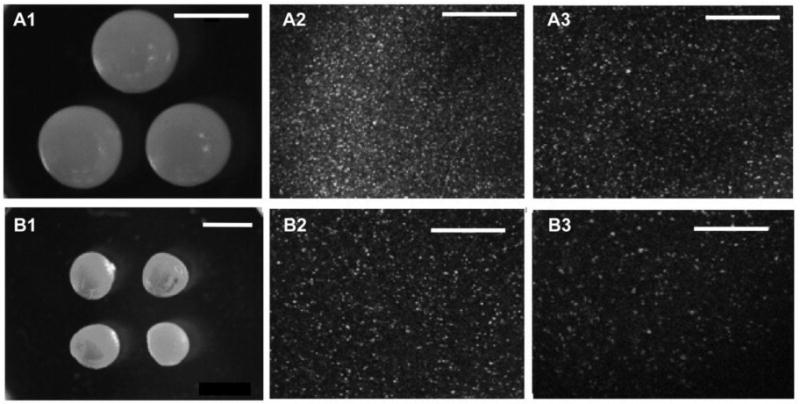
Microphotographs of optical microscopic analysis (A1 and B1) and confocal microscopic images (A2, A3, B2, and B3) of pectin hydrogel beads. Volume of beads: A1–A3, 50 μl; B1–B3, 10 μl. Bacterial content: A1 and B1: 0, A2: 10 Log, A3 and B2: 9 Log, B3: 8 Log. Bar in A1=3 mm, Bar in B1=2 mm, Bar in A2–3 and B2–3=100 μm. Field width: A1=10.5 mm, B1=21 mm, A2, A3, B2, and B3=400 μm.
Since all operation parameters (time of reaction, solidification, and washing, stirring rate, volume of reaction and washing media, ratio of reactants, temperature, etc.) were under precise control, batch-batch variations in the properties of the resultant hydrogel beads, in terms of composition, crosslinking density, porosity, and encapsulation rate, could be limited. Thus in the following evaluation experiments, the impact of batch-to-batch differences on the encapsulation, such as encapsulation efficiency, bacterial and bead stability, and bacterial viability, was ignored. Table 1 shows the encapsulation efficiency of pectin-derived hydrogel beads. 100% of bacteria in pectin solutions were encapsulated in the resultant beads. Furthermore, the inclusion of glucose in viscous pectin had no impact on the encapsulation rate.
Table 1.
Comparison of LGG amount in pectin drops and in hydrogel beads
| Type of beads | Suspended in solution (Log CFU/mL) | Encapsulated in bead (Log CFU/mL) |
|---|---|---|
| Pectin without glucose | 8.71 ± 0.11 | 8.70 ± 0.09 |
| Pectin with 10% glucose | 9.11 ± 0.10 | 9.10 ± 0.13 |
The effect of the length of lyophilization time on growth of encapsulated LGG was examined (Figure 2). An initial quick drop in LGG growth was observed for the first four hours for all formulations, which was followed by a continuous decrease at the rates much slower. For LGG encapsulated in bare pectin beads, the reduction of growth was measured less than 25% after 1 day lyophilization, showing a cryo-resistant character.
Figure 2.
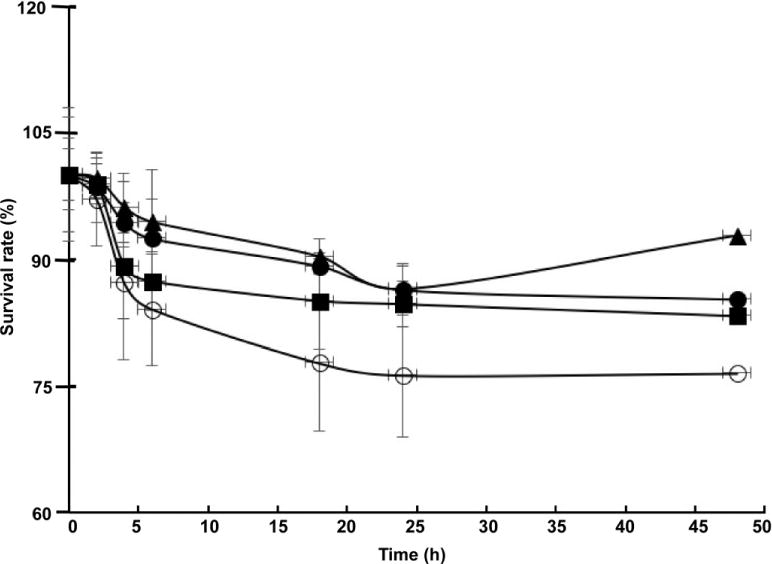
The effect of glucose on LGG growth during freeze-drying. Glucose content: 0% (○), 5% (■), 10% (▲), 20% (●)
As a cryopreservative agent and metabolized sugar, glucose has been shown to have different protective effects for different bacterial species on their survival from low temperature or acidic stress (41–46), the influence of glucose on LGG in pectin hydrogel beads was investigated in this study. As shown in Figure 2, the inclusion of glucose further enhanced the cryo-resistance of LGG. Among the samples containing 5, 10, and 20% glucose, a slight difference in bacterial growth was observed, the beads with 10% glucose seems function best to retain LGG’s survivability. At high concentration, such as 10%, the non-permeable additive glucose may prevent injurious eutectic freezing of cell fluids by trapping salts in a highly viscos phase or glassy state, and bind to water molecules that inhibits intracellular and extracellular ice formation (41). However, when the glucose concentration becomes too high, the protectant turns to be toxic to cells (42). Thus, the present study found the crucial of the inclusion of an adequate amount of glucose in preservation of the survival of LGG during lyophilization.
We then studied the weight loss of the beads during lyophilization and compared the results obtained (Figure 3) to the survival reduction of the encapsulated LGG (Figure 2). It was found that under experimental conditions the majority of water (99%) was removed from the pectin hydrogel beads after submission to lyophilization for 4 hours. During this time period the survival reduction for bacteria encapsulated in glucose including pectin beads was negligible. The survival reduction for LGG in the beads without glucose was about 7%. A major survival reduction was found as with continuous lyophilization. The data indicated the remaining traces of water were critical in the process to preserving the ability of LGG to survive, and the inclusion of glucose could relieve the problem (43). In the following experiments, LGG encapsulating pectin hydrogel beads were lyophilized for 4 hours, and then stored at room temperature or in frozen for further examination.
Figure 3.
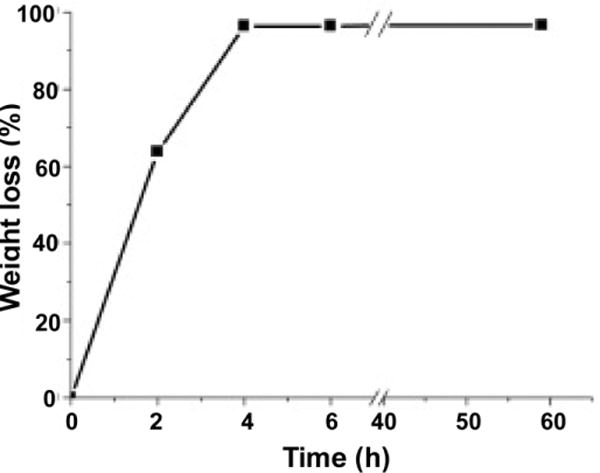
Time-dependent weight loss of pectin hydrogel beads during lyophilization.
Encapsulated LGG was stored at ambient temperature for 6 days, then cultured in MRS broth followed by examination for structural and growth properties of LGG. The SEM microphotographs of the surfaces and the fracture sections of the beads before and after incubation were examined (Figure 4). The hydrogel beads were shrunk to pellets with irregular shape after lyophilization (Figure 4A), but still possessed a porous structure with pores of various sizes connecting to form channels (Figure 4B). It appeared that the pores resulted from lyophilization in which the continuous solid phase of ice was removed from another continuous solid phase of pectin, which were interpenetrated each other. LGG were detected on the surfaces (Figure 4C) of the beads and inside the pores, but most were dwelling in the inner walls of the pores (Figure 4D). Upon culturing of LGG in MRS broth for 24 hours, the spaces in pectin beads were almost filled with the bacteria (Figure 4E and F). As bacterial growth continued, the beads gradually disintegrated after culturing for 72 hours. We have found that LGG culture reaches the stationary phase after 24 hour-culture at 35 °C. The growth of encapsulated LGG showed the same growth character (Figure 5).
Figure 4.
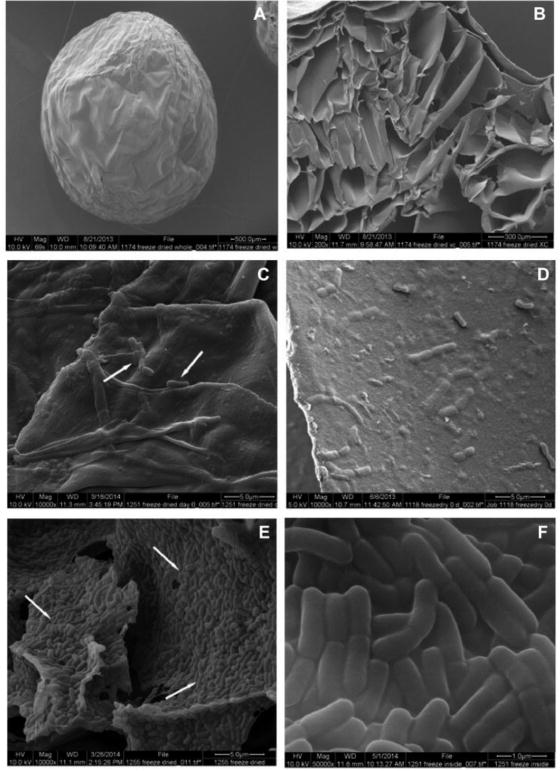
SEM images of hydrogel beads encapsulated with LGG after freeze-dry: surface view (A, C) and fractural section (B, D); and re-incubated in MRS broth for 24 hours (E, F). Magnification: A=70X, B=200X, C, D, and E=10,000X, F=50,000X.
Figure 5.
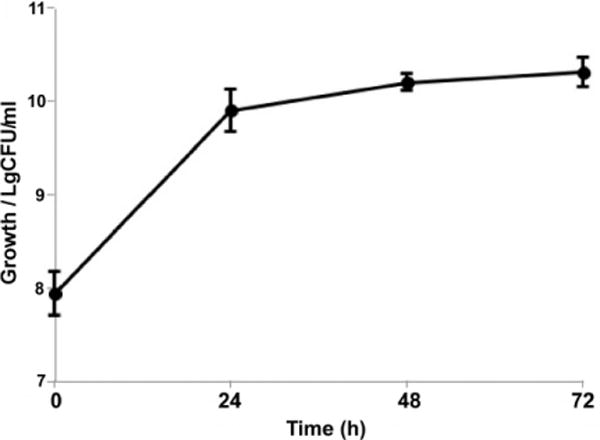
Time curve of encapsulated LGG growth. Encapsulated LGG was cultured in MRS broth under anaerobic conditions at 35 °C.
We next tested the effects of the encapsulation approach generated in this study on improving long-term storage of probiotics. Encapsulated LGG and non-encapsulated LGG with freeze-drying were stored at the ambient condition for up to 4 weeks. We then cultured encapsulated LGG and non-encapsulated LGG that have been stored for 1, 2 and 4 weeks and detected the growth rate and production of p40 in the culture supernatant. We have reported that p40 has protective effects on intestinal epithelial cells (35, 37). We also found that the highest amount of p40 is recovered in LGG culture broth at the condition of OD600 =2. Thus, we cultured 108 of encapsulated and non-encapsulated LGG in 5 ml of MRS broth at 35 °C. The growth rate was determined by the time required for researching the condition of OD600 =0.6. Production of p40 was evaluated using Western blot analysis of 20 μl of culture broth with OD600=2 using an anti-p40 antibody.
Our results showed that encapsulated LGG with 1, 2, and 4-week storage time grew in MRS broth at the same growth rate as freshly prepared LGG. Although the growth rate of non-encapsulated LGG with 1 and 2-week storage time was the same as that of encapsulated LGG, non-capsulated LGG with 4-week storage time did not grow in MRS broth. Based on the ability of LGG growth, which has been widely used as a standard for assessing the effects of encapsulation approaches for probiotics, our data suggest that the encapsulation system developed by our study can increase LGG’s shelf time when stored at the ambient condition.
We further studied the function of LGG after storage. The amount of p40 produced by non-encapsulated LGG was significant lower than that by encapsulated LGG (Figure 6). We also found that the amount of p40 produced by encapsulated LGG was decreased as the storage time increased (Figure 6).
Figure 6.
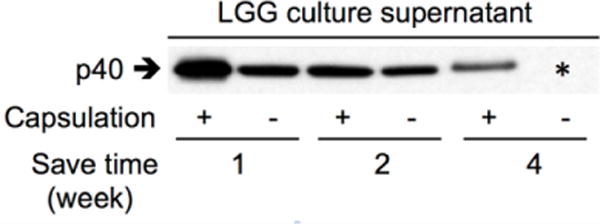
Increased production of p40 by encapsulated LGG. LGG with and without encapsulation was cultured in MRS broth at 35 °C after storage at the ambient condition for indicated times. Culture supernatants were collected from LGG culture with OD600=2. 20 μl of culture supernatant was used for Western blot analysis of p40 using an anti-p40 antibody. * non-capsulated LGG with 4-week storage time did not grow in MRS broth.
Our data suggest that this encapsulation method protects LGG’s ability to produce p40, as compared to non-capsulated LGG. However, the fact that the ability of LGG to produce p40 becomes decreased as increasing the storage time emphasizes the need for improving this method to better preserve the function of probiotics.
The survival of encapsulated LGG under conditions mimicking the physiological environment of the gastrointestinal tract was also evaluated using an in vitro assay. As shown in Figure 7, non-capsulated LGG lost survival when incubated in simulated gastric solution at pH 1.6 for 2 hours (reduced 7.5 log). The LGG encapsulated in bare pectin beads partially lost their activity (reduced 2 log for 2 hour incubation in gastric simulation). LGG encapsulated in pectin beads containing 10% glucose were able to survive in the stomach conditions for 2 hours at pH 1.6. Most remaining bacteria were able to travel through the small intestine simulations (2 hours, at pH 6.5) to the colon simulations, where the change in LGG activity after 2 hours was negligible. Since the concentration of non-capsulated LGG was extremely low when it was transferred to the small intestinal simulation, to better understand the effect of pectin and glucose on protease stability of LGG, in a separate experiment, we incubated non-capsulated LGG, LGG with glucose or pectin, and the encapsulated LGG in the simulated small intestine solution, skipping the stage of conditioning in the simulated stomach environment. As shown in Table 2, encapsulation of LGG in pectin beads protected LGG from protease digestion. The inclusion of glucose in the beads further enhanced the stability of LGG. The addition of glucose or pectin to the cultures of LGG with proteases enhanced the survival of LGG, but the effect of pectin was less than glucose. These data indicates the protective activity of both glucose and pectin for LGG in small intestine tract.
Figure 7.
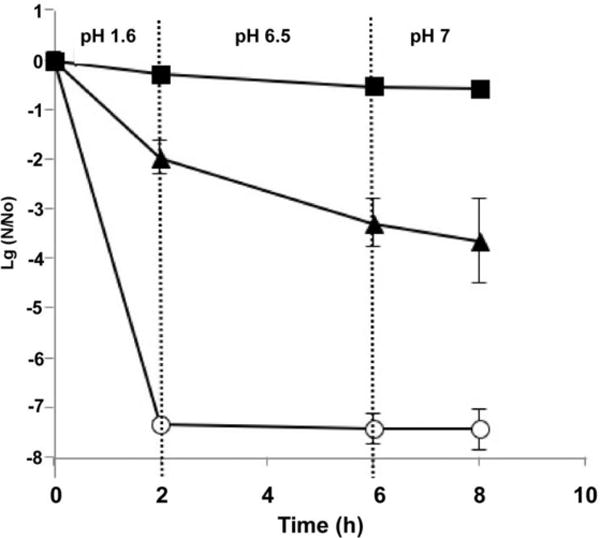
Survival of non-capsulated LGG (○) and LGG encapsulated in pectin beads with 10% glucose (■) and without glucose (▲) in simulated GIT medium under anaerobic condition.
Table 2.
LGG survival from culture in small intestinal simulations
| 0 h (Log CFU/mL) |
4 h (Log CFU/mL) |
|
|---|---|---|
| Non-capsulated LGG | 9.76 (0.04) | 3.25 (0.27)* |
| Non-capsulated LGG with glucose | 9.70 (0.04) | 6.52 (0.16)* |
| Non-capsulated LGG with pectin | 9.85 (0.03) | 5.36 (0.21)* |
| LGG in pectin beads with 10% glucose | 10.05 (0.05) | 9.81 (0.13) |
| LGG in Pectin beads without Glucose | 7.22 (0.05) | 5.97 (0.47)* |
p < 0.05 compared to the 0 h group.
We then investigated the protective activity of glucose and pectin in their free forms for the survival of non-capsulated LGG in acidic environments. As shown in Table 3, at pH 3 no significant changes in LGG survivability could be detected regardless either the medium contained glucose, or pectin, or glucose and pectin together, or none of them. The result was consistent with that previously reported (21). It could be attributed to the unique galactose-rich exopolysaccharides (EPS) produced by living LGG that form a protective shield on the surfaces of the bacteria, which reportedly promoted the survival and persistence of LGG in murine GIT, and showed a relatively high competitive index of EPS mutant in stomach (44). At pH 2, without the addition of either glucose or pectin, LGG lost its survivability significantly (reduced from 10 log to 3 log); whereas, only less than 1 log reduction was detected for LGG incubated in the presence of glucose or the galacturonan-rich pectin, either both together or individually. As the solution pH reached 1.5, the survivability of LGG reduced by 7.5 log after incubation for 2 h; the application of glucose saved LGG survivability to some degree (reduced 6 log). The application of free pectin had no effect (reduced 7.5 Log), in contrast to the use of cross-linked pectin (comparison data in Figure 7 to Table 3). Overall, in comparison of glucose to pectin, the former seemed to have a higher protective activity than the latter in keeping LGG from acidic deactivation.
Table 3.
Effects of glucose and pectin on LGG survival in solution with various pH
| pH | Glucose | Pectin | Viability of LGG (Log CFU/mL) |
|
|---|---|---|---|---|
| Zero time | 2 h | |||
| 3 | YES | YES | 9.81(0.05) | 9.91(0.47) |
| 3 | YES | NO | 9.80(0.01) | 9.69(0.41) |
| 3 | NO | YES | 9.84(0.04) | 9.53(0.16) |
| 3 | NO | NO | 9.91(0.05) | 9.64(0.06) |
| 2 | YES | YES | 9.85(0.04) | 9.83(0.05) |
| 2 | YES | NO | 9.89(0.03) | 9.63(0.33) |
| 2 | NO | YES | 9.82(0.05) | 9.45(0.05) |
| 2 | NO | NO | 9.83(0.02) | 3.36 (0.06) |
| 1.5 | YES | YES | 9.85(0.03) | 3.54(0.50) |
| 1.5 | YES | NO | 9.88(0.06) | 3.60(0.25) |
| 1.5 | NO | YES | 9.81(0.05) | 2.25(0.34) |
| 1.5 | NO | NO | 9.84(0.03) | 2.35(0.28) |
| 1 | YES | YES | 9.84(0.05) | <1 |
| 1 | YES | NO | 9.87(0.03) | <1 |
| 1 | NO | YES | 9.89(0.05) | <1 |
| 1 | NO | NO | 9.80(0.06) | <1 |
D-glucose could serve as an energy resource for LGG, and so the solution pectin could function as prebiotic (45–47), this may explain why the presence of both glucose and pectin could enhance the survival of LGG in solution at pH 2 or in simulated small intestinal solution. It has been reported that the inclusion of sugars could provide ATP to F0F1-ATPase via glycolysis, enabling proton exclusion and thereby enhancing LGG survival during gastric transit (46). Supposedly, the difference in the metabolic efficiency between glucose and pectin by LGG impacted their protective activity. The small molecule of glucose, but not the macromolecular pectin, can be metabolized efficiently.
Pectin beads provide a physical barrier to protect core materials from the attack of macromolecular antagonistics, such as for LGG in the present study. Pectin in cross-linking with Ca++ also protected LGG from acidic deactivation, in contrast to its free form. Supposedly, as in acidic environment, an ion exchange reaction may occur between the chelated Ca++ and the migrated H+ ions that help to create a microenvironment within the beads, which is less acidic than outside solution. Therefore, the encapsulated LGG were more “acid-resistant” than non-capsulated LGG with pectin in the free form (Figure 7 and Table 3).
3.2. Detection of the effects of encapsulation on LGG’s in vivo activity
As reported previously, we developed a pectin/zein hydrogel bead system to specifically deliver p40, a LGG-derived protein, to the colon. Using this delivery system, we showed that p40 prevents and treats experimental colitis in mice (35, 37). In the present study, we fed mice with encapsulated LGG in pectin-derived beads. Non-capsulated LGG was used as a control. The p40 level in feces was detected using ELISA. Non-capsulated LGG treatment increased the p40 level in feces at the first day after treatment (p<0.05), as compared to that in the control and bead only treated groups. p40 production was further increased at the first day after treatment by encapsulated LGG (p<0.05) (Figure 8). These data suggest that the encapsulation of LGG enhanced p40 production, thereby increasing the ability of LGG to exert its functions in vivo.
Figure 8.
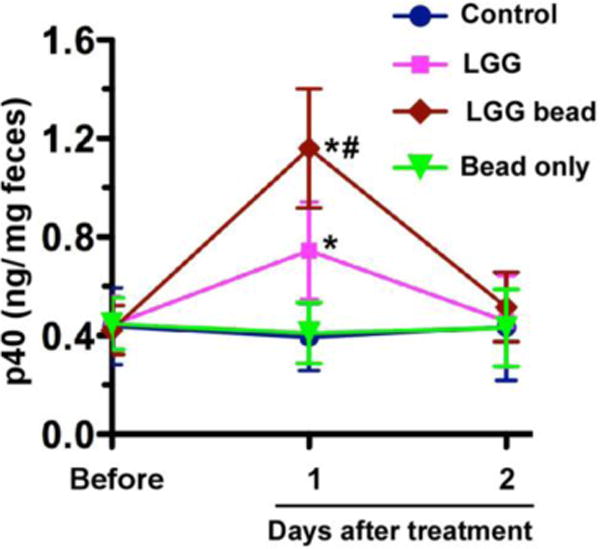
Encapsulation of LGG increases the p40 level in mouse feces. Mice were gavaged with 108 of freshly prepared LGG in water or encapsulated LGG (saved at 4°C for less than 2 weeks). Non-treatment was used as control. Beads only were used as control for encapsulated LGG. Feces were collected before and at the indicated times after treatment for ELISA analysis of fecal p40 levels. *p < 0.05 compared to that in the same group before treatment and the bead only group at 1 and 2 days after treatment. # p < 0.05 compared to that in LGG-treated group at 1 day after treatment. N=5 in each group.
Next, DSS-reduced intestinal injury and acute colitis model was used to evaluate whether encapsulated LGG exerted higher level of the preventive effect on DSS-induced colitis. DSS treatment induced injury and acute colitis with massive colon ulceration and severe inflammation. These abnormalities were significantly reduced by treatment with encapsulated LGG (Figure 9A). The scores of epithelial injury and inflammation were assessed by a pathologist blind to the treatment protocol. The scores of the group receiving DSS and DSS with bead only were 7.3 ± 1.5 and 7.8 ± 1.3, respectively, which were not significant different (p > 0.05). As compared to scores of DSS treated group and the group with DSS and bead only, the score of the group with encapsulated LGG treatment was significantly decreased (4.9 ± 1.4, p < 0.05), and the score of non-encapsulated LGG and DSS group (6.6 ± 1.3) was reduced, but it was not significant (p > 0.05). (Figure 9B).
Figure 9.
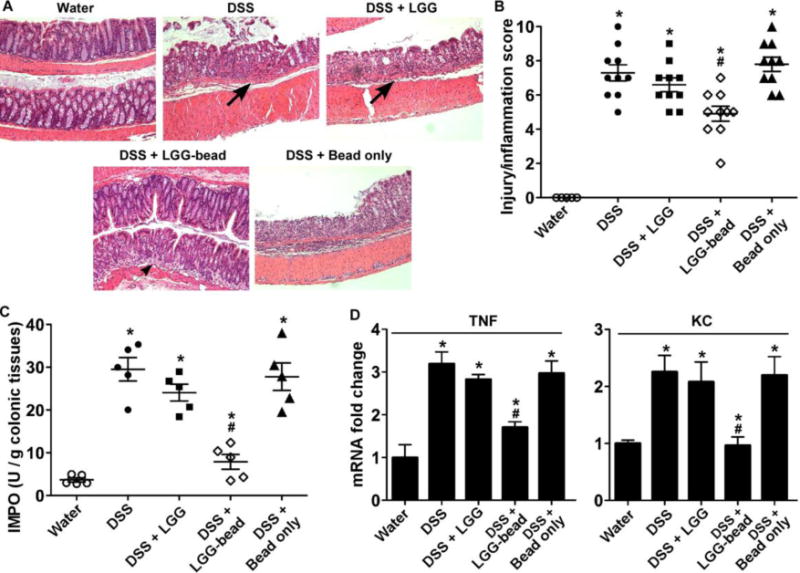
LGG encapsulated in pectin hydrogel beads prevents DSS-induced colitis in mice. Colonic injury and colitis were induced by treating mice with 3% DSS in drinking water for 4 days. Mice were gavaged with 108 of non-capsulated LGG in water or encapsulated LGG/day, starting 3 days before DSS treatment until the end of the experiment. Beads only were used as control for encapsulated LGG. (A) Paraffin-embedded colon sections were stained with H&E for light microscopic assessment of epithelial damage. Original magnification: 10X. (B) Injury and inflammation scores are shown. (C) MPO activity in the colonic tissue was detected. (D) Colonic tissues were isolated for real-time PCR analysis of the indicated cytokine mRNAs. The average cytokine mRNA expression level in the water group was set as 1, and mRNA expression levels in other groups were compared to obtain the fold change. * p < 0.05 compared to the water group, # P < 0.05 compared with DSS, DSS with non-encapsulated LGG-treated and bead only treated groups. N=5 in each group in D.
DSS induces neutrophil infiltration in the colon leading to increasing colonic MPO activity, which is therefore an inflammatory marker for colitis. Treatment of mice with encapsulated LGG, but not non-capsulated LGG or bead only, significantly reduced DSS-stimulated MPO activity in the colon (p<0.01) (Figure 9C).
Increased proinflammatory cytokine production is a hallmark of DSS-induced colitis. Therefore, we tested the effects of encapsulated LGG on inflammatory cytokine production in the DSS-induced colitis model. We used Real-Time PCR analysis of RNA isolated from the colon to detect cytokine mRNA levels. Our previously studies demonstrated that p40 treatment down-regulated DSS-induced production of several proinflamatory cytokines, including tumor necrosis factor (TNF) and KC (6, 12). Treatment with encapsulated LGG, but not non-capsulated LGG or bead only, led to down-regulation of DSS-induced mRNA levels of TNF (p<0.05) and KC (p<0.05) (Figure 9D). These results suggest that oral administration of LGG in the pectin-derived beads has potential effects on prevention of intestinal inflammation.
Although many studies which were focused on encapsulation of probiotics have been reported, they are mainly contributed the development of the methods and lack proof of concept for final applications, such as the functions of encapsulated probiotics in maintaining health and disease prevention. Our studies applied in vivo assays to evaluate the functions of encapsulated LGG in prevention of colitis. Thus, results from our studies provide information for understanding the beneficial effects encapsulation on clinical efficacy of probiotics.
4. Conclusion
The present studies demonstrated that LGG could be encapsulated in the pectin-derived delivery system which preserved viability of LGG when administrated orally. The growth of the encapsulated LGG was further enhanced by the inclusion of glucose in the pectin formulation. LGG encapsulated in hydrogel beads exhibited longer shelf life and higher potential for prevention of intestinal inflammation. Therefore, pectin with glucose may have more advantages as encapsulation materials for protecting probiotics from acid condition and protease digestion in the human gastrointestinal tract. Furthermore, this encapsulation approach may benefit the functions of probiotics, thereby having the potential application for improving efficacy of probiotics.
Acknowledgments
The authors gratefully acknowledge the technical support of Dr. Lihan Huang, Mr. Joseph Uknalis, and Ms. Audrey Thomas-Gahring of ERRC-USDA. This work was supported by NIH grants R01DK081134 (F.Y.) and core services performed through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404.
References
- 1.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser MJ. Harnessing the power of the human microbiome. Proc Natl Acad Sci USA. 2010;107:6125–6126. doi: 10.1073/pnas.1002112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EMM, Sartor RB, Sherman PM, Mayer E. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques TM, Cryan JF, Shanahan F, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota modulation and implications for host health: dietary strategies to influence the gut-brain axis. Innov Food Sci Emerg. 2014;22:239–247. [Google Scholar]
- 5.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 7.Yan F, Cao H, Cover TL. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, Chaturvedi R, Israel DA, Wang B, Peek RM, Jr, Wilson KT, Polk DB. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G504–14. doi: 10.1152/ajpgi.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas U, Ranganathan N. Probiotics, Prebiotics, and Synbiotics: Gut and Beyond. Gastroenterol Res Pract. 2012;1:1–16. doi: 10.1155/2012/872716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders ME, Marco M. Food Formats for Effective Delivery of Probiotics. Food Science and Technology. 2010;1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- 11.Prakash S, Martoni C. Toward a new generation of therapeutics. Applied Biochemistry and Biotechnology. 2006;128:1–21. doi: 10.1385/abab:128:1:001. [DOI] [PubMed] [Google Scholar]
- 12.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 13.Lammers KM, Vergopoulos A, Babel N, Gionchetti P, Rizzello F, Morselli C, Caramelli E, Fiorentino M, d’Errico A, Volk HD. Probiotic therapy in the prevention of pouchitis onset: decreased interleukin-1beta, interleukin-8, and interferon-gamma gene expression. Inflamm Bowel Dis. 2005;11:447–454. doi: 10.1097/01.mpa.0000160302.40931.7b. [DOI] [PubMed] [Google Scholar]
- 14.Gorbach SL, Goldin BR, Barry R. US Patent 4,839. Lactobacillus strains and method of selection. 1989 Jun 13;281
- 15.Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, Dreborg S, Goodman R, Kuitunen M, Haahtela T, Heine RG, Lack G, Osborn DA, Sampson H, Tannock GW, Lee BW. Clinical use of probiotics in radiatric allergy. WAO Journal. 2012;11:148–167. doi: 10.1097/WOX.0b013e3182784ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojsak I, Snovak N, Abdović S, Szajewska H, Mišak Z, Kolaček S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2009;29(3):312–6. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Manley KJ, Fraenkel MB, Mayall BC, Power DA. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med J Aust. 2007;186(9):454–457. doi: 10.5694/j.1326-5377.2007.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 18.Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoine JM. Current challenges for probiotics in food. In: Lathinen S, Ouwehand AC, Salminen S, Wright AV, editors. Lactic acid bacteria: microbiological and functional aspects. 4th. CRC Press; London, UK: 2011. pp. 202–221. [Google Scholar]
- 20.Ortakci F, Sert S. Stability of free and encapsulated Lactobacillus acidophilus ATCC 4356 in yogurt and in an artificial human gastric digestion system. J Dairy Sci. 2012;95:6918–6925. doi: 10.3168/jds.2012-5710. [DOI] [PubMed] [Google Scholar]
- 21.Golding BR, Gorbach SL, Saxielin M, Barakat S, Gualtier L, Salminen S. Survival of lactobacillus species (strain GG) in human gastrointestinal tract. Digestive Diseases and Sciences. 1992;37:121–128. doi: 10.1007/BF01308354. [DOI] [PubMed] [Google Scholar]
- 22.Okuro PK, Thomazini M, Balieiro JCC, Liberal RD, Fávara-Trindade CS. Co-encapsulation of lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Research International. 2013;53:96–103. [Google Scholar]
- 23.Goderska K, Zybala M, Czarnecki Z. Characterization of microencapsulated lactobacillus rhamnosus LR7 strain. Polish Journal of Food and Nutrition Sciences. 2001;53(3):21–24. [Google Scholar]
- 24.Sunny-Roberts EQ, Knorr D. The protective effect of monosodium glutamate on survival of lactobacillus rhamnosus GG and lactobacillus rhamnosus E-97800 (E800) strains during spray-drying storage in trehalose-containing powders. International Dairy Journal. 2009;19:209–214. [Google Scholar]
- 25.Capela P, Hay TKC, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organism in yoghurt and freeze-dried yoghurt. Food Research International. 2006;39:203–211. [Google Scholar]
- 26.Brodkorb A, Doherty S, Wang LZ, Ross PR, Fitzgerald GF, Stanton C. Improved viability of probiotic by whey protein products. XVth International Workshop on Bioencapsulation; Vienne, AU. September 6, 2007; pp. S3–5. [Google Scholar]
- 27.Ananta E, Volkert M, Knorr D. Cellular injuries and storage stability of spray-dried lactobacillus rhamnosus GG. International Diary Journal. 2005;15:399–409. [Google Scholar]
- 28.Weinbreck F, Bodnár I, Marco ML. Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? International Journal of Food Microbiology. 2010;135:364–367. doi: 10.1016/j.ijfoodmicro.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Gabara C, Chaves KS, Ribeiro MCE, Souza FN, Grosso CRF, Gigante ML. Viability of Lactobacillus acidophilus La5 in pectin-whey protein microparticles during exposure to simulated gastrointestinal conditions. Food Research International. 2013;51:872–878. [Google Scholar]
- 30.Sandoval-Castilla O, Lobato-Calleros C, Gacía-Galindo HS, Alvarez-Ramírez J, Vernon-Carter EJ. Textural properties of alginate-pectin beads survivability of entrapped Lb. casei in simulated gastrointestinal conditiona and in yoghurt. Food Research International. 2010;43:111–117. [Google Scholar]
- 31.Gouin S. Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol. 2004;15:330–347. [Google Scholar]
- 32.Hansen LT, Allan-Wojtas PM, Jin YL, Paulson AT. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002;19:35–45. [Google Scholar]
- 33.Liu LS, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24:3333–3343. doi: 10.1016/s0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu LS, Fishman ML, Hicks KB. Controlled release systems for agricultural and food applications. In: Parris N, Liu LS, Song C, Shastri VP, editors. New Delivery Systems for Controlled Drug Release from Naturally Occurring Materials. ACS; Washington, D.C: 2008. pp. 265–283. (ACS Symposium Series 992). [Google Scholar]
- 35.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu LS, Chaturvedi R, Peek RM, Jr, Wilson K, Polk B. Colon specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGF receptor-dependent mechanism. Journal of Clinical Investigation. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu LS, Kost J, Yan F, Spiro RC. Hydrogels from Biopolymer Hybrid for Biomedical, Food, and Functional Food Applications. Polymers. 2012;4:997–1011. [Google Scholar]
- 37.Yan F, Liu L, Dempsey PJ, Tsai YH, Raines EW, Wilson CL, Cao H, Cao Z, Liu LS, Polk DB. A Lactobacillus rhamnosus GG-derived Soluble Protein, p40, Stimulates Ligand Release from Intestinal Epithelial Cells to Transactivate Epidermal Growth Factor Receptor. J Biol Chem. 2014;288:30742–30751. doi: 10.1074/jbc.M113.492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60:413–417. doi: 10.1016/j.ejpb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Juneja VK. Thermal inactivation of Escherichia coli O157:H7 in ground beef supplemented with sodium lactate. J Food Protection. 2003;66(4):664–667. doi: 10.4315/0362-028x-66.4.664. [DOI] [PubMed] [Google Scholar]
- 40.Liu LS, Kende M, Gordon R, Fishman ML, Hicks KB. Pectin/zein beads for potential colon-specific drug delivery: synthesis and in vitro evaluation. Drug Delivery. 2006;13:417–423. doi: 10.1080/10717540500394935. [DOI] [PubMed] [Google Scholar]
- 41.Ravula RR, Shah NP. Microencapsulation of probiotic bacteria and their survival in frozen fermented dessert. The Australian Journal of Dairy Technology. 2000;55(3):139–144. [Google Scholar]
- 42.Hubálek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46:205–229. doi: 10.1016/s0011-2240(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 43.Capela P, Hay TKC, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Research International. 2006;39:203–211. [Google Scholar]
- 44.Lebeer S, Claes IJJ, Verhoeven TLA, Vanderleyden J, De Keersmaecker SCJ. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microbial Biotechnology. 2010;4:368–374. doi: 10.1111/j.1751-7915.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nazzaro F, Fratianni F, Orlando P, Coppola R. Biochemical traits, survival and biological properties of the probiotic Lactobacillus plantarum Grown in the presence of prebiotic inulin and pectin as energy source. Pharmaceuticals. 2012;5:481–492. doi: 10.3390/ph5050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corcoran BM, Stanton C, Fitzgerald GF, Ross PP. Survival of probiotic Lactobacilli in acid environments is enhanced in the presence of metabolizable sugars. Applied and Environmental Microbiology. 2005;76:3060–3067. doi: 10.1128/AEM.71.6.3060-3067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pak D, Muthaiyan A, Story R, O’Bryan CA, Lee SO, Grandall PG, Ricke S. Fermentative capacity of three strains of Lactobacillus using different sources of carbohydrates: in vitro evaluation of synbiotic effects, resistance and tolerance to bile and gastric juices. J Food Research. 2013;2:158–167. [Google Scholar]


