Introduction
Cancer is the second leading cause of death in the United States (US) [1]. The National Cancer Institute (NCI), the Centers for Disease Control, and the National Center for Health Statistics predicted a total of 1,638,910 new cancer cases and 577,190 cancer deaths in 2014 [2]. The most commonly diagnosed cancers among men are prostate, lung, and colorectal; the most fatal types of cancer in men are lung, prostate, colorectal, and liver. The most commonly diagnosed cancers among women are breast, lung, and colorectal; the most fatal types of cancer in women are lung, breast, and colorectal [3]. The most commonly known and discussed risk factor for cancer is a positive family history; 89% of Americans believe that inherited predisposition or cancer genes have a significant effect in developing cancer [4]. First, second, or third degree familial history of specific cancers increases individual risk of cancer at both the same and other locations [5]. This link has been proven in colorectal, breast, ovarian, and uterine cancer for women and prostate cancer for men [5,6,7,8,9]. However, the majority of cancer patients (more than 85% of women with breast cancer) do not have a family member with the disease suggesting that other, lifestyle-related risk factors that are modifiable are at play [10,11].
Obesity, defined as having a body mass index (BMI) ≥30 kg/m2, is a major public health problem. In 2013, 35% of adults in the US were obese with an additional 34% considered overweight (BMI between 25–29.9 kg/m2) and at risk of becoming obese [11,12]. Each year in the US, 400,000 deaths and $117 billion in health care and related costs are attributed to obesity [12,13]. Obesity has been identified as a major, modifiable risk factor for cancer and has been associated with cancer development at several organ sites for both men and women [4]. The link between food, nutrition, physical activity and cancer risk was initially explored in a comprehensive review published by the World Cancer Research Fund (WCRF) in 2005 and 2007 [14]. A meta-analysis by Renehan et al. extended the WCRF findings to confirm, among men, strong relationships exist between excess BMI (based on 5 kg/m2 increase) and esophageal adenocarcinoma, thyroid cancer, kidney cancer, and colon cancer [15]. Among women, results also confirmed strong relationships between endometrial cancer, gallbladder cancer, kidney cancer, and esophageal cancer, similar to the 2007 WCRF report [15]. Obesity has been shown to increase the risk of cancer as well as cancer mortality rates by 10% per 5 kg/m2 weight increase [15]. The association between obesity and cancer is so significant that national cancer prevention and cancer survivorship guidelines have recommended an active lifestyle and maintaining a healthy body weight (BMI between 20–25 kg/m2) for the prevention of cancer and optimal health as a cancer survivor [16, 17]. Engaging in healthy behaviors, including consuming a healthy diet, getting adequate physical activity, and participating in annual cancer screenings, can play a significant role in improving health outcomes and quality of life.
Awareness of one’s risk of developing cancer is an essential first step in taking action to change behaviors to lower the risk of developing cancer. Previous research has shown that there is a significant association between family history of cancer and cancer risk perceptions [18]. The 2015 American Institute for Cancer Research (AICR) Cancer Risk Awareness Survey Report documented that 89% of respondents cited family history (or cancer genes) as a risk factor for cancer [4]. Women with a personal and family history of cancer were more likely than individuals without any cancer history to worry about getting cancer or believe that they will get cancer in the future, and disagree that cancer is caused by behavioral or lifestyle factors [19]. This link has been studied primarily in women regarding breast cancer. Women with a family history of breast cancer report having greater perceived breast cancer risk compared to women without breast cancer family history, which may be attributable to the increased attention to, and availability of, the breast cancer susceptibility genetic test [18, 20, 21, 22]. Yet, other prominent and modifiable risk factors for cancer may not alter cancer risk perceptions. Despite obesity being identified as a significant risk factor for cancer development and recurrence, awareness of obesity as a risk factor for cancer is suboptimal and those who are obese often do not have heightened perception of risk [12, 23, 24]. In fact, the AICR report documented that only little more than half of respondents (52%) correctly identified obesity as a risk factor for cancer [4]. However, the study only assessed average risk of getting cancer as opposed to one’s personal risk based off of his or her personal characteristics and behaviors. The research evaluating weight status and personal cancer risk perceptions is extremely limited. This data is needed to develop effective, theory-driven interventions to promote a healthy lifestyle before and after a cancer diagnosis. To determine if absolute and relative risk perceptions of cancer vary in women by weight status, data from the NCI’s Health Information National Trends Survey (HINTS 4, Cycle 1) were analyzed [25, 26].
Methods
This study analyzed data from the HINTS 4 (Cycle 1), a nationally representative survey that collected data on the American public’s need for, access to, and use of health-related information and health-related behaviors, perceptions, and knowledge. HINTS 4 (Cycle 1) recruited American adults aged 18 and older, between October 2011 and February 2012, using a self-administered mailed questionnaire. Additional details about the HINTS survey, sampling framework, and study purposes have been published previously [27,28]. The HINTS 4 (Cycle 1) sample consisted of 3,959 respondents and the final response rate was 36.7%. In this analysis, respondents that did not provide data on their age (n=68), absolute cancer risk perceptions (n=120), relative cancer risk perceptions (n=130), family history of cancer (n=322), and weight status (n=229) were excluded. Subjects with a BMI less than 18.5 kg/m2 were excluded due to limited ability to make meaningful comparisons to the other groups in the models (n=69). We also excluded respondents with a positive personal history of cancer (n=563), as that would likely alter their risk perceptions. A total of 2,585 cases subjects were included in this analysis.
Variables
Main Independent Variable
Weight status was determined using the calculated BMI determined from self-reported height and weight measures. We were able to categorize subjects into three weight categories: healthy weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (BMI≥30 kg/m2).
Outcome Variables
Absolute risk perception was measured using one question: How likely are you to get cancer in your lifetime? Relative risk perception was assessed using the question: Compared to other people your age, how likely are you to get cancer in your lifetime? Response options to both questions were a five point Likert scale, which ranged from very unlikely to very likely.
Patient Characteristics
Socio-demographics
Socio-demographic items included gender (male or female), age (18–39, 40–49, 50–59, 60–69, and 70+), race/ethnicity (Non-Hispanic White, Non-Hispanic Black and/or African American, Hispanic, and Other), educational attainment (less than high school, high school/equivalent, some college, 4-year college+) and income (<$20,000, $20,000–$49,999, $50,000–$99,999, >$100,000).
Medical History
Family history of cancer was assessed using one question: Have any of your family members ever had cancer? Two cancer history groups (positive and negative family history of cancer) were utilized to determine the association between family history and cancer beliefs.
Behavioral Risk Factors
Smoking status was determined using two questions: Have you smoked at least 100 cigarettes in your entire life and how often do you smoke cigarettes? Subjects were defined as a smoker if they answered “yes” to the first question and either “every day” or “some days” to the second question. Physical activity was assessed using two questions: 1. In a typical week how many days do you do any physical activity or exercise of at least moderate intensity, such as biking, walking, etc. and 2. How much time do you spend doing the activities from the previous question (minutes and hours). Adherence to the physical activity goal was defined, according to national physical activity recommendations, as participating in at least 150 minutes per week of moderate intensity exercise [16].
Statistical Analysis
All data were weighted to yield nationally representative estimates by using final sample weights and a set of jackknife replicate weights from the HINTS database. Means and standard deviations (SDs) or frequencies were used to summarize socio-demographics, weight, physical activity goal adherence, smoking status, and absolute and relative cancer risk perceptions. Percentages summarized the level of perceived risk (absolute and relative), among those who were a healthy weight versus overweight or obese. Proportional odds logistic regression assessed the association of BMI status with perceived risk (absolute and relative) of cancer. Additional logistic regression models examined family history as a predictor of absolute and relative risk perceptions for cancer. Backward stepwise selection identified control variables (i.e., race and age) that had statistically significant effects on the outcome variables (perceived absolute and relative risk of cancer). Statistical significance was assessed at an alpha of 0.05. The analysis was carried out in SAS® Version 9.3.
Results
Our study sample consisted of 2,585 participants who satisfied the study inclusion criteria. The mean reported age was 51.55 (±0.62) years and the mean BMI was 28.15 (±0.25) kg/m2. The study demographics categorized by weight status are shown in Table 1. There were significantly more females included in this study than males. There were also significant gender differences between all three BMI categories, in particular between the healthy and overweight groups. The healthy weight respondents were significantly younger than the overweight respondents as well as the obese respondents. Non-Hispanic Black and Hispanic groups were significantly more overweight and obese compared to the Non-Hispanic White group. There were statistically significant differences in education levels across all three BMI categories, most notable between the healthy and obese groups. Compared to obese participants, healthy and overweight participants were more physically active.
Table 1.
Characteristics of Study Population: Overall and By Weight Status
| Overall | Healthy Weight | Overweight | Obese | Between healthy and overweight | Between healthy and obese | Between overweight and obese | Between healthy, overweight and obese | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable | |||||||||||
| Categories | |||||||||||
|
| |||||||||||
| n | n | % | n | % | n | % | p value | p value | p value | p value | |
|
| |||||||||||
| Gender | .0044* | .2050 | .0896 | .0168* | |||||||
| Male | 1051 | 287 | 27.31 | 454 | 43.20 | 310 | 29.50 | ||||
| Female | 1534 | 569 | 37.09 | 481 | 31.36 | 484 | 31.55 | ||||
|
| |||||||||||
| Age | .0007* | .0009* | .9664 | .0024* | |||||||
| 18–39 | 637 | 266 | 41.76 | 199 | 31.24 | 172 | 27.00 | ||||
| 40–49 | 511 | 166 | 32.49 | 185 | 36.20 | 160 | 31.31 | ||||
| 50–59 | 576 | 170 | 29.51 | 212 | 36.81 | 194 | 33.68 | ||||
| 60–69 | 499 | 124 | 24.85 | 210 | 42.08 | 165 | 33.07 | ||||
| 70+ | 336 | 121 | 36.01 | 121 | 36.01 | 94 | 27.98 | ||||
|
| |||||||||||
| Race/Ethnicity | .0078* | <.0001* | .0640 | <.0001* | |||||||
| NH White | 1591 | 565 | 35.51 | 571 | 35.89 | 455 | 28.60 | ||||
| NH Black | 382 | 71 | 18.59 | 126 | 32.98 | 185 | 48.43 | ||||
| Hispanic | 330 | 82 | 24.85 | 144 | 43.64 | 104 | 31.52 | ||||
| Other | 186 | 105 | 56.45 | 55 | 29.57 | 26 | 12.98 | ||||
|
| |||||||||||
| Household Income | .5479 | .3404 | .1755 | .1975 | |||||||
| <$20,000 | 500 | 154 | 30.80 | 163 | 32.60 | 183 | 36.60 | ||||
| $20,000–$49,999 | 732 | 213 | 29.10 | 264 | 36.07 | 255 | 34.84 | ||||
| $50,000–$99,999 | 699 | 244 | 34.91 | 256 | 36.62 | 199 | 28.47 | ||||
| >$ 100,000 | 450 | 180 | 40.00 | 167 | 37.11 | 103 | 22.89 | ||||
|
| |||||||||||
| Education | .1243 | .0001* | .0835 | .0021* | |||||||
| <HS | 231 | 63 | 27.27 | 84 | 36.36 | 84 | 36.36 | ||||
| HS | 472 | 127 | 26.91 | 162 | 34.32 | 183 | 38.77 | ||||
| Post HS/Some College | 780 | 218 | 27.95 | 309 | 39.62 | 253 | 32.44 | ||||
| College Degree+ | 1087 | 436 | 40.11 | 363 | 33.39 | 261 | 24.01 | ||||
|
| |||||||||||
| Physical Activity Goal Met | .6107 | .0001* | <.0001* | <.0001* | |||||||
| Yes | 326 | 133 | 40.80 | 136 | 41.72 | 57 | 17.48 | ||||
| No | 2269 | 723 | 31.86 | 799 | 35.21 | 737 | 32.48 | ||||
|
| |||||||||||
| Smoker | .3792 | .9819 | .3132 | .4770 | |||||||
| Yes | 433 | 156 | 36.03 | 150 | 34.64 | 127 | 29.33 | ||||
| No | 2151 | 700 | 32.54 | 785 | 36.49 | 666 | 30.96 | ||||
|
| |||||||||||
| Family History of Cancer | .9908 | .0901 | .0537 | .1121 | |||||||
| Yes | 1837 | 587 | 31.95 | 650 | 35.38 | 600 | 32.66 | ||||
| No | 748 | 269 | 35.96 | 285 | 38.10 | 194 | 25.93 | ||||
Note: NH = Non-Hispanic, HS = High School,
= significant difference at p<0.05.
Absolute and relative risk perceptions of developing cancer differed significantly between those with and without family history of cancer (Table 2). Participants with family history of cancer were significantly more likely to have increased absolute (OR=4.30) and relative (OR=3.82) risk perceptions of cancer and believe that they are more susceptible to cancer compared to individuals without a family history of cancer.
Table 2.
Absolute and Relative Risk Perceptions of Developing Cancer By Cancer History Status
| Overall | Family History of Cancer | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| None | Family | Difference between history groups | |||||
|
| |||||||
| N | % | N | % | N | % | p value | |
|
| |||||||
| How likely are you to get cancer in your lifetime | <.0001* | ||||||
| Very Unlikely | 254 | 9.31 | 129 | 18.16 | 125 | 5.70 | |
| Unlikely | 380 | 13.51 | 193 | 25.48 | 187 | 8.65 | |
| Neither | 1104 | 43.58 | 306 | 41.96 | 798 | 44.24 | |
| Likely | 693 | 28.35 | 95 | 11.91 | 598 | 35.04 | |
| Very Likely | 130 | 5.24 | 15 | 2.48 | 115 | 6.36 | |
|
| |||||||
| Compared to other people your age, how likely are you to get cancer in your lifetime | |||||||
| Very Unlikely | 250 | 9.02 | 126 | 18.44 | 124 | 5.19 | <.0001* |
| Unlikely | 504 | 19.59 | 228 | 29.81 | 276 | 15.43 | |
| Neither | 1107 | 44.21 | 291 | 40.97 | 816 | 45.52 | |
| Likely | 589 | 22.11 | 79 | 8.72 | 510 | 27.56 | |
| Very Likely | 111 | 5.07 | 14 | 2.06 | 97 | 6.29 | |
Note:
=significant difference at p<0.05.
Absolute and relative risk perceptions of developing cancer by weight status are shown in Table 3. Absolute risk perception of cancer increased as weight increased yet was not statistically different between weight groups. Relative risk perceptions of cancer also trended upwards as weight increased and did vary by weight status with obese subjects significantly more likely to state that they were likely/very likely to get cancer in their lifetime compared to other people their age than healthy weight subjects (OR=1.51).
Table 3.
Absolute and Relative Risk Perceptions of Developing Cancer by Weight Status
| Healthy | Overweight | Obese | Between Healthy, Overweight and obese | Between healthy and overweight | Between healthy and obese | Between overweight and obese | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | % | n | % | n | % | p value | p value | p value | p value | |
|
| ||||||||||
| How likely are you to get cancer in your lifetime | ||||||||||
| Very Unlikely | 87 | 9.66 | 89 | 9.37 | 78 | 8.79 | ||||
| Unlikely | 132 | 13.72 | 142 | 13.56 | 106 | 13.20 | .1089 | .1262 | .1633 | .0706 |
| Neither | 384 | 47.50 | 412 | 45.01 | 308 | 37.01 | ||||
| Likely | 202 | 23.19 | 251 | 29.18 | 240 | 33.81 | ||||
| Very Likely | 47 | 5.94 | 31 | 2.88 | 52 | 7.20 | ||||
|
| ||||||||||
| Compared to other people your age, how likely are you to get cancer in your lifetime | .0244* | .1046 | .0027* | .1516 | ||||||
| Very Unlikely | 87 | 10.14 | 88 | 8.76 | 75 | 7.94 | ||||
| Unlikely | 189 | 22.11 | 172 | 17.39 | 143 | 19.06 | ||||
| Neither | 368 | 45.80 | 426 | 47.70 | 313 | 38.07 | ||||
| Likely | 167 | 15.21 | 210 | 22.29 | 212 | 30.50 | ||||
| Very Likely | 41 | 6.74 | 29 | 3.86 | 41 | 4.43 | ||||
Note:
= significant difference at p<0.05.
Absolute and relative risk perceptions are stratified by weight status and family history of cancer in Figure 1. For both absolute and relative risk perceptions, subjects with a family history of cancer were significantly more likely to have an increased risk perception across all three BMI groups (p<.0001). Within subjects that had no family history of cancer, there were no significant differences in absolute or relative risk perception between BMI groups. Within subjects that had a reported family history of cancer, obese subjects were more likely to report an increased relative risk perception compared to healthy weight subjects (p=.0081). In a multivariate model adjusted for age, gender, race and education, this association remained significant (p=0.0310).
Figure 1.
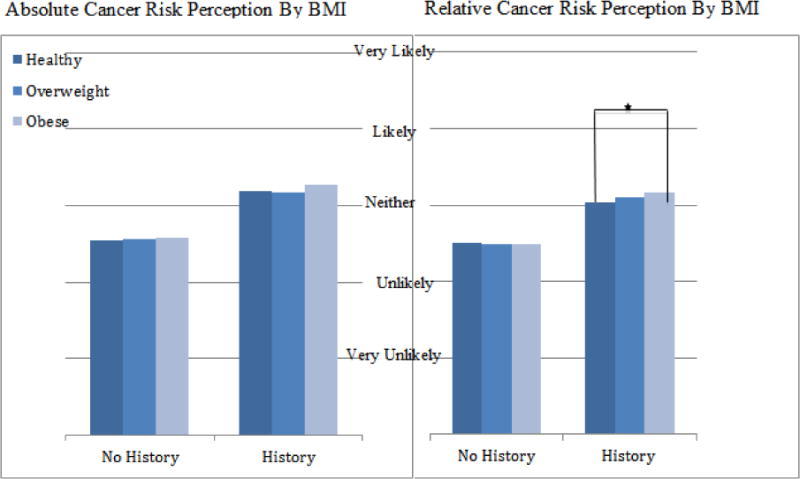
Note: * = significant difference at p=.0081.
Discussion
Family history is a well-known risk factor for cancer; those who have a family history of cancer are more likely to perceive their own personal risk as higher compared to those without a family history of cancer [5, 12, 18, 19, 20, 21]. In our study, those with a family history of cancer were 4.24 times more likely to have an increased absolute risk perception and 3.78 times more likely to have an increased relative risk perception of getting cancer compared to those without a positive family history. Kowalkowski et al. found similar results in their analysis of the HINTS 2007 data; individuals with family history of cancer were 3.55 times more likely to worry about getting cancer and 8.81 times more likely to agree that they will develop cancer in the future [18].
Our HINTS data showed a trend in increasing cancer risk perceptions with increased BMI. However, we only demonstrated a significant difference in relative risk perception between healthy and obese participants (p=.0027). Obese subjects were 13% more likely to have an increased relative risk perception and stated that they were likely or very likely to get cancer in their lifetime compared to other people their age, compared to those of a healthy weight. This difference was also seen when the data was stratified by family history of cancer (p=.0081); among subjects with a positive family history, obese subjects were 14% more likely to have an increased relative risk perception of cancer and stated that they were likely or very likely to get cancer in their lifetime compared to other people their age, compared to healthy weight subjects. Educational interventions with an explanation of an individual’s risk have been shown to reduce the gap between actual risk and perceived risk [29]. Improving accuracy of risk perception may lead to increased awareness of risk-reducing behaviors.
In our study, absolute risk perceptions of developing cancer did not differ based on weight status suggesting that those with a higher BMI did not understand the increased cancer risk associated with excess weight. This lack of increased awareness is consistent with an AICR report, which found that only 49% of female respondents and 56% of male respondents noted that obesity is a cancer risk factor [4]. When stratified by family history, there was still no significant difference in absolute risk perception between any of the weight categories. Prior research has shown that those with a cancer history are more likely to believe that cancer is caused by genetics alone and disagree with the idea that cancer is caused by a person’s behaviors [16,30]. Therefore, it is especially important to educate overweight and obese patients with and without a positive family history of cancer about their increased risk of cancer and the lifestyle modifications required to reduce risk of cancer. Informing those with an elevated risk of cancer that their risk can be lowered by altering behaviors that effect their weight, including maintaining a healthy diet and an adequate level of physical activity, may be impactful [29,31].
Race, age, and smoking status were also found to be significant predictors of both absolute and relative cancer risk perceptions. These variables are known to influence both cancer risk and cancer risk perceptions [4,20,32]. When these potential confounders were included in a multivarite model, there was still a significant difference in relative risk perception between obese and healthy weight subjects. Surprisingly, there were no statistically significant differences based on educational attainment even though the HINTS data showed significant differences in weight status by education. While previous work has shown that those who are less educated perceived themselves as at lower risk of developing cancer this association was not seen in our study [30,31].
The HINTS survey is a nationally representative survey with a relatively large sample size. Very few participants were excluded from our study. During analysis, the jackknife procedure was utilized to ensure that the analyses were appropriately weighted. While there are many potential confounders to perceived cancer risk, we were able to analyze the effect of gender, age, race, income, and education. However, our study was limited by the questions asked in the HINTS 4 (Cycle 1) survey. While the survey obtained information on family history of cancer, the type of cancer was not recorded. The subject pool consisted of significantly more subjects with a family history of cancer than national norms (71% in our study versus <15% in other studies) indicating that future studies evaluating the association between weight status and cancer risk perceptions should explore the role of family history as a covariate further among subjects with a negative family history [6,11]. Additionally, cancer risk perception data was collected on one’s risk of cancer in general so future studies should assess risk perceptions by types of cancer. The HINTS sample was largely Non-Hispanic White and highly educated (>high school). Research has shown that obesity rates are higher among Non-Hispanic Black and Hispanic populations as well as those with lower educational attainment. Further research is needed to better understand cancer risk perceptions among more diverse groups.
Conclusion
Obesity is a major, modifiable risk factor for a multitude of cancers among both men and women yet individuals are much less likely to identify obesity as a risk factor for cancer compared to family history of cancer. Public health education programs are needed to improve awareness of the impact of elevated weight on cancer risk. Health care providers should view the patient encounter as an opportunity to emphasize the role of weight management in cancer prevention, particularly among obese patients. Improved awareness may stimulate behavioral modification necessary to reduce cancer risk.
References
- 1.Leading Causes of Death. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; Feb 6, 2015. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. Atlanta: American Cancer Society; (American Cancer Society Surveillance Research). [Google Scholar]
- 3.Cancer Facts For Demographic Groups. Centers for Disease Control and Prevention; Sep 02, 2014. [Google Scholar]
- 4.The 2015 Cancer Risk Awareness Survey Report. American Institute for Cancer Research; 2015. [Google Scholar]
- 5.Slattery M, Richard K. Family history of cancer and colon cancer risk: the Utah Population Database. Journal of the National Cancer Institute. 1994;86(21):1618–1626. doi: 10.1093/jnci/86.21.1618. [DOI] [PubMed] [Google Scholar]
- 6.Gary Steinberg D, et al. Family history and the risk of prostate cancer. The Prostate. 1990;17(4):337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 7.Slattery M, Richard K. A comprehensive evaluation of family history and breast cancer risk: the Utah Population Database. JAMA. 1993;270(13):1563–1568. [PubMed] [Google Scholar]
- 8.La Vecchia C, et al. Family history and the risk of stomach and colorectal cancer. Cancer. 1992;70(1):50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Cramer D, et al. Determinants of ovarian cancer risk. I. Reproductive experiences and family history. Journal of the National Cancer Institute. 1983;71(4):703–709. [PubMed] [Google Scholar]
- 10.What Are the Risk Factors for Breast Cancer?The. The American Cancer Society; Mar 23, 2015. What Are the Risk Factors for Breast Cancer? [Google Scholar]
- 11.Scott Ramsey D, et al. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genetics in Medicine. 2006;8(9):571–575. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FastStats, Obesity and Overweight. Centers for Disease Control and Prevention; Jan 14, 2015. [Google Scholar]
- 13.Bassett M, Perl S. Obesity: The Public Health Challenge of Our Time. American Journal of Public Health. 2004;94(9):1477. doi: 10.2105/ajph.94.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 15.Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 16.Kushi LH, Doyle C, McCullough M, Rock CL, Denmark-Wahnfried W, Bandera EV, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Survivorship. http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf.
- 18.Kowalkowski MA, Hart SL, Du XL, Baraniuk S, Latini DM. Cancer perceptions: implications from the 2007 Health Information National Trends Survey. Journal of Cancer Survivorship. 2012 Mar 29; doi: 10.1007/s11764-012-0217-y. [DOI] [PubMed] [Google Scholar]
- 19.Cappelli M, et al. Psychological and social determinants of women’s decisions to undergo genetic counseling and testing for breast cancer. Clinical genetics. 1999;55(6):419–430. doi: 10.1034/j.1399-0004.1999.550605.x. [DOI] [PubMed] [Google Scholar]
- 20.Lipkus I, et al. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiology Biomarkers & Prevention. 1999;8(6):533–539. [PubMed] [Google Scholar]
- 21.Lloyd S, et al. Familial breast cancer: a controlled study of risk perception, psychological morbidity and health beliefs in women attending for genetic counselling. British journal of cancer. 1996;74(3):482. doi: 10.1038/bjc.1996.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 23.Redeker C, et al. The launch of Cancer Research UK’s ‘Reduce the Risk’campaign: baseline measurements of public awareness of cancer risk factors in 2004. European Journal of cancer. 2009;45(5):827–836. doi: 10.1016/j.ejca.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Consedine N, et al. Obesity and awareness of obesity as risk factors for breast cancer in six ethnic groups. Obesity research. 2004;12(10):1680–1689. doi: 10.1038/oby.2004.208. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. (Cycle 1 Methodology Report).Health Information National Trends Survey 4 (HINTS 4) [Google Scholar]
- 26.Nelson DE, Kreps GL, Hesse BW, et al. The Health Information National Trends Survey (HINTS): development, design, and dissemination. Journal of Health Communication. 2004;9:443–60. doi: 10.1080/10810730490504233. discussion 81–4. [DOI] [PubMed] [Google Scholar]
- 27.Hesse B, et al. The health information national trends survey: research from the baseline. Journal of Health Communication. 2006;11(S1):vii–xvi. doi: 10.1080/10810730600692553. [DOI] [PubMed] [Google Scholar]
- 28.Nelson D, et al. The health information national trends survey (HINTS): Development, design, and dissemination. Journal of health communication. 2004;9(5):443–460. doi: 10.1080/10810730490504233. [DOI] [PubMed] [Google Scholar]
- 29.Alexander N, et al. The effect of an educational intervention on the perceived risk of breast cancer. Journal of general internal medicine. 1996;11(2):92–97. doi: 10.1007/BF02599584. [DOI] [PubMed] [Google Scholar]
- 30.Pavelka J, et al. Morbid obesity and endometrial cancer: surgical, clinical, and pathologic outcomes in surgically managed patients. Gynecologic oncology. 2004;95(3):588–592. doi: 10.1016/j.ygyno.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 31.Von Gruenigen V, et al. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma. Cancer. 2006;107(12):2786–2791. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 32.Woloshin S, et al. Women’s Perceptions of Breast Cancer Risk How You Ask Matters. Medical Decision Making. 1999;19(3):221–229. doi: 10.1177/0272989X9901900301. [DOI] [PubMed] [Google Scholar]


