Abstract
Aims
Endothelin-1 (ET-1) is an autocrine inhibitor of collecting duct (CD) Na+ and water reabsorption. CD ET-1 production is increased by a high salt diet and is important in promoting a natriuretic response. The mechanisms by which a high salt diet enhances CD ET-1 are being uncovered. In particular, elevated tubule fluid flow, as occurs in salt loading, enhances CD ET-1 synthesis. Tubule fluid solute content and interstitial osmolality can also be altered by a high salt diet, however their effect on CD ET-1 alone, or in combination with flow, is poorly understood.
Main methods
ET-1 mRNA production by a mouse inner medullary CD cell line (mIMCD3) in response to changing flow and/or osmolality was assessed.
Key findings
Flow or hyperosmolality (using NaCl, mannitol or urea) individually caused an ~2-fold increase in ET-1 mRNA, while flow and hyperosmolality together increased ET-1 mRNA by ~14 fold. The hyperosmolality effect alone and the synergistic effect of flow + hyperosmolality was inhibited by chelation of intracellular Ca2+, however were not altered by blockade of downstream Ca2+-signaling pathways (calcineurin or NFATc), inhibition of cellular Ca2+ entry channels (purinergic receptors or polycystin-2), or blockade of the epithelial Na+ channel. Inhibition of NFAT5 with rottlerin or NFAT5 siRNA greatly reduced the stimulatory effect of osmolality alone and osmolality + flow on mIMCD3 ET-1 mRNA levels.
Significance
Both flow and osmolality individually and synergistically stimulate mIMCD3 ET-1 mRNA content. These findings may be relevant to explaining high salt diet induction of CD ET-1 production.
Keywords: Endothelin, collecting duct, osmolality, flow
Introduction
Collecting duct (CD) endothelin-1 (ET-1) is an important regulator of arterial pressure and urinary Na+ and water excretion. ET-1 is a potent autocrine inhibitor of CD Na+ and water reabsorption (1, 2); CD-specific knockout of ET-1 causes Na+ and water retention and hypertension (3-5). ET receptor antagonist-induced fluid retention, a major factor in clinical trials (6, 7), is substantially due to inhibition of CD ET receptors (8).
Increased salt intake increases CD ET-1 production; this response is important in maintaining body fluid volume (BFV) homeostasis (9). The mechanism(s) by which elevated salt intake enhances CD ET-1 synthesis are beginning to be understood. Circulating hormones (aldosterone, atrial natriuretic peptide, angiotensin II and vasopressin) do not appear to be involved (10-12), suggesting a role for local factors. Recent studies by our group showed that tubule fluid flow, which is increased by high salt intake, stimulates both cortical CD (CCD) (13, 14) and inner medullary CD (IMCD) (14) ET-1 mRNA accumulation. The stimulatory effect of flow on CCD ET-1 synthesis is mediated, at least in part, by Na+ delivery and the epithelial Na+ channel (ENaC) (14), however flow-stimulated IMCD ET-1 production is independent of ENaC (14). Since the IMCD produces and binds more ET-1 than any other cell type in the kidney (9, 15), we sought to further explore how IMCD ET-1 is regulated. One possible IMCD ET-1 regulatory factor, in addition to flow, is osmolality. Renal interstitial and tubule fluid NaCl concentration can be increased by a high salt diet. Increased renal medullary interstitial NaCl concentration has been shown to enhance thick ascending limb ET-1 production (16). Increased media osmolality has also been reported to stimulate IMCD ET-1 production (17-19), however other studies have not observed this and/or have described a dependence upon the specific solute studied (9, 19). Furthermore, the effect of osmolality on the IMCD ET-1 response to flow is unknown. Consequently, the current study was undertaken to investigate whether osmolality alone, or together with flow, can affect IMCD ET-1. We hypothesized that since both flow and osmolality are increased by high salt intake, that these factor work together to augment CD ET-1 biosynthesis.
Materials and Methods
Reagents
Calcineurin inhibitory peptide and cyclosporine A were obtained from Tocris Bioscience (Ellisville, MO). All other drugs and chemicals were obtained from Sigma (St. Louis, MO) unless stated otherwise.
Cell culture
The mouse IMCD cell line, mIMCD3, was used for all studies. Mouse IMCD3 cells with polycystin-2 knockdown were provided by Drs. Rajeev Rohatgi and Luca Gusella at the Icahn School of Medicine at Mount Sinai, NY (20). Cells were grown to confluence on 10-cm2 plastic culture plates in a 5% CO2 incubator at 37°C; 50:50 DMEM/F-12 supplemented with 10% fetal bovine serum, 1 mg/ml penicillin and 1 mg/ml streptomycin was used as growth medium. For the control studies done under stationary conditions, cells were grown in 12-well plates under identical conditions. In each study, sample size (N) refers to separate plates. No more than 2 plates came from a given cell split.
Flow and osmolality studies
Rectangular parallel plate polycarbonate flow chambers (Catalog number 31-010, Glycotech, Gaithersburg, MD) were attached to 10-cm cell culture plates containing confluent mIMCD3 cells using vacuum and silastic gaskets to form a channel with the dimensions: 0.25 mm depth, 1 cm width and 5.9 cm in length, having a total surface area of 5.9 cm2. The flow chamber has two ports through which perfusate enters and exits the channel. The liquid was pumped with a peristaltic pump (Ismatec, Glattburg, Switzerland). HBSS (pH 7.4) was used as the perfusate for control experiments and was supplemented with drugs and/or chemicals for additional experiments. RNA was extracted from cells exposed to flow and from control no-flow cells. All experiments were performed at 37°C.
Previous studies by our group determined that a 2 hr exposure to 2 dyne/cm2 shear stress optimally stimulated ET-1 mRNA accumulation in mIMCD3 cells (14), hence all experimental periods lasted 2 hr and all shear stress was 2 dyne/cm2. All pharmacologic agents were added 30 min before, and continued throughout, the experimental period. Similarly, studies on hyperosmolality involved increasing perfusate osmolality 30 min before and throughout the experimental period. Each experimental period involved exposing cells to either stationary conditions or to flow. For stationary studies, cells were placed in flow chambers in a manner identical to flow studies, however the chambers were only filled with perfusate and not exposed to flow.
RNA analysis and real-time PCR
RNA from cultured cells was isolated using the RNeasy Mini Kit and reverse transcribed with Omniscript RT Kit (Qiagen, Valencia, CA). ET-1, GAPDH and NFAT5 mRNA levels were determined by real-time PCR (StepOne Plus, Applied Biosystems, Foster City, CA) using the Taqman Gene Expression Assay with ET-1 (catalog number Mm00438656_m1), GAPDH (catalog number Mm03302249_m1) and NFAT5 (catalog number Mm00467257_m1) primers, respectively.
siRNA studies
Mouse NFAT5 siRNA (catalog number MSS225643) and negative controls (scrambled siRNA sequences) were purchased from Invitrogen (Waltham, MA). Cells were grown on 25 mm glass coverslips (Warner Instruments, Hamden, CT), transfection was carried out for 48 hr using Lipofectamine RNAiMax as the transfection agent (Invitrogen), and flow studies were performed in polycarbonate flow chambers (catalog number 64-1860, Warner instruments, Hamden CT) according to conditions described above. RNA was extracted from cells exposed to flow or no flow conditions. All experiments were performed at 37°C.
Statistics
Data are represented as mean ± SE. One-way ANOVA was used to compare the differences between groups. The Shapiro-Wilk test was used to evaluate for normal distribution. P < 0.05 was considered significant.
Results
Effect of flow and osmolality on mIMCD3 ET-1 mRNA
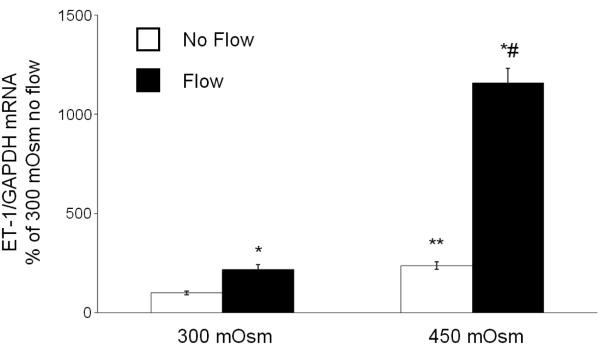
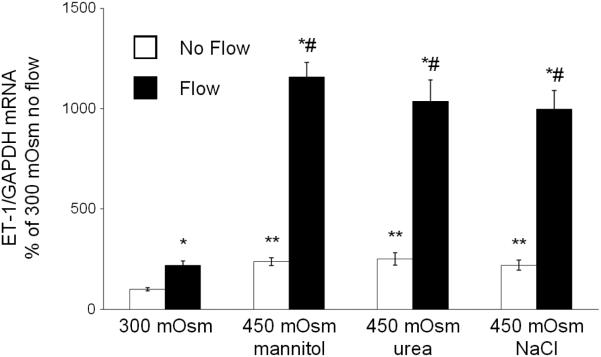
ET-1 mRNA very closely reflects ET-1 protein levels (9); since ET-1 protein levels were below the level of detection in the flow system, only ET-1 mRNA values are reported. As previously described (14), flow increased mIMCD3 ET-1 mRNA ~2-fold as compared to cells under stationary (no flow) conditions (Figure 1). Increasing media osmolality to 450 mOsm with mannitol augmented ET-1 mRNA ~2-fold in cells under stationary conditions, while hyperosmolality and flow synergistically increased ET-1 mRNA by ~14-fold (Figure 1). Increasing osmolality with urea or NaCl to 450 mOsm gave a similar ET-1 mRNA response to hyperosmolality ± flow as that seen with mannitol (Figure 2).
Figure 1.
Effect of hyperosmolality (mannitol) on stationary and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
Figure 2.
Effect of hyperosmolality with mannitol, urea or NaCl on stationary and flow-stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
Role of ENaC or Ca2+ pathways in flow- and osmolality-stimulated mIMCD3 ET-1 mRNA
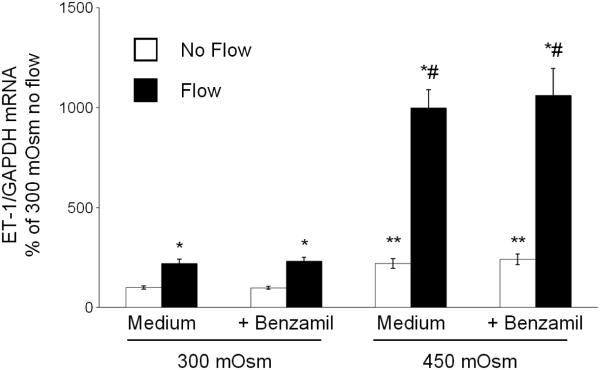
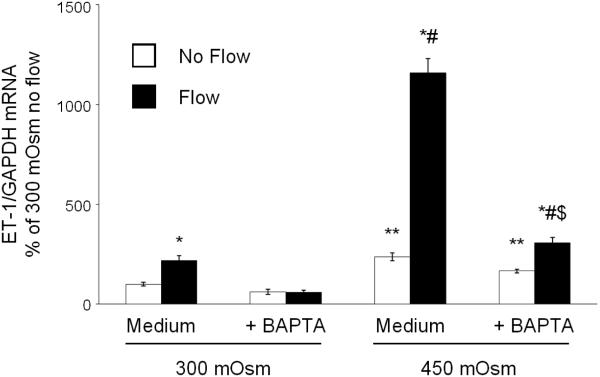
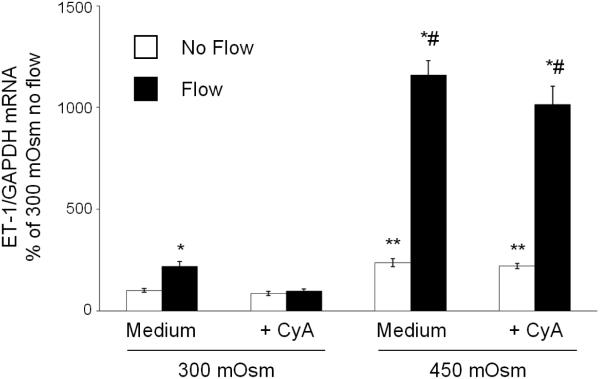
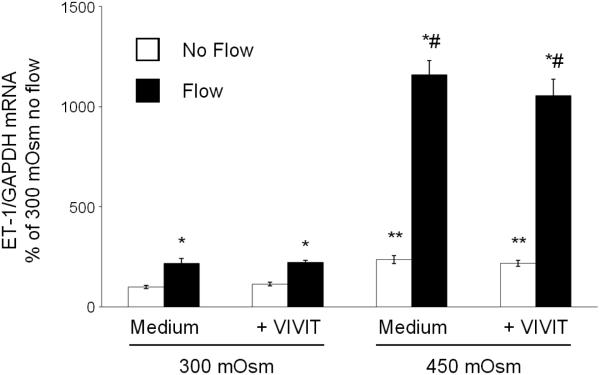
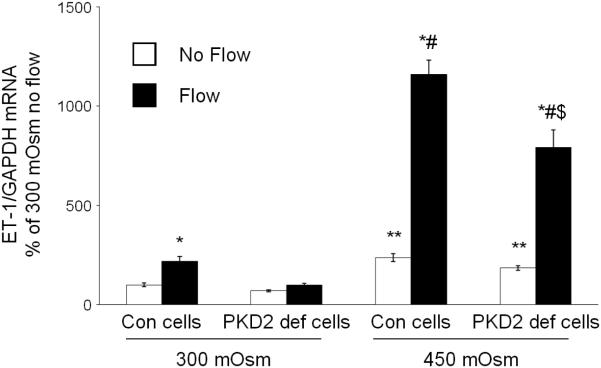
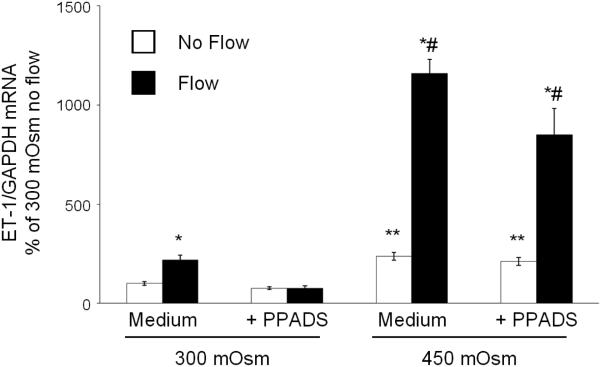
As previously described (14), ENaC inhibition did not affect the mIMCD3 ET-1 flow response (Figure 3). In addition, ENaC blockade had no effect on osmolality stimulated ET-1 mRNA levels (Figure 3). Chelation of intracellular Ca2+ with BAPTA-AM inhibited the ET-1 response to flow, hyperosmolality and flow + hyperosmolality (Figure 4). Since Ca2+ appears to play a central role, the effect of modifying various Ca2+-signaling pathways and Ca2+ channels that have been implicated as having a role in flow-stimulated mIMCD3 ET-1 mRNA production (14) was assessed. Inhibition of calcineurin with cyclosporine A prevented the flow stimulated ET-1 mRNA increase (Figure 5), however it had no effect on hyperosmolality or hyperosmolality + flow stimulated ET-1 mRNA. Since nuclear factor of activated T-cells (NFAT) 1-4 (NFATc) has been implicated in ET-1 regulation in IMCD cells (21), the effect of inhibition of NFATc (VIVIT peptide) was examined; VIVIT peptide had no effect on flow and/or hyperosmolality stimulation of ET-1 mRNA (Figure 6). The effect of blockade of two Ca2+ cellular entry pathways (polycystin-2 and purinergic receptors) was examined since both pathways have been implicated in modulation of IMCD ET-1 production (14). Flow stimulated ET-1 mRNA was largely abolished in polycystin-2 deficient mIMCD3 cells (Figure 7), while the hyperosmolality effect was not changed in stationary cells and mildly reduced in cells exposed to flow and hyperosmolality. Similarly, inhibition of purinergic receptors (PPADS) abolished the ET-1 flow response, but had no effect on hyperosmolality or hyperosmolality + flow stimulated ET-1 mRNA content (Figure 8).
Figure 3.
Effect of inhibition of ENaC (0.2 µM benzamil) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
Figure 4.
Effect of chelation of intracellular calcium (50 µM BAPTA-AM) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm, $p<0.05 vs. flow 450 mOsm.
Figure 5.
Effect of inhibition of purinergic receptors (30 µM PPADS) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
Figure 6.
Effect of inhibition of NFATc inhibitor peptide (0.2 µM VIVIT peptide) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
Figure 7.
Effect of Pkd2 gene (encodes polycystin-2) deficiency on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm, $p<0.05 vs. flow 450 mOsm.
Figure 8.
Effect of calcineurin inhibition (3 µg/ml cyclosporine A) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm.
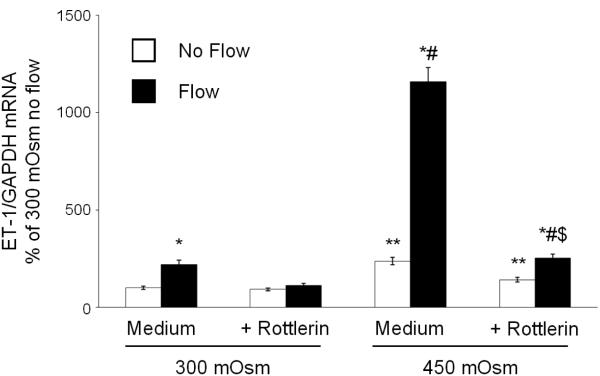
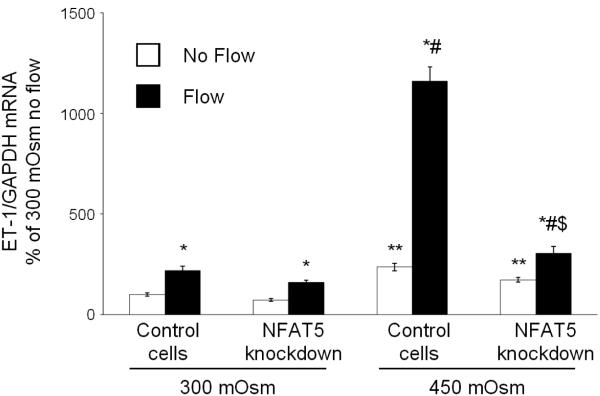
Since hyperosmolality stimulation of mIMCD3 ET-1 production is through, at least in part, mechanisms independent of flow-enhanced mIMCD3 ET-1 production, the effect of blockade of NFAT5, a factor known to be largely induced by hyperosmolality, on mIMCD3 ET-1 mRNA content was determined. Rottlerin, a relatively non-specific NFAT5 inhibitor (22-24), markedly reduced the ET-1 mRNA response to flow and/or hyperosmolality as compared to cells transfected with scrambled siRNA (Figure 9). In order to more specifically determine a role for NFAT5, studies were conducted with NFAT5 siRNA. NFAT5 siRNA reduced NFAT5 mRNA levels by 65% (data not shown) and was associated with marked inhibition of the ET-1 mRNA hyperosmolality, but not the flow, response (Figure 10). Notably, NFAT5 siRNA almost completely abolished the stimulatory effect of flow + hyperosmolality.
Figure 9.
Effect of rottlerin (10 µM) on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=10-12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm, $p<0.05 vs. flow 450 mOsm.
Figure 10.
Effect of NFAT5 siRNA on osmolality (mannitol) and flow stimulated ET-1 mRNA content in mIMCD3 cells. n=12. *p<0.05 vs. no flow control same conditions; **p<0.05 vs. no flow control 300 mOsm: #p<0.05 vs. flow 300 mOsm, $p<0.05 vs. flow 450 mOsm.
Discussion
The key findings in the current study are that: 1) hyperosmolality stimulates IMCD ET-1 mRNA; 2) hyperosmolality synergizes with flow to increase IMCD ET-1 mRNA; and 3) the hyperosmolality effect depends upon [Ca2+]I and NFAT5. We previously reported that flow augments IMCD ET-1 mRNA (14); that hyperosmolality also increases IMCD ET-1 biosynthesis raises some interesting, albeit speculative, possibilities. As stated earlier, high salt intake can increase renal interstitial NaCl concentration which augments thick ascending limb ET-1 production (16). If increased interstitial osmolality were the major factor in stimulating CD ET-1 production, then one would expect that IMCD ET-1 production would be greatest under conditions where interstitial osmolality is highest, i.e., during volume contraction/water deprivation. However, IMCD ET-1 production is greatest during BFV expansion (9). Since flow and perfusate hyperosmolality synergized to increase ET-1 synthesis, it may be that the osmolality of the tubule fluid is an important factor. Tubule fluid osmolality, largely due to NaCl, can be increased during high salt intake; notably, this is a relatively unique physiologic condition wherein tubule fluid flow and solute concentration are increased together. Thus, the combination of elevated tubule fluid flow and solute concentration may represent a specific and strong signal to the IMCD indicating that BFV is expanded and induction of natriuretic and diuretic ET-1 is needed to compensate. Note that these considerations apply only to physiologic conditions; under pathologic states with reduced nephron number, tubule fluid flow and osmolality could be increased together in order to attempt to maintain BFV homeostasis. It is tempting to speculate that such a scenario may contribute to increased renal ET-1 production typically seen in chronic kidney disease (25); additional studies are needed to test this possibility.
The stimulatory effect of hyperosmolality on mIMCD3 ET-1 mRNA was independent of the specific solute and ENaC, suggesting that it is hyperosmolality per se which augments the IMCD ET-1 synthetic process. Notably, urea increased ET-1 mRNA; while urea is cell permeable, it does not immediately equilibrate across the plasma membrane and so acute osmolality effects could be exerted. The urea effect is of unclear significance in regard to salt or water loading responses, however it is conceivable, albeit not studied in vivo, that changes in dietary protein could modulate CD ET-1 through changes in kidney urea levels. One potential limitation of these studies is that we are unable to define the time course of osmolality on stimulating ET-1 mRNA levels. Since changes in ET-1 mRNA levels are not detectable before 2 hr, it is possible that ET-1 gene transcription is stimulated quite early after increasing osmolality (i.e., minutes, not hours).
The hyperosmolality effect was dependent on [Ca2+]I; such a finding is perhaps not surprising given previous studies showing that Ca2+ is important in initiating signaling pathways involved in ET-1 synthesis in IMCD cells under stationary (26) and flow (14) conditions, as well as in CCD (13). Since previous studies implicated two specific Ca2+ channels (purinergic P2X receptors and polycystin-2) in mediating flow-induced ET-1 mRNA in mIMCD3 cells (14), we investigated the role for these channels in the hyperosmolality response. In contrast to the flow response, hyperosmolality-induced ET-1 mRNA was unaffected by blockade of purinergic receptors or polycystin-2. We did not further examine how [Ca2+]I increases in response to hyperosmolality; little is known how this occurs in any cell type.
The signaling pathways downstream of hyperosmolality-stimulated increases in [Ca2+]I in mIMCD3 are uncertain. We found no evidence suggesting a role for calcineurin or classically Ca2+-dependent NFAT isoforms. In contrast, the current studies indicate that NFAT5 is centrally important in mediating the ET-1 hyperosmolality response. While not specifically investigated herein, induction of NFAT5 activity has been observed to be dependent, at least in some systems, upon increases in [Ca2+]I (27). Further, NFAT5 has been extensively implicated in mediating induction of a number of genes by hyperosmolality (28). However, to our knowledge, there are no published reports on NFAT5 stimulation of ET-1 production. While the current study was not designed to investigate whether NFAT5 directly regulates ET-1 gene transcription, it is notable that the ET-1 promoter contains a NFAT5 consensus recognition site within 1-2 kb of the transcriptional start site.
A striking finding was our observation that hyperosmolality and flow markedly synergize to increase mIMCD3 ET-1 mRNA. How such synergy occurs is unclear, particularly since flow and osmolality appear to increase ET-1 mRNA by separate pathways. Previous studies by our group (14), as confirmed in the current study, indicate that flow-dependent induction of mIMCD3 ET-1 mRNA requires purinergic receptors, polycystin-2 and calcineurin, while hyperosmolality alone, as well as the synergistic effect of hyperosmolality + flow, does not depend upon these pathways. Clearly, further studies are required to determine the molecular mechanisms involved in this synergistic effect.
Conclusion
Hyperosmolality and flow separately and synergistically increase IMCD ET-1 biosynthesis. The mechanisms by which the separate effects occur are partly different, with both flow and hyperosmolality being dependent upon [Ca2+]I, while the hyperosmolality effect is uniquely dependent upon NFAT5. The pronounced synergistic effect of hyperosmolality and flow raises the possibility that these two factors are important in mediating increased IMCD ET-1 production in response to high salt intake.
Acknowledgements
We thank Rajeev Rohatgi and Luca Gusella for providing the polycystin-2 deficient cells. The research was supported in part by NIH P01 HL095499 to D.E.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–70. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomita K, Nonguchi H, Terada Y, Marumo F. Effects of ET-1 on water and chloride transport in cortical collecting ducts of the rat. Am J Physiol. 1993;264:F690–F6. doi: 10.1152/ajprenal.1993.264.4.F690. [DOI] [PubMed] [Google Scholar]
- 3.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–11. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005;288:F912–20. doi: 10.1152/ajprenal.00432.2004. [DOI] [PubMed] [Google Scholar]
- 5.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2008;295:F1635–40. doi: 10.1152/ajprenal.90279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton M, Kohan DE. Endothelin antagonists in clinical trials: lessons learned. J Clin Invest. 2011;172:255–60. doi: 10.1159/000328859. [DOI] [PubMed] [Google Scholar]
- 7.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–35. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart D, Chapman M, Rees S, Woodward SK, Kohan DE. Myocardial, smooth muscle, nephron and collecting duct gene targeting reveals the organ sites of endothelin A receptor antagonist fluid retention. J Pharmacol Exp Ther. 2013;346:182–9. doi: 10.1124/jpet.113.205286. [DOI] [PubMed] [Google Scholar]
- 9.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285:F664–73. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Huang Y, Kohan DE. Role of the renin-angiotensin-aldosterone system in collecting duct-derived endothelin-1 regulation of blood pressure. Can J Physiol Pharmacol. 2008;86:329–36. doi: 10.1139/Y08-028. [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Nonoguchi H, Tomita K. Physiological effects of vasopressin and atrial natriuretic peptide in the collecting duct. Cardiovasc Res. 2001;51:470–80. doi: 10.1016/s0008-6363(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 13.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol. 2011;300:F650–6. doi: 10.1152/ajprenal.00530.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit MM, Strait KA, Matsuda T, Kohan DE. Na delivery and ENaC mediate flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol. 2012;302:F1325–30. doi: 10.1152/ajprenal.00034.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 16.Herrera M, Garvin J. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol. 2005;288:F58–F64. doi: 10.1152/ajprenal.00209.2004. [DOI] [PubMed] [Google Scholar]
- 17.Kramer HJ, Hashemi T, Backer A, Bokemeyer D. Hyperosmolality induced by betaine or urea stimulates endothelin synthesis by differential activation of ERK and p38 MAP kinase in MDCK cells. Kidney Blood Press Res. 2002;25:65–70. doi: 10.1159/000063510. [DOI] [PubMed] [Google Scholar]
- 18.Migas I, Backer A, Meyer-Lehnert H, Kramer HJ. Endothelin synthesis by porcine inner medullary collecting duct cells. Effects of hormonal and osmotic stimuli. Am J Hypertens. 1995;8:748–52. doi: 10.1016/0895-7061(95)00115-6. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Terada Y, Nonoguchi H, Ujiie K, Tomita K, Marumo F. Effect of hyperosmolality on production and mRNA expression of endothelin-1 in inner medullary collecting duct. Am J Physiol. 1993;264:F684–F9. doi: 10.1152/ajprenal.1993.264.4.F684. [DOI] [PubMed] [Google Scholar]
- 20.Flores D, Battini L, Gusella GL, Rohatgi R. Fluid shear stress induces renal epithelial gene expression through polycystin-2-dependent trafficking of extracellular regulated kinase. Nephron Physiol. 2011;117:27–36. doi: 10.1159/000321640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strait KA, Stricklett PK, Kohan RM, Kohan DE. Identification of two nuclear factor of activated T-cells (NFAT)-response elements in the 5'-upstream regulatory region of the ET-1 promoter. J Biol Chem. 2010;285:28520–8. doi: 10.1074/jbc.M110.153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn BK, Jeong SK, Kim HS, Choi KJ, Seo JT, Choi EH, Ahn SK, Lee SH. Rottlerin, a specific inhibitor of protein kinase C-delta, impedes barrier repair response by increasing intracellular free calcium. J Invest Dermatol. 2006;126:1348–55. doi: 10.1038/sj.jid.5700244. [DOI] [PubMed] [Google Scholar]
- 23.Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28:453–8. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Tian W, Cohen DM. Rottlerin inhibits tonicity-dependent expression and action of TonEBP in a PKCdelta-independent fashion. Am J Physiol Renal Physiol. 2002;282:F710–7. doi: 10.1152/ajprenal.00303.2001. [DOI] [PubMed] [Google Scholar]
- 25.Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86:896–904. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2007;293:F601–6. doi: 10.1152/ajprenal.00085.2007. [DOI] [PubMed] [Google Scholar]
- 27.Gajghate S, Hiyama A, Shah M, Sakai D, Anderson DG, Shapiro IM, Risbud MV. Osmolarity and intracellular calcium regulate aquaporin-2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. J Bone Mineral Res. 2009;24:992–1001. doi: 10.1359/JBMR.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillon J, Morancho B, Santiago V, Lopez-Rodriguez C. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72:1597–604. doi: 10.1016/j.bcp.2006.07.002. [DOI] [PubMed] [Google Scholar]