Abstract
Aims
To determine 1) the prospective associations of conduct problems during early adolescence with tobacco, alcohol and cannabis use in young adulthood and 2) to what extent these associations are due to overlapping genetic versus environmental influences.
Design
A prospective twin study using biometric twin modelling.
Setting
Finland.
Participants
1847 Finnish twins (943 males and 904 females) were interviewed in early adolescence, of which 73% (N=1353, 640 males and 713 females) were retained in young adulthood.
Measurements
Symptom counts of conduct disorder (CD) criteria were obtained from a semi-structured clinical interview in early adolescence (age 14–15 years, M=14.2, SD=0.15). Frequency of alcohol, tobacco, and cannabis use was obtained from a semi-structured clinical interview in young adulthood (age 19.9–26.6 years, M=22.4, SD=0.7).
Findings
We found modest to moderate phenotypic correlations (r=0.16 to 0.35) between early adolescent CD symptoms and substance use in young adulthood. In males, the phenotypic correlations of CD symptoms with all three substance use variables are largely explained by overlapping genetic influences. In females, overlapping shared environmental influences predominantly explain the phenotypic correlation between CD symptoms and tobacco and cannabis use.
Conclusions
Conduct disorder symptoms in early adolescence appear to moderately predict substance use in early adulthood. In males, genetic influences seem to be most important in explaining the relationship between conduct disorder symptoms and substance use whereas in females, shared environmental influences seem to be most important.
Introduction
Adolescence is a period in which many youth initiate the use of psychoactive substances. To illustrate, between ages 15 and 21, a sharp increase is seen in the incidence of alcohol, tobacco, and cannabis use, with estimates of, respectively, 90, 70, and 25–50 percent in American and European 21-year-olds [1]. Increased levels of substance use are associated with problems later in life, including poor educational performance, health problems and substance use disorders [2–5].
Early conduct problems, including aggression towards people or animals, destruction of property, deceitfulness or theft and serious violations of rules [6], have been associated with various measures of substance use later in life. For instance, White et al. [7] found that higher levels of conduct problems among boys in early adolescence predict higher levels of alcohol and marijuana use from age 13 to 18. In addition, Elkins et al. [8] reported that endorsing conduct symptoms by age 11 predicts initiation of tobacco, alcohol, and illicit drug use, and that a diagnosis of Conduct Disorder (CD) between age 11 and 14 predicts all three types of substance use disorders by age 18. The established association between conduct problems and substance use may be explained by a common aetiology.
Twin studies represent an adequate method to test for common aetiology by determining the extent to which genetic and environmental factors are shared by two or more traits. For both conduct problems and substance use, the contribution of genetic and environmental factors has been demonstrated. With regard to conduct problems, moderate heritability has been shown, with reported estimates ranging from .31 to .61 [9–11]. Heritability estimates of substance use have been found to vary across stages of substance use involvement, with strongest genetic influences for heavier stages of substance use [12–13]. For persistent smoking and lifetime cannabis use, meta-analyses point towards moderate heritability, with estimates of .46 in females and .59 in males for persistent smoking [14] and of .40 in females and .48 in males for cannabis use [13]. For level of alcohol consumption, genetic factors also appear to have a moderate influence, with estimates around .50 [15–16].
Previous twin research to understand the phenotypic association between CD and substance use involvement has mainly focused on substance use disorders [e.g. 17–19]. However, studies testing the extent to which the association between conduct problems and level of substance use is due to overlapping genetic or environmental influences are relatively scarce. In a cross-sectional study, Miles et al. found that the moderate phenotypic correlation (r=.38 in males and .31 in females) between CD and marijuana use in adolescents was due to overlapping genetic and unique environmental influences on both phenotypes, while shared environmental influences only explained marijuana use [10]. The use of longitudinal data provides the opportunity to also study early manifestations of genetic and environmental influences potentially underlying future substance use. This way, genetically informative designs can improve our understanding of how the relationship between conduct problems and substance use unfolds over time, further elucidating the mechanisms underlying the development of substance use behaviour. For instance, Korhonen et al. [20] found that part of the additive genetic and shared environmental factors underlying externalizing behaviour in early adolescence is also involved in smoking behaviour at age 14 and illicit drug use by age 17. Likewise, Silberg et al. [21] demonstrated that early conduct disturbance is related to mid-adolescent substance use through common genetic and shared environmental risk factors.
Here, we used data on conduct problems and substance use from a large sample of Finnish twins, collected with structured interviews at two time points covering the crucial period from early adolescence (age 14) to young adulthood (age 19–26). Detailed information was available on CD symptoms and levels of use of the three most commonly used psychoactive substances: tobacco, alcohol, and cannabis. The aims of this study were to determine 1) the prospective associations of conduct problems during early adolescence with tobacco, alcohol, and cannabis use in young adulthood, and 2) the extent to which these associations were due to overlapping genetic and environmental influences. This information guides further research into the specific genetic and environmental origins of the shared liability for conduct problems and substance use, which may contribute to the development of effective prevention or intervention programs targeting substance use in adolescence and young adulthood.
Methods
Participants
The study sample consisted of identical (monozygotic; MZ) and non-identical (dizygotic; DZ) Finnish twins, participating in the FinnTwin12 (FT12) study. FT12 is a population-based twin study that consists of five consecutive birth cohorts (1983–1987) [22]. The Central Population Registry was used to identify families with twins. A total of 2724 families (87% of all identified eligible families) returned the family questionnaire, and from these families, 2567 twin pairs completed the baseline questionnaires [23].
For the current study, data were used from an intensive subsample of FT12, consisting of 1) a sample randomly drawn from the 1983 cohort, with the exclusion of those living in the most remote rural areas of Finland (13%), 2) a random sample from the 1984–1987 cohorts (59%), and 3) all remaining twins from the 1984–1987 cohorts at elevated familial risk for alcohol problems based on parental reports of alcohol use (28%) [17]. Previously it was shown that the modest sample enrichment for familial alcohol use did not systematically affect the genetic and environmental variance components for various behavioural variables, including measures of drinking frequency, smoking initiation, and behaviour problems [17]. The intensive subsample included 1035 twin pairs who were invited to complete a full psychiatric face-to-face interview in early adolescence (age 14) and young adulthood (age 19–26). At age 14, 90% of the twins completed the assessment [17,22] and at age 19–26, 73% of the twins studied at age 14 were retained. Attrition was not selective on early adolescent conduct problems or substance use in early or mid-adolescence (all p-values >0.05).
Measures
Conduct problems
At age 14, face-to-face interviews were conducted using a translated version of the Child Semi-Structured Assessment for the Genetics of Alcoholism, Adolescent version (C-SSAGA-A). This polydiagnostic instrument marks lifetime diagnoses on a variety of psychiatric disorders, including CD, based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IIIR [24]. For this study, symptom counts of thirteen CD criteria were categorized into no conduct problems, some conduct problems (endorsement of one or two symptoms), and three or more conduct symptoms.
Tobacco, alcohol, and cannabis use
In young adulthood, the translated, adult version of the SSAGA, a comprehensive, semi-structured interview to assess substance dependence and related psychiatric disorders was administered [25]. The first questions of the substance use sections were developed to assess lifetime use and frequency of use of tobacco, alcohol and cannabis. For this study, frequency of tobacco use was defined as the number of events per day, including cigarettes, cigars, pipes and snus (Swedish type of smokeless tobacco). Four categories of daily tobacco use were distinguished, ranging from no daily tobacco use to 11 or more cigarettes, cigars, pipes or snus portions a day. Alcohol use was defined as the typical number of drinks a week, including beer, wine, spirits, and other alcoholic drinks. Five categories of alcohol use were distinguished, ranging from no/low levels of alcohol use (<3 alcoholic drinks a week) to high levels of use (15 or more drinks a week). Cannabis use was categorized as no lifetime use of cannabis, 1–2 times used during lifetime, and lifetime cannabis use of 3 times or more. For each substance, the number of categories was based on the distribution of the frequency of use. Table 1 presents the categories for each substance.
Table 1.
Descriptive statistics on the levels of conduct problems in early adolescence and substance use in young adulthood.
| Phenotype | Response Categories | Prevalence | ||
|---|---|---|---|---|
| Males | Females | |||
| Conduct | 0 | No symptoms | 451 (48.2%) | 574 (63.7%) |
| 1 | 1–2 symptoms | 346 (37.0%) | 251 (27.9%) | |
| 2 | 3 or more symptoms | 138 (14.8%) | 76 (8.4%) | |
| Tobacco | 0 | No tobacco use | 256 (40.6%) | 380 (53.8%) |
| 1 | 1–5 cigarettes per day | 62 (9.8%) | 116 (16.4%) | |
| 2 | 6–10 cigarettes per day | 155 (24.6%) | 135 (19.1%) | |
| 3 | 11 or more cigarettes per day | 158 (25.0%) | 75 (10.6%) | |
| Alcohol | 0 | 0–2.5 drinks per week | 89 (14.0%) | 188 (26.6%) |
| 1 | 3–4.5 drinks per week | 72 (11.4%) | 131 (18.5%) | |
| 2 | 5–8.5 drinks per week | 93 (14.7%) | 232 (32.8%) | |
| 3 | 9–14.5 drinks per week | 189 (29.8%) | 123 (17.4%) | |
| 4 | 15 or more drinks per week | 191 (30.1%) | 33 (4.7%) | |
| Cannabis | 0 | Never used | 435 (69.0%) | 535 (76.1%) |
| 1 | Used 1–2 times in lifetime | 87 (13.8%) | 79 (11.2%) | |
| 2 | Used 3 times or more in lifetime | 108 (17.1%) | 89 (12.7%) |
Statistics
Genetic model fitting
We used the classical twin design to separate the variance in conduct symptoms during early adolescence and use of tobacco, alcohol, and cannabis in young adulthood into that due to additive genetic (A), shared (family) environmental (C), and residual (E) influences (see[26–27]). A represents the variance resulting from the sum of allelic effects across all segregating genes. C refers to environmental influences that have the effect of making twins more similar to one another, such as shared home environment, parenting style, and residential area. The E term includes environmental influences that have the effect of making twins different from one another, stochastic biological effects, as well as measurement error. These variance components can be estimated because MZ twins share all their genetic variation, while DZ twins share on average 50% of their segregating genetic variation. If the variance in a phenotype were completely due to genetic variance, we would expect a twin pair correlation of 1.0 for MZ twins and 0.5 for DZ twins. Both MZ and DZ twin pairs share the C influences, so if C were the only source of variance, we would expect a twin pair correlation of 1.0 for both MZ and DZ pairs. E factors are unique for each individual, so if E were the only source of variance, we would expect a twin pair correlation of zero for both MZ and DZ pairs. In reality, phenotypic variance is generally due to a combination of A, C, and E influences and structural equation modelling is used to determine the combination of influences that best matches the observed data.
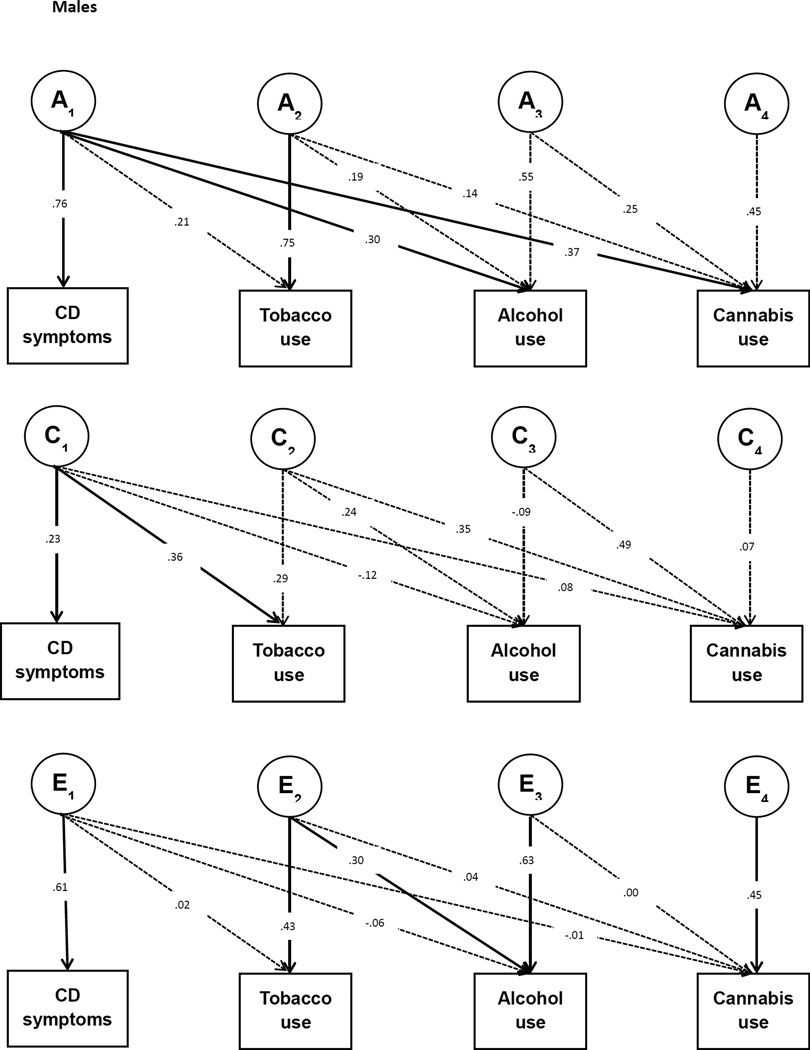
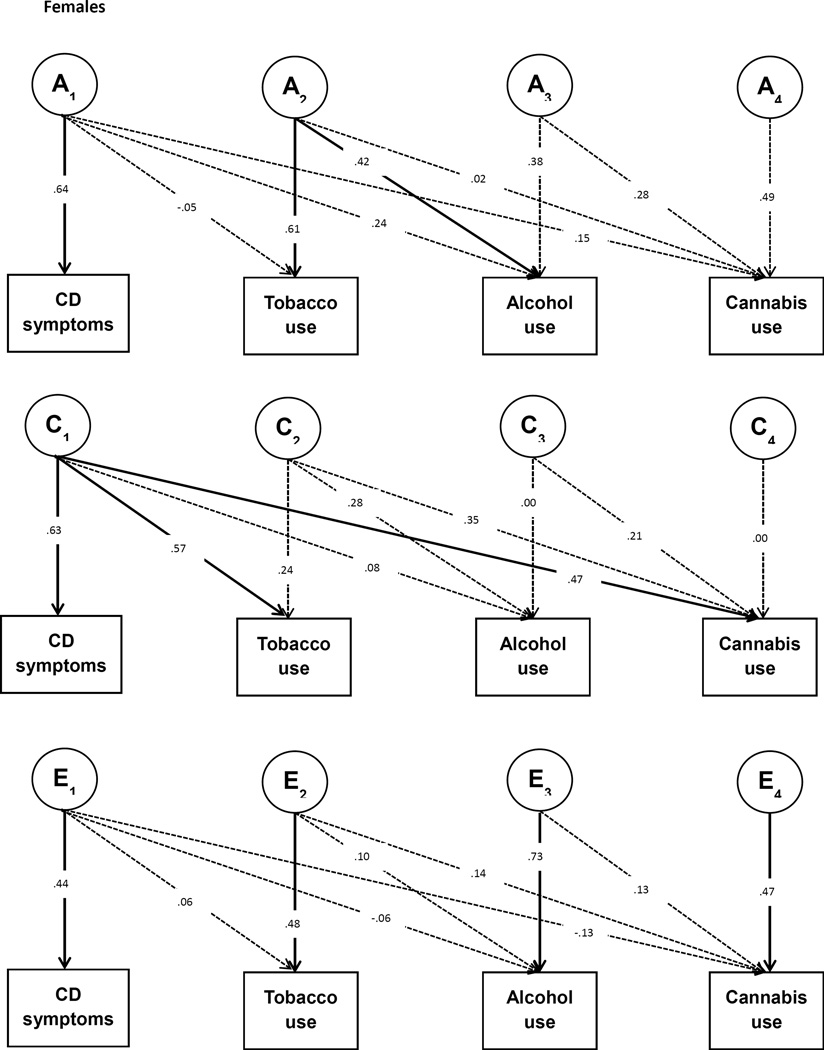
In the same way, the cross-twin cross-trait correlations (CTCT; e.g. the correlation between conduct problems of twin 1 and cannabis use of twin 2) are used to partition the covariance between conduct symptoms and use of tobacco, alcohol, and cannabis into A, C, and E influences. When the CTCT correlation is higher for MZ twins than for DZ twins this indicates that part of the phenotypic correlation between the two traits is due to overlapping genetic influences. A parallel logic follows for the interpretation of C and E paths that contribute to the covariance between the traits. To model this, we fitted a multivariate Cholesky decomposition with the four phenotypes, in which we estimated A, C, and E contributions to each of the variables as well as the A, C, and E contributions to the phenotypic covariation between the phenotypes (See Figure 1). In this way we obtained a measure of overlap in the genetic and environmental variation underlying conduct symptoms during adolescence and the use of substances in young adulthood.
Figure 1.
Multivariate Cholesky Decomposition. The boxes represent the observed variables and the circles represent the latent variables that influence the observed variables. A, C, and E denote latent genetic, shared environmental and residual influences, respectively. A1 represents the genetic influences on CD symptoms, and the crosspaths from A1 to tobacco, alcohol, and cannabis use indicate the extent to which these genetic influences are shared with each substance use trait. A2, A3, and A4 represent genetic influences on tobacco, alcohol and cannabis use that are not shared with CD symptoms. Crosspaths from A2 to alcohol and cannabis use, and from A3 to cannabis use capture the covariation between these substance use traits. The same structure applies for the C and E factors. Dashed lines indicate non-significant pathways (p>0.05). Note that the pathways from E1 to CD symptoms, E2 to tobacco use, E3 to alcohol use, and E4 to cannabis use were not tested for significance; these pathways cannot be dropped from the model as measurement error is always present.
Structural equation modelling of twin data is performed in the flexible matrix algebra program Mx [28], which employs maximum likelihood modelling procedures. We fitted models to the raw ordinal data, where it is assumed that a normally distributed continuum of liability underlies the observed categories of the variables. Age and sex effects on the phenotypes were accounted for. The goodness-of-fit of a model is summarised by a statistic distributed as a chi-square (χ2). By testing the change in model fit (Δχ2) against the change in degrees of freedom (Δdf), we tested whether constraining parameters to zero or constraining them to be equal across groups, significantly worsened the model fit. This way we tested the significance of each pathway in the model.
Results
Descriptive Statistics
The final study sample included 1847 twin individuals (51% male) with data on at least one variable. Participants were aged between 14.0 and 15.0 years at time of the first survey (M=14.2, SD=0.15) and between 19.9 and 26.6 at the second survey (M=22.4, SD=0.7). The overall sample included 910 complete twin pairs (181 MZ males, 184 MZ females, 152 DZ males, 128 DZ females, and 265 DZ opposite-sex pairs) and 27 single twins whose co-twin did not participate. Single twins were included because they contribute to the estimation of the thresholds. Note that sample sizes differ per variable. Table 1 shows the descriptive statistics for conduct symptoms and each of the substance use variables.
Preliminary analyses
Before estimating the variance components, the effects of sex, age and zygosity on the thresholds of each variable were tested using an α-level of 0.01. Levels of conduct symptoms and substance use did not significantly differ between MZ and DZ twins in either sex. We found a significant sex effect on the thresholds for conduct problems, alcohol and tobacco use (all p<0.001, respectively), indicating that males report more conduct problems and use more alcohol and tobacco than females. No significant sex effects were found for cannabis use, suggesting no differences in levels of cannabis use between sexes. No significant age effects were found for any of the variables. In subsequent genetic analyses thresholds were estimated separately for males and females and age was included as a covariate.
Table 2 shows the polychoric twin pair correlations for each zygosity group. For each variable, the MZ twin pair correlations were higher than the DZ twin pair correlations, suggesting the influence of genetic factors.
Table 2.
Polychoric twin pair correlations (and 95% confidence intervals) for conduct symptoms, and alcohol, cannabis, and tobacco use, estimated in Mx (corrected for age and sex effects).
| Zygosity | N pairs | Conduct symptoms |
Tobacco use | Alcohol use | Cannabis use |
|---|---|---|---|---|---|
| MZ M | 113–178 | 0.60 (0.46; 0.71) | 0.81 (0.71; 0.88) | 0.53 (0.36; 0.66) | 0.79 (0.63; 0.89) |
| DZ M | 85–151 | 0.39 (0.21; 0.55) | 0.45 (0.21; 0.64) | 0.26 (0.02; 0.46) | 0.63 (0.36; 0.80) |
| MZ F | 145–183 | 0.83 (0.73; 0.89) | 0.77 (0.66; 0.84) | 0.48 (0.32;0.60) | 0.73 (0.52; 0.86) |
| DZ F | 98–128 | 0.51 (0.30; 0.68) | 0.55 (0.33; 0.70) | 0.21 (−0.01; 0.41) | 0.52 (0.23; 0.73) |
| DZ OS | 168–262 | 0.33 (0.16; 0.48) | 0.55 (0.39; 0.68) | 0.29 (0.12; 0.43) | 0.47 (0.27; 0.63) |
F=female, M=male, MZ =monozygotic, DZ=dizygotic, OS=opposite-sex
Genetic modelling
We first fitted general sex-limitation models for each of the variables, allowing for qualitative and quantitative differences in the sources of variation between the sexes. Subsequently, we fitted common effects sex-limitation models, which only allow for quantitative sex differences in the variance components. Comparing the model fit between these models indicated no significant deterioration in fit for any of the traits (all p>0.05), implying no differences in the source of genetic variation between males and females. We then tested for quantitative sex differences by constraining the variance estimates to be equal across sexes. Male and female path estimates could not be equated for conduct disorder (Δχ2(2)=10.04, p=0.007). Therefore, in subsequent modelling we estimated the variance components separately for males and females.
Table 3 shows the A, C, and E estimates as obtained from the univariate common effects sex-limitation models. Genetic influences on conduct symptoms are 38% for males and 81% for females, whereas genetic influences on the substance use phenotypes range from 31% to 61%. Shared environmental influences play a role in some traits; C explains approximately 20% of the individual difference in conduct symptoms in males, and between 0% (for alcohol use, both sexes) and 47% (for cannabis use in males) in the different substance use variables.
Table 3.
Estimates of the standardised genetic (A), shared environmental (C), and residual variance (E) components for conduct symptoms, and tobacco, alcohol, and cannabis use as obtained from univariate twin models (95% confidence intervals between brackets).
| Phenotype | Males | Females | ||||
|---|---|---|---|---|---|---|
| A | C | E | A | C | E | |
| Conduct symptoms | 0.38 (0.00; 0.72) | 0.21 (0.00; 0.56) | 0.41 (0.28; 0.55) | 0.81 (0.25; 0.89) | 0.02 (0.00; 0.54) | 0.17 (0.11; 0.26) |
| Tobacco use | 0.61 (0.29; 0.83) | 0.20 (0.01; 0.49) | 0.19 (0.12; 0.29) | 0.43 (0.09; 0.76) | 0.34 (0.04; 0.64) | 0.23 (0.16; 0.33) |
| Alcohol use | 0.51 (0.05; 0.66) | 0.02 (0.00; 0.41) | 0.47 (0.34; 0.63) | 0.47 (0.05; 0.60) | 0.01 (0.00; 0.36) | 0.52 (0.40; 0.67) |
| Cannabis use | 0.31 (0.00; 0.83) | 0.47 (0.00; 0.81) | 0.21 (0.11; 0.38) | 0.57 (0.00; 0.83) | 0.17 (0.00; 0.73) | 0.26 (0.14; 0.49) |
Table 4 shows the phenotypic correlations between conduct symptoms and the three substance use variables as well as the cross-twin-cross-trait (CTCT) correlations (e.g. correlation of conduct symptoms twin 1 with alcohol use twin 2). We found significant modest to moderate phenotypic correlations between conduct symptoms in early adolescence and all three measures of substance use in young adulthood, ranging between 0.16 and 0.35. The CTCT correlations provide a first impression of the extent to which the phenotypic correlations between conduct symptoms and the substance use variables are due to A, C, and E (this is formally modelled below). For males, the CTCT correlations appear higher for MZ twins than for DZ twins for conduct-alcohol and conduct-cannabis, suggesting that overlapping genetic influences may explain the phenotypic correlation. The CTCT correlation for conduct-tobacco use for males and all female CTCT correlations appear comparable between MZ and DZ twins, suggesting that the phenotypic correlations between these variables may be more influenced by shared environmental factors.
Table 4.
Phenotypic and cross-twin-cross-trait (CTCT) correlations of conduct symptoms with tobacco, alcohol, and cannabis use (95% confidence intervals between brackets).
| Tobacco use | Alcohol use | Cannabis use | |
|---|---|---|---|
| Phenotypic correlation males | 0.25 (0.15; 0.35) | 0.18 (0.08; 0.27) | 0.29 (0.18; 0.40) |
| Phenotypic correlation females | 0.35 (0.25; 0.45) | 0.16 (0.06; 0.25) | 0.33 (0.21; 0.44) |
| CTCT Monozygotic males | 0.22 (0.10; 0.34) | 0.21 (0.08; 0.34) | 0.30 (0.16; 0.42) |
| CTCT Dizygotic males | 0.22 (0.07; 0.37) | 0.09 (−0.07; 0.25) | 0.18 (0.00; 0.34) |
| CTCT Monozygotic females | 0.32 (0.20; 0.43) | 0.16 (0.04; 0.27) | 0.37 (0.24; 0.50) |
| CTCT Dizygotic females | 0.39 (0.24; 0.52) | 0.15 (−0.01; 0.30) | 0.39 (0.20; 0.55) |
| CTCT Dizygotic opposite-sex | 0.20 (0.07; 0.32) | 0.09 (−0.04; 0.21) | 0.16 (0.02; 0.30) |
Path coefficients of the multivariate Cholesky decomposition can be found in Figure 1 and results are summarised in Table 5. To test the significance of each path in the Cholesky decomposition, genetic and environmental parameters were dropped from the model and model fit was compared with the full model using an α-level of 0.05. Because the parameter estimates here are based on multivariate relationships, the estimates may differ from the estimates obtained in the univariate models and the expectations from the bivariate phenotypic correlations presented in Table 4.
Table 5.
Results from the multivariate Cholesky decomposition of conduct symptoms and tobacco, alcohol, and cannabis use. Shown are the standardised genetic (A), shared environmental (C), and residual variance (E) components for each trait as well as the phenotypic correlations (Phen r) and the genetic, shared environmental, and residual contribution to the phenotypic correlation between the traits.
| Variance components | Phenotypic correlation with conduct disorder | |||||||
|---|---|---|---|---|---|---|---|---|
| A | C | E | Total Phen r | A component of Phen r |
C component of Phen r |
E component of Phen r |
||
| Males | Conduct | 0.57 | 0.05 | 0.38 | -- | -- | -- | -- |
| Tobacco | 0.60 | 0.21 | 0.19 | 0.26 | 0.16 | 0.08 | 0.01 | |
| Alcohol | 0.43 | 0.08 | 0.49 | 0.17 | 0.23 | −0.03 | −0.03 | |
| Cannabis | 0.43 | 0.37 | 0.20 | 0.30 | 0.28 | 0.02 | 0.00 | |
| Females | Conduct | 0.41 | 0.39 | 0.19 | -- | -- | -- | -- |
| Tobacco | 0.37 | 0.39 | 0.24 | 0.35 | −0.03 | 0.36 | 0.03 | |
| Alcohol | 0.37 | 0.08 | 0.54 | 0.18 | 0.15 | 0.05 | −0.03 | |
| Cannabis | 0.33 | 0.39 | 0.27 | 0.33 | 0.09 | 0.30 | −0.06 | |
Based on the multivariate Cholesky decomposition, we obtained the contribution of A, C, and E influences to the phenotypic correlation between the traits (see Table 5). Following the tracing rules of pathway analysis, the parameters in Figure 1 can be converted into the A, C, and E influences on each trait and the genetic and environmental covariance between traits (also see ref. 28). For example, the genetic covariance between conduct symptoms and tobacco use can be traced through the path from conduct to A1 times the path from A1 to tobacco (i.e. for males: 0.76*0.21=0.16). The genetic correlation can then also be calculated by dividing the genetic covariance between two traits by the square root of the product of the genetic variances of the two traits.
For males the phenotypic correlation of conduct symptoms with alcohol and cannabis use can be mostly explained by overlapping A influences, whereas the phenotypic correlation between conduct symptoms and tobacco use is predominantly due to overlapping C influences. For females, the phenotypic correlations between conduct symptoms and tobacco and cannabis use are predominantly due to overlapping C influences. Although not significant, the phenotypic correlation between conduct symptoms and alcohol use in females seems mostly explained by A influences.
Discussion
Using a prospective, longitudinal twin design, we examined the co-occurrence of early adolescent conduct problems derived from structured face-to-face interviews and frequency of tobacco, alcohol, and cannabis use in young adulthood, also obtained from structured interviews. Furthermore, we determined the genetic and environmental influences on the four phenotypes, and assessed the extent to which genetic and environmental factors contribute to the covariance between the phenotypes.
Results of the multivariate analyses showed that, in males and females, both additive genetic effects and shared environmental effects contribute to the variance in adolescent conduct problems and tobacco, alcohol and cannabis use in early adulthood. Because the multivariate models include more information, the estimates obtained from the multivariate analyses should be regarded as superior to the univariate estimates. The heritability estimates for conduct problems based on the multivariate models 57% in males, 41% in females) are roughly similar to previously reported estimates in partly overlapping samples [17, 20, 30] and in accordance with estimates from previous research [9–11]. Heritability estimates for tobacco (60% in males, 37% in females), alcohol (43% in males, 37% in females), and cannabis use (43% in males, 33% in females) are also broadly comparable to estimates from previous studies [13–16].
Modest to moderate correlations (ranging from 0.16 to 0.35) were found between early conduct problems and tobacco, alcohol and cannabis use in young adulthood. These phenotypic correlations are broadly comparable to earlier established correlations between externalizing/conduct problems in early adolescence and later tobacco, alcohol and other substance use [20–21]. Moderate correlations between conduct problems and cannabis use have also been reported cross-sectionally [10]. This combination of findings may suggest that the strength of the association between early adolescent behavioural problems and subsequent substance use is not attenuated much over time.
Consistent with Korhonen et al. [20] who studied the co-occurrence of early externalizing behaviour, based on teacher ratings, and mid-adolescent tobacco and drug use in a partly overlapping sample, we found that common genetic and shared environmental influences explained the co-occurrence between adolescent conduct problems and tobacco, alcohol, and cannabis use in young adulthood. More specifically, we found that shared environmental factors contribute to the association between adolescent conduct problems and subsequent tobacco and cannabis use in females, and with tobacco use in males. Silberg et al. [21] also indicated the importance of common shared environmental influences for conduct disturbance and substance use in both sexes. They further illustrated that family dysfunction and affiliation with deviant peers are implicated in these shared environmental effects. According to Silberg et al., common genetic influences are also involved in the co-occurrence of conduct problems and substance use in both sexes [21]. This is in contrast to our findings where only in males the co-occurrence of conduct symptoms and substance use can be mostly explained by overlapping genetic influences. A highly heritable latent phenotype, generally referred to as “behavioural disinhibition” [31], may be implicated in these shared genetic effects. Based on the findings of the present study, this trait seems of particular importance for understanding conduct problems and substance use in males. Future research is needed to replicate the gender differences in the causes of covariation between early conduct problems and later substance use.
Our findings confirm that the presence of conduct problems in early adolescence is an indicator of liability to substance use later in life. This finding adds to the literature on precursors of substance use phenotypes, including externalizing behaviour [20], early age of alcohol use initiation [32], and early cannabis use [33]. Knowledge on the precursors of substance use can guide the selection of high-risk youths for selective prevention programs.
Our findings may also inform future genome-wide association (GWA) analyses that will help further elucidation of the complex genetic architecture of conduct behaviour and substance use. The overlapping genetic influences on CD symptoms and substance use (especially in males) indicate that genetic variants identified to play a role in conduct behaviour may also contribute to the genetic architecture of vulnerability to externalizing behaviour in general and the other way around. Multivariate GWA studies could distinguish between genetic variants that are trait-specific versus those that contribute to externalizing behaviour in general and this may deepen our understanding of the genetic aetiology of these phenotypes.
Strengths of the present prospective study are the relatively large sample and the use of structured interviews conducted by well-trained staff to assess conduct problems and substance use. A feature that may limit the generalizability of the findings to the general population is the modest oversampling for familial alcoholism risk. However, given that the oversampling had no systematic effect on the estimates of the variance components for several risk-relevant outcomes in early adolescence [17], we do not expect it has strongly affected our results. In addition, although examining levels of use of the various substances as opposed to lifetime incidence is more informative in young adulthood, this approach assumes that the same factors influence no versus any use and any versus more frequent use. Multi-stage modelling showed that there is a substantial overlap in genetic and environmental factors influencing earlier and later stages of substance use, but also that there are factors that contribute specifically to later stages of use [34–35]. Finally, although our dataset is relatively large, the power to discriminate between the different variance components was limited due to the use of a threshold model (as necessitated by the highly skewed data) [36].
In conclusion, this study demonstrated that modest to moderate correlations between early adolescent conduct problems and frequency of tobacco, alcohol, and cannabis use in early adulthood can be explained by common genetic and shared environmental effects. Our findings point in the direction of sex differences in the causes of covariation between early conduct problems and later substance use frequency. Future research in genetically informative samples is needed to replicate these sex differences. Moreover, since our findings imply that there may be common biological and environmental processes underlying early adolescent conduct problems and later substance use, research directed at the identification of the specific genetic and environmental factors that underlie conduct problems and substance use will improve our understanding of the underlying mechanisms. This may eventually lead to better prevention and treatment interventions.
Acknowledgments
We gratefully acknowledge prof. Conor Dolan for his statistical support.
KJHV and ACH are supported by the Netherlands Organization for Health Research and Development, ZonMw 31160212. KJHV is supported in part by a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. Data collection and analysis in FinnTwin12 has been supported by National Institute of Alcohol Abuse and Alcoholism (grants AA-12502, AA-00145, and AA-09203 to RJ Rose and AA15416 and K02AA018755 to DM Dick), the Academy of Finland (grants 100499, 205585, 118555, 141054 and 264146 to JK). JK is supported by Academy of Finland grants 265240, 263278.
Footnotes
The authors have no conflicts of interest.
References
- 1.Degenhardt L, Chiu W-T, Sampson N, Kessler RC, Anthony JC, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook JS, Brook DW, Zhang C, Cohen P. Tobacco Use and Health in Young Adulthood. The Journal of Genetic Psychology: Research and Theory on Human Development. 2004;165:310–323. doi: 10.3200/GNTP.165.3.310-323. [DOI] [PubMed] [Google Scholar]
- 3.King KM, Meehan BT, Trim RS, Chassin L. Marker or mediator? The effects of adolescent substance use on young adult educational attainment. Addiction. 2006;101:1730–1740. doi: 10.1111/j.1360-0443.2006.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCambridge J, McAlaney J, Rowe R. Adult Consequences of Late Adolescent Alcohol Consumption: A Systematic Review of Cohort Studies. PLoS Med. 2011;8:e1000413. doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose RJ, Winter T, Viken RJ, Kaprio J. Adolescent alcohol abuse and adverse adult outcomes: evaluating confounds with drinking-discordant twins. Alcohol Clin Exp Res. 2014 Aug;38(8):2314–2321. doi: 10.1111/acer.12491. Epub 2014 Jul 17. PubMed PMID: 25040879; PubMed Central PMCID: PMC4146743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.White HR, Zie M, Thompson W, Loeber R, Stouthamer-Loeber M. Psychopathology as a predictor of adolescent drug use trajectories. Psychol Addict Behav. 2001;15:210–218. [PubMed] [Google Scholar]
- 8.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 9.Bertoletti E, Michelini G, Moruzzi S, Ferrer G, Ferini-Strambi L, Stazi MA, Ogliari A, Battaglia M. A general population twin study of conduct problems and the auditory P300 waveform. Journal of Abnormal Child Psychology. 2014;42:861–869. doi: 10.1007/s10802-013-9836-7. [DOI] [PubMed] [Google Scholar]
- 10.Miles DR, van den Bree MB, Pickens RW. Sex differences in shared genetic and environmental influences between conduct disorder symptoms and marijuana use in adolescents. American Journal of Medical Genetics. 2002;114:159–168. [PubMed] [Google Scholar]
- 11.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behaviour: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- 12.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 13.Verweij KJH, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, Boomsma DI, Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behaviour in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 15.Heath AC, Martin NG. Genetic influences on alcohol consumption patterns and problem drinking: results from the Australian NH&MRC twin panel follow-up survey. Ann N Y Acad Sci. 1994;708:72–85. doi: 10.1111/j.1749-6632.1994.tb24699.x. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Nurnberger JI, Jr, Kaprio J. Genetic and environmental effects on conduct disorder, alcohol dependence symptoms, and their covariation at age 14. Alcoholism: Clinical and Experimental Research. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- 18.Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. http://dx.doi.org/10.1037/0021-843X.107.3.363. [DOI] [PubMed] [Google Scholar]
- 19.Grant JD, Lynskey MT, Madden PAF, Nelson EC, Few LR, Bucholz KK, Statham DJ, Martin NG, Heath AC, Agrawal A. The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders. Psychological Medicine. 2015;45:3505–3515. doi: 10.1017/S0033291715001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korhonen T, Latvala A, Dick DM, Pulkkinen L, Rose RJ, Kaprio J, Huizink AC. Genetic and environmental influences underlying externalizing behaviours, cigarette smoking and illicit drug use across adolescence. Behaviour Genetics. 2012;42:614–625. doi: 10.1007/s10519-012-9528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silberg J, Rutter M, D’Onofrio B, Eaves L. Genetic and environmental risk factors in adolescent substance use. J Child Psychol Psychiatry. 2003;44:664–676. doi: 10.1111/1469-7610.00153. [DOI] [PubMed] [Google Scholar]
- 22.Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res Hum Genet. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- 23.Dick DM, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol. 2005;33:219–229. doi: 10.1007/s10802-005-1829-8. [DOI] [PubMed] [Google Scholar]
- 24.Kuperman S, Schlosser SS, Kramer JR, Bucholz KK, Hesselbrock V, Reich T, et al. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. American Journal of Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- 25.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Studies Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht: Kluwer; 1992. [Google Scholar]
- 27.Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- 28.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. VCU Box 900126, Richmond: VA 23298: Department of Psychiatry; 2006. [Google Scholar]
- 29.Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26(1):65–69. [Google Scholar]
- 30.Dibble A. Antisocial Behavior from Adolescence to Early Adulthood: Heritability, Stability, and Correlates using a Longitudinal Twin Sample. VCU Theses and Dissertations. 2013 http://scholarscompass.vcu.edu/etd/3025. [Google Scholar]
- 31.Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioural disinhibition. Am J Med Genet. 2000;96:684–695. [PubMed] [Google Scholar]
- 32.Ystrom E, Kendler KS, Reichborn-Kjennerud T. Early age of alcohol initiation is not the cause of alcohol use disorders in adulthood, but is a major indicator of genetic risk. A population-based twin study. Addiction. 2014;109:1824–1832. doi: 10.1111/add.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal A, Neale M, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal A, Neale M, Jacobson K, Prescott CA, Kendler KS. Illicit drug use and abuse/dependence: modeling of two-stage variables using the CCC approach. Addict Behav. 2005;30:1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: A multi-stage model from cannabis availability, cannabis initiation, and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]