Abstract
Background
Distinct monocyte subsets predict cardiovascular risk and contribute to heart failure progression in murine models but have not been examined in clinical acute decompensated heart failure (ADHF).
Methods and Results
Blood samples were obtained from 11 healthy controls (HC) and at admission and discharge in 19 ADHF patients. Serological markers of inflammation were assessed on admission and discharge. Monocyte populations were defined using flow cytometry for cell-surface expression of CD14 and CD16: CD14++CD16− (classical), CD14++CD16+ (intermediate), and CD14+CD16++ (non-classical). In ADHF patients, C-reactive protein (CRP) and IL-6 were higher compared with HC (both p<0.001), and decreased from admission to discharge (CRP: 12.1±10.1 to 8.6±8.4 mg/L, p=0.005; IL-6: 19.8±34.5 to 7.1±4.7 pg/ml, p=0.08). In ADHF patients, the admission proportion of CD14++CD16-monocytes was lower (68% vs. 85%, p< 0.001) and CD14++CD16+ (15% vs. 8%, p=0.002) and CD14+CD16++ (17% vs. 7%, p=0.07) monocytes higher compared with HC. Additionally, the proportion of CD14++CD16− monocytes increased (68% to 79%, p=0.04) and the CD14+CD16++ monocytes decreased (17% to 7%, p=0.049) between admission and discharge.
Conclusions
Following standard treatment of ADHF, the monocyte profile and circulating inflammatory markers shifts to more closely resemble those of HC, suggesting a resolving acute inflammatory state. Functional studies are warranted to understand how specific monocyte subsets and systemic inflammation may contribute to ADHF pathophysiology.
Keywords: heart failure, innate immunity, monocytes, inflammation
INTRODUCTION
Over 5 million Americans currently have heart failure (HF), and acute decompensated heart failure (ADHF) is the most common reason for hospital admission among persons older than 65.1 With the increasing incidence of HF risk factors (e.g. age, obesity, and diabetes), ADHF hospitalizations are likely to increase in the coming years. Over one-third of patients who survive ADHF hospitalization are rehospitalized or die within 90 days of initial discharge2, and 1-year mortality is over 30% 3. Unfortunately, current therapy for ADHF is empiric and largely based on expert consensus.4, 5
Abnormalities in the inflammatory cascade have been associated with both the initiation and the progression of HF.6 The detrimental consequences of sustained inflammation in HF models led to several controlled trials aimed at neutralizing proinflammatory cytokines in HF patients. However, results were overall neutral in these studies and an appreciable number of patients had worsening of HF 7, 8, raising important questions about how the immune system contributes to ADHF. The innate immune system could modulate the inflammatory component of HF through several mechanisms, including the production of inflammatory cytokines (e.g. TNF-α, IL-6) and reactive oxygen species, activation of the complement system, and modulation of endothelial cell function and myeloid cell recruitment.
Monocytes are key innate immune system mediators of inflammatory responses. Monocyte dysregulation has been implicated in the pathogenesis of diverse diseases including diabetes, tumor metastasis, pulmonary fibrosis, myocardial infarction, and atherosclerosis. Functionally distinct monocyte subsets can be characterized using flow cytometry for surface expression of FcγIII receptor CD16 and the lipopolysaccharide receptor CD14. The Nomenclature Committee of the International Union of Immunological Societies defines three subsets by this method: CD14++CD16− (classical), CD14++CD16+ (intermediate), and CD14+CD16++ (non-classical).9 The distribution of these monocyte subsets changes with age,10 and affects the risk for cardiovascular events even if clinically evident vascular disease is not present.11, 12 Recently, Ismahil et. al. found that differential monocyte subpopulations causally contribute to disease pathogenesis in a murine model of HF,6 but data in human HF are sparse and cross-sectional.13, 14 We sought to identify changes in monocyte subsets over the course of HF hospitalization, as this information could suggest further avenues for the study of ADHF pathophysiology.
MATERIALS AND METHODS
Study design
The goals of this study were to compare serologic inflammatory markers and monocyte subsets between ADHF patients and healthy adult controls (HC), and to determine how these parameters change over the course of hospital treatment for ADHF. For the purposes of this study, three monocyte subsets were considered: CD14++CD16− (classical), typically accounting for 80-90% of cells in the circulation, and the CD14++CD16+ (intermediate), and CD14+CD16++ (non-classical) subsets, which account for the remaining 10-20%.9 Since changes in monocyte subsets during ADHF have not previously been reported, and few studies have examined differences between HF patients and healthy controls, we powered the study based on IL-6, a marker of systemic inflammation. Based on a prior cross-sectional study of symptomatic HF outpatients vs. HC, we predicted a decrease in plasma IL-6 from 40 to 15 pg/ml between admission and discharge with standard deviation of ±25 pg/ml and mean IL-6 of 3.0 pg/ml in HC. Using these assumptions, 20 or more ADHF subjects and 5 or more HC were believed sufficient to demonstrate significant between-group and pre-post ADHF hospitalization differences in plasma IL-6 levels at α = 0.05 and β = 0.20.
Enrolled patients
Patients hospitalized at the University of Michigan Health System with an admitting diagnosis of ADHF were eligible for this study. Baseline exclusion criteria were acute coronary syndrome, severe renal dysfunction (dialysis or estimated glomerular filtration rate < 15 ml/min/1.73m2), cirrhosis or other evidence of severe hepatic dysfunction, active infection or inflammatory disorder (e.g. rheumatoid arthritis, lupus), active smoking, active solid organ or hematologic malignancy, use of immunosuppressive therapy (including corticosteroids), and planned mechanical circulatory support, cardiac transplantation, or other surgical procedure during the index hospitalization. Patients who were found to have active infectious or inflammatory conditions (e.g. pneumonia, gout flare) following initial enrollment and those who had surgical procedures, blood product transfusion, or immunosuppressive therapy during hospitalization were excluded from the analysis. Nonsmoking older adult (aged ≥ 65 years) HC were recruited through a database managed by the University of Michigan Claude D. Pepper Older Americans Independence Center.
Sample collection and analysis
ADHF patients with an anticipated hospital length of stay longer than 48 hours were enrolled and admission/baseline blood samples were drawn within 24 hours of hospital admission. Discharge blood samples were drawn on the date of hospital discharge, or within 24 hours prior to an anticipated weekend discharge according to laboratory facility availability. Blood samples were drawn from HC subjects at a single baseline visit.
Analyses for basic laboratory parameters including renal function, electrolytes, high-sensitivity C-reactive protein (hs-CRP), and complete blood count with differential were performed by the University of Michigan Pathology Laboratory using standard techniques. The University of Michigan Cancer and Immunology Core Laboratory measured plasma IL-6, IL-10, TNF-α, and TGF-β using commercially available high-sensitivity Luminex assays (EMD Millipore, Billerica, MA) and soluble CD-14, MCP-1, and RANTES levels using a commercially-available ELISA assay.
Monocyte isolation and flow cytometry
Monocyte populations were analyzed in all patients and assessed for expression of CD14 and CD16 to define the circulating monocyte populations. 8ml of whole blood was collected by venipuncture in Cell Preparation Tube (CPT) with Sodium Citrate (BD Vacutainer, Franklin Lake, NJ) for each study participant at the time points outlined above. The CPT tubes were centrifuged at 2000×g for 30 min, inverted 5×, and stored overnight. The next day, the top layer was removed, split into 2 × 4ml aliquots, diluted up to 12 ml with ice cold PBS to wash, and centrifuged at 300×g for 10 minutes. After centrifugation, the supernatant was aspirated and the cell pellets were washed again with ice cold PBS. The cell pellet was resuspended in 2ml 1× RBX Lysis Buffer (Biolegend, San Diego, CA) and incubated for 10 minutes at room temperature in the dark. The RBC lysis buffer was neutralized with deionized water and then the cells were pelleted at 300×g and resuspended in Cell Staining Buffer (Biolegend, San Diego, CA). Cells were blocked for 10 minutes on ice with Human TruStain FcX™ (Fc Receptor Blocking Solution; Biolegend, San Diego, CA). Approximately 1 × 106 cells in 300ul of buffer were mixed with 15ul of antibody (Alexa Fluor® 647 anti-human CD14 Antibody #325611, Alexa Fluor® 488 anti-human CD16 Antibody #302022, Biolegend, San Diego, CA, or the appropriate isotype controls) diluted 1:200 in Cell Staining Buffer and incubated for 30 minutes in the dark on ice. Antibodies were titrated to determine optimal concentrations for staining. After incubation, the cells were washed two times with cold Cell Staining Buffer and analyzed using an Accuri C6 Flow Cytometer (Accuri Instruments, Inc., Ann Arbor, MI). At least 500,000 cells per sample were analyzed. Flow cytometry data was further analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Dead cells were excluded using propidium iodide (PI) as previously described (ref). In all patients, the percent of dead cells as defined using PI was less than 5%. The percentage of CD14 and CD16 cells were determined in relation to the isotype control samples. Forward scatter height against forward scatter area was first determined to eliminate doublets. Then forward and side scatter were used to grossly define the monocyte population and then further stratified using CD14 and CD16 to determine CD14++CD16− (classical), CD14++CD16+ (intermediate), and the CD14+CD16++ (non-classical) monocyte subsets as previously described.15 The analysis of monocyte subsets in peripheral blood mononuclear cell stains and the gating strategy used are depicted in Supplemental Figure 1. Populations were determined by gating on the CD14− or CD14+ population and measuring the number of events in that population compared to total number of events collected. Percent positive after staining with Alexa Fluor® 647 anti-human CD14 or Alexa Fluor® 488 anti-human CD16 antibody for each sample was determined by gating on the CD14− or CD14+ population in unstained cells, displaying the data as a histogram, and measuring the shift in the histogram above 5% after staining. Linear mixed-models analysis was used to estimate least squares means for monocyte population size and percent positive of each population after antibody staining (SAS 9.2; SAS Institute).
Statistical analysis
We used Wilcoxon signed-rank testing to evaluate differences in HF patients between hospital admission and discharge, including differences in proportion of monocyte subsets, and Mann-Whitney U testing to evaluate differences between HF patients and HC. GraphPad Prism 6 (GraphPad Software, La Jolla, CA) was used for between-group statistical comparisons.
RESULTS
Enrolled study population
A total of 28 ADHF patients were initially enrolled in the study; two were excluded from baseline analysis after enrollment due to lack of clinical ADHF or subsequently meeting exclusion criteria (< 24 hour length of stay with no clinical worsening from baseline in 1, biopsy-proven liver cirrhosis in 1). An additional seven HF patients were excluded from admission-discharge analysis (inpatient death in 1, left ventricular assist device scheduled or placed while inpatient in 3, patient withdrawal from study prior to hospital discharge in 2, and failure to obtain blood sample within discharge time window in 1). The baseline characteristics of the HC and ADHF patients are shown in Table 1. The ADHF patients were on average younger and as expected, had significant comorbidity burden.
Table 1.
Baseline clinical characteristics
| Variables | HF patients (n = 19) | Healthy controls (n = 11) |
|---|---|---|
| Age, years | 56 ± 11 | 60 ± 16 |
| Female | 4 (21%) | 3 (27%) |
| Body mass index (kg/m2) | 34 ± 6 | 25 ± 4* |
| Ejection fraction, % | 20 ± 15 | n/a |
| History of: | ||
| Coronary disease | 8 (47%) | 0* |
| Hypertension | 11 (65%) | 0* |
| Chronic kidney disease | 10 (59%) | 0* |
| Diabetes mellitus | 11 (65%) | 0* |
| Admission/baseline: | ||
| Systolic blood pressure, mmHg | 112 ± 18 | 129 ± 12* |
| WBC count (K/μl) | 7.4 ± 1.9 | 5.7 ± 1.3* |
| Monocyte count (K/μl) | 0.6 ± 0.2 | 0.4 ± 0.1* |
| Hemoglobin, g/dl | 12.5 ± 3.5 | 13.8 ± 0.9* |
| Urea nitrogen, mg/dl | 36 ± 24 | 18 ± 5* |
| Serum creatinine, mg/dl | 1.4 ± 0.5 | 0.9 ± 0.2* |
| Estimated GFR† (ml/min/1.73m2) | 58 ± 22* | 80 ± 25* |
| B-type natriuretic peptide (pg/ml) | 994 ± 665 |
Abbreviations: GFR, glomerular filtration rate; HF, heart failure; WBC, white blood cell
p<0.05 between HF patient and healthy control groups
Using Modification of Diet in Renal Disease equation
Data are presented as mean ± standard deviation.
Circulating cytokines and inflammatory biomarkers
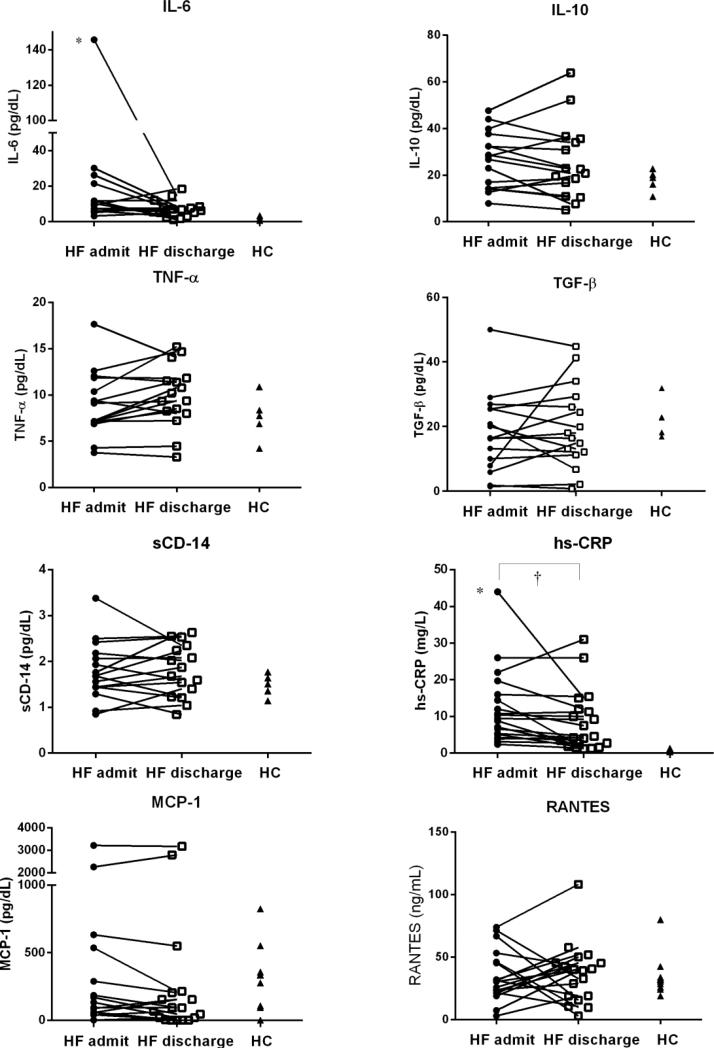
On admission, ADHF patients had significant differences in levels of hs-CRP and IL-6 compared with HC (hs-CRP: 12.1±10.1 vs. 0.6±0.3 mg/L; IL-6 19.8±34.5 vs. 1.7±1.0 pg/ml, both p<0.001). Additionally, in ADHF patients, hs-CRP significantly decreased from admission to discharge (hs-CRP: 8.6±8.4 mg/L, p=0.005; median change −1.1 mg/L, IQR of change −5.3-0.2 mg/L) and IL-6 trended lower (IL-6: 7.1±4.7 pg/ml, p=0.08; median change −2.6 pg/ml, IQR of change −14.2-1.7 pg/ml). IL-10, TGF-β, TNF-α, sCD-14, MCP-1, and RANTES levels did not significantly differ between ADHF patients and HC or between admission and discharge in ADHF (Figure 1).
Figure 1.
Cytokines and Biomarkers
Abbreviations: HC, healthy controls; HF, heart failure
* p<0.05 HF admit vs. HC; † p<0.05 HF discharge vs. admit
Monocyte subsets
We observed differences in the distribution of monocyte subsets between ADHF patients upon hospital admission and HC. Specifically, the proportion of CD14++/CD16− (classical) monocytes was lower (68 vs. 85%, p<0.001) and that of CD14++/CD16+ (intermediate) and CD14/CD16++ (non-classical) cells were higher (15 vs. 8%, p=0.002 and 17% vs. 7%, p=0.07, respectively) in ADHF patients at admission compared with HC. We also found that the monocyte subsets transitioned as ADHF patients were treated during their hospitalization. Compared with admission, we observed an increase in the CD14++/CD16− (classical) proportion (68 to 79%, p=0.04; median change 4%, IQR of change −3 to 21%) and decrease in the CD14+/CD16++ (non-classical) proportion (17 to 7%, p=0.049; median change −2%, IQR of change −7 to 1%) upon hospital discharge, so that the overall monocyte subset distribution more closely resembled that observed in the HC (Figure 2). We found no correlations between baseline left ventricular ejection fraction, admission B-type natriuretic peptide, blood urea nitrogen, or serum sodium levels and baseline or changes in monocyte distribution. Similarly, we found no significant correlations between baseline or change in CRP, IL-6, MCP-1, or RANTES levels and baseline or changes in monocyte distribution.
Figure 2.
Monocyte Subset Distribution
Abbreviations: HC, healthy controls; HF, heart failure
* p<0.05, HF vs. HC; † p≤0.05, HF discharge vs. admit
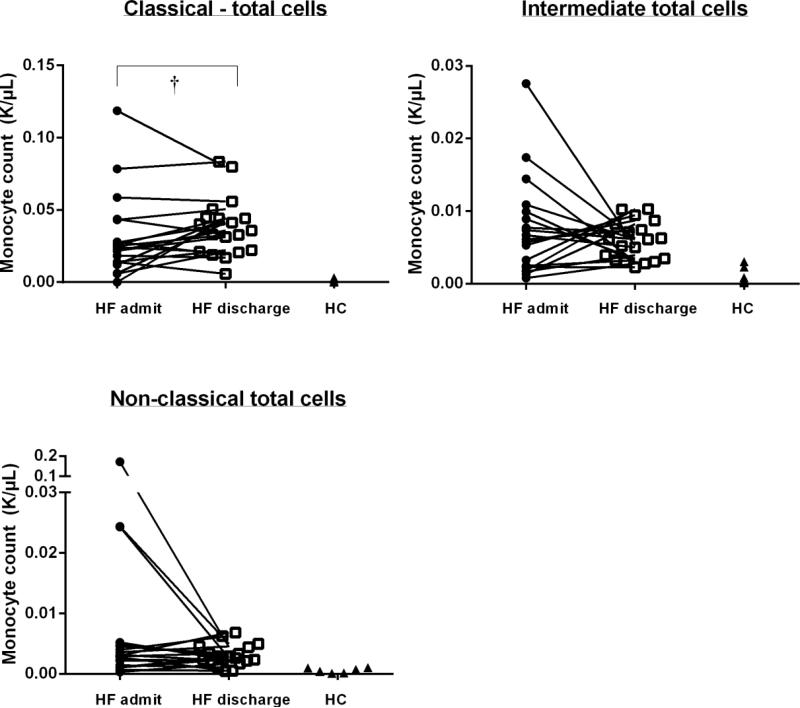
In the ADHF group, 16 of 19 patients had total monocyte counts by automated differential available at both admission and discharge. Total monocyte count was higher in ADHF patients compared with HC, but did not change from admission to discharge in ADHF patients (ADHF admission: 0.6±0.2 K/μL; ADHF discharge 0.7±0.3 K/μL, p=0.22; HC: 0.4±0.1 K/μL, p<0.01 vs. ADHF admission and ADHF discharge). Regardless if obtained at admission or discharge, all monocyte counts in each subset were more numerous in ADHF patients than in HC (p<0.003 for all). By total counts, the CD14++/CD16− (classical) subset significantly increased between admission and discharge in ADHF patients (0.02±0.02 K/μL to 0.03±0.02 K/μL, p=0.02). Changes in the CD14++CD16+ (intermediate) subset, and CD14+CD16++ (non-classical) subsets by total counts were not significant (p>0.05) between admission and discharge in ADHF; trends were in the same direction as for the subset proportions, but displayed substantial variability (Figure 3).
Figure 3.
Total Monocyte Count
Abbreviations: HC, healthy controls; HF, heart failure
† p<0.05 HF discharge vs. admit; all HF vs. HC comparisons p<0.003
DISCUSSION
In this study, we demonstrate that 1) patients admitted with ADHF have serological evidence of inflammation (specifically, elevated levels of CRP and IL-6), 2) ADHF patients have a distinct monocyte subset profile compared with HC, and 3) following treatment of ADHF, the monocyte subset distribution of ADHF patients shifts to more closely resemble the HC monocyte profile. To our knowledge, this study is the first to explore how circulating monocyte subsets evolve over the course of ADHF treatment.
Recent basic science studies have implicated activation of myeloid cells (monocytes and macrophages) in the pathobiology of HF but few studies have investigated this issue in clinical HF.6 In one study of 30 ambulatory HF patients, absolute monocyte count increased with New York Heart Association class and predicted worsening HF or death over the subsequent 20 months. Furthermore, myeloid cells from these patients had higher CD11b and lower CXCR1 receptor expression than healthy controls. A retrospective analysis from the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial demonstrated that absolute monocyte count predicted all-cause mortality and HF hospitalization on a univariate basis but not after adjusting for clinical variables. The authors hypothesized that specific monocyte subpopulations might predict HF outcomes better than absolute monocyte levels and called for prospective studies to address this and other related questions.16
The identification of distinct monocyte subsets in preclinical models provides a unique opportunity to investigate the immune system's role in human cardiovascular disease.17 CD16+ monocyte counts are increased in many inflammatory conditions, and are believed to modulate the inflammatory component of coronary artery disease. Even in patients without overt vascular disease, monocyte subsets carry important prognostic information for cardiovascular events. Rogacev and colleagues prospectively followed 119 patients with chronic kidney disease for an average of 4.9 years. They found that the CD14++/CD16+ (intermediate) monocyte subset predicted combined death and major cardiovascular events (myocardial infarction, stroke, amputation, revascularization) independent of other risk factors, including prevalent vascular disease. The same group followed 951 patients referred for coronary angiography for a mean of 2.6 years in the HOM SWEET HOMe (Heterogeneity of Monocytes in Subjects Who Undergo Elective Coronary Angiography—The Homburg Evaluation) study. Patients in the highest quartile of CD14++/CD16+ (intermediate) monocyte count had three times the risk of combined death and major cardiovascular events, even after extensive adjustment for overall leukocyte counts, traditional cardiovascular risk factors, and the presence of vascular disease at baseline.12
Only two prior reports have described monocyte subset distribution in human HF. In an outpatient cross-sectional study, Barisione and colleagues found that the proportions of CD14++/CD16+ (intermediate) monocytes were increased and CD14+/CD16+ (non-classical) decreased in HF compared to controls without HF. The CD14++/CD16+ (intermediate) proportion increased with New York Heart Association class, and was inversely related to left ventricular ejection fraction. In another cross-sectional study, Amir et. al also reported decreased proportion of CD14+/CD16+ (non-classical) monocytes in patients with stable HF, but found no difference in CD14++/CD16+ (intermediate) cells when compared to age-matched controls.14 In contrast to both studies in chronic HF patients, we found that ADHF patients upon hospital admission had substantially higher proportion of CD14+/CD16+ (non-classical) monocytes than HC; this proportion then decreased over the course of hospitalization to more closely resemble that found in HC. To our knowledge, our study is the first to report monocyte subset distribution in ADHF and changes during hospitalization. The novel aspect of our findings is that monocyte subsets in ADHF patients evolved over a short timeframe during standard inpatient treatment, despite no presence of infection or use of direct immune-modulating therapies. This observation suggests that the immune system acutely responds to both HF decompensation and its treatment.
This concept is supported by the work of Colombo and colleagues, who suggest that vascular inflammation is a key factor in ADHF.18 They first demonstrated that severely ill ADHF patients requiring inotropic support had increased venous endothelial expression of pro-inflammatory genes that improved following clinical re-compensation.19 A subsequent study in healthy young people showed that venous congestion substantially alters endothelial gene expression in several relevant domains, including leukocyte adhesion molecules (VCAM-1) and chemokines (CXCL-2, secreted by monocyte/macrophage lineage cells).20
Several studies have demonstrated immune-modulating effects of commonly used HF medications including angiotensin-converting enzyme inhibitors and beta blockers.21, 22 Recently, genetic ablation of the mineralocorticoid receptor, the molecular target for spironolactone and eplerenone, in macrophages and endothelial cells demonstrated a profound impact on cardiac remodeling in preclinical models of HF.23 These studies provide possible mechanistic links to the circulating monocyte findings in our study. However, these chronic HF medications did not change substantially during hospitalization (see Supplementary Table 1) and would be unlikely to explain the monocyte alterations observed over the short-time frame of ADHF treatment. The vast majority of ADHF patients worldwide are treated with intravenous loop diuretics to remove volume overload, including all ADHF patients in our study. Failure to resolve symptomatic congestion during ADHF hospitalization increases the risk for rehospitalization and death,24 but high doses of diuretics and worsening renal function also predict poor outcomes.25 Further investigation of the relationship between monocyte phenotypes, endothelial activation, heart failure therapeutics, and volume status may clarify the mechanisms through which the immune system modulates ADHF and suggest new treatment approaches.
Similar studies in obesity and trauma demonstrate that monocyte subsets have prognostic value with regards to disease severity and clinical outcomes26, 27. Importantly, in all of these diseases, our current understanding has progressed first from an appreciation that absolute monocyte levels are predictive, to a more nuanced appreciation that monocyte heterogeneity and ultimately the functional consequences of this heterogeneity contribute to disease pathogenesis. Recent preclinical studies have demonstrated causal contributions of circulating myeloid cells to HF directly implicating these cells and the systemic inflammatory response to HF pathogenesis.6 Interestingly, the authors found that transfer of immune cells from animals with HF recapitulated multiple facets of cardiac remodeling in normal mice again supporting the notion that circulating immune cells modulate cardiac responses present in HF. We believe our hypothesis-generating results from clinical HF populations support these notions and further contribute to the expanding body of knowledge linking immune dysfunction and HF.
Limitations
This pilot study includes a small number of patients, and we were unable to examine the relationships between monocyte profiles and clinical variables or outcomes. The HC group was slightly, but not significantly, older than the ADHF cohort. However, healthy aging skews monocytes towards CD16+ subsets, and accordingly this difference should have biased towards no difference in monocyte profiles between ADHF patients and HC. Additionally, we also were unable to obtain total monocyte counts at both admission and discharge in three ADHF patients. The data were analyzed both by excluding those patients as well as by carrying over the single available monocyte count (from admission or discharge) with no obvious change in the reported trends.
The ADHF patient cohort we analyzed was quite advanced, with low blood pressure, markedly elevated B-type natriuretic peptide levels, nearly half not tolerating angiotensin-converting enzyme inhibitor or angiotensin receptor blockade at hospital discharge, and requiring high loop diuretic doses for clinical stability. Whether our findings would apply to a less ill ADHF population is unknown. As mentioned above, monocyte distribution did not appear related to circulating cytokines/chemokines or several clinical markers of HF severity. Our results suggest that functional assessment of monocytes may help clarify the relationships between traditional markers of HF severity and the innate immune system.
Finally, there exist many methodologies to characterize circulating monocyte populations and these methodologies are rapidly evolving along with our appreciation of the tremendous plasticity of monocyte populations9, 17, 28. Over the recent years, considerable effort has been dedicated to more accurately define monocyte subsets and to ascribe functional significance to these flow cytometric-based classifications. For the purpose of this exploratory study, we focused primarily on morphological characteristics of peripheral blood mononuclear cells to identify monocyte populations and then used flow cytometry of cell surface markers to further define the monocyte subsets. There exist many methodological approaches to characterize monocyte subsets. As with all approaches, there is always the possibility of contamination by other circulating immune cells (neutrophils, NK cells) in our monocyte populations. The small contamination from these other circulating cells would be more problematic if our analysis included genomic profiling which can amplify transcripts from small populations of cells; however, because we focused on the cell-surface markers and how these evolve with standard HF treatment, we do not believe these will fundamentally skew the conclusions presented here. Studies are already underway to further characterize these monocyte populations using FACS sorting and purification to allow for more precise genomic and proteomic characterization of these circulating monocyte populations.
CONCLUSION
In this study of patients hospitalized for ADHF, serological markers of inflammation were elevated and monocyte subset distribution substantially differed from HC. Following standard ADHF treatment, CD14+/CD16++ (non-classical) monocytes decreased and CD14++/CD16− (classical) monocytes increased so that the overall subset profile more closely resembled that of the HC. Further studies are warranted to clarify the relationship between circulating monocyte subsets and ADHF pathophysiology.
Supplementary Material
Highlights.
Inflammation is known to be associated with heart failure.
Decompensated heart failure (ADHF) patients had elevated inflammatory markers.
ADHF patients had distinct monocyte subsets compared with healthy controls (HC).
Treatment of heart failure made monocyte profiles more closely resemble that of HC.
ACKNOWLEDGEMENTS
This study was supported by an Inaugural Grant from the University of Michigan Samuel and Jean Frankel Cardiovascular Center. Healthy controls were recruited through a registry maintained by the University of Michigan's Claude D. Pepper Older Americans Independence Center. Dr. Scott Hummel is supported by NIH/NHLBI K23HL109176 and NIH/NIA R21AG047939. Dr. Sascha Goonewardena is supported by NIH/NHLBI K08HL123621. The authors would like to acknowledge the invaluable assistance of Ms. Joanna Wells.
ABBREVIATIONS
- ADHF
acute decompensated heart failure
- HC
healthy controls
- HF
heart failure
- hs-CRP
high-sensitivity C-reactive protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J. Am. Coll. Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 3.Kommuri NVA, Koelling TM, Hummel SL. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am. J. Med. 2012;125:209.e209–209.e215. doi: 10.1016/j.amjmed.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE, McMurray JJV, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2013 doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 6.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann D, McMurray JJV, Packer M, Swedberg K, Borer J, Colucci W, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen D, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 8.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure:results of the Anti-tnf Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 10.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults.(research article). BMC Immunology. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. Cd14++cd16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 12.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Große-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Böhm M, Fliser D, Heine GH. Cd14++cd16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Barisione C, Garibaldi S, Ghigliotti G, Fabbi P, Altieri P, Casale M, Spallarossa P, Bertero G, Balbi M, Corsiglia L, Brunelli C. Cd14cd16 monocyte subset levels in heart failure patients. Dis. Markers. 2010;28:115–124. doi: 10.3233/DMA-2010-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir O, Spivak I, Lavi I, Rahat MA. Changes in the monocytic subsets cd14(dim)cd16(+) and cd14(++)cd16(-) in chronic systolic heart failure patients. Mediators Inflamm. 2012;2012:616384. doi: 10.1155/2012/616384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. Ccr2 on cd14(+)cd16(+) monocytes is a biomarker of hiv-associated neurocognitive disorders. Neurology(R) neuroimmunology & neuroinflammation. 2014;1:e36. doi: 10.1212/NXI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene SJ, Harinstein ME, Vaduganathan M, Subacius H, Konstam MA, Zannad F, Maggioni AP, Swedberg K, Butler J, Gheorghiade M. Prognostic value of monocyte count in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST trial). Am. J. Cardiol. 2012;110:1657–1662. doi: 10.1016/j.amjcard.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo PC, Onat D, Sabbah HN. Acute heart failure as “acute endothelitis” -- interaction of fluid overload and endothelial dysfunction. Eur. J. Heart Fail. 2008;10:170–175. doi: 10.1016/j.ejheart.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 20.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 2014;35:448–454. doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa b activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 22.Kiemer AK, Vollmar AM. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann. Rheum. Dis. 2001;60(Suppl 3):iii68–70. doi: 10.1136/ard.60.90003.iii68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J. Clin. Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr., Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur. Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J. Am. Coll. Cardiol. 2010;55:872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautes-Fridman C, Clement K, Cremer I. Cd14dimcd16+ and cd14+cd16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 27.West SD, Goldberg D, Ziegler A, Krencicki M, Du Clos TW, Mold C. Transforming growth factor-beta, macrophage colony-stimulating factor and c-reactive protein levels correlate with CD14(high)CD16+ monocyte induction and activation in trauma patients. PLoS One. 2012;7:e52406. doi: 10.1371/journal.pone.0052406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appleby LJ, Nausch N, Midzi N, Mduluza T, Allen JE, Mutapi F. Sources of heterogeneity in human monocyte subsets. Immunol. Lett. 2013;152:32–41. doi: 10.1016/j.imlet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.