Abstract
Amyotrophic lateral sclerosis (ALS) is a rare degenerative disorder characterized by loss of upper and lower motor neurons. Neuroimaging has provided noticeable evidence that ALS is a complex disease, and shown that anatomical and functional lesions extend beyond precentral cortices and corticospinal tracts, to include the corpus callosum; frontal, sensory, and premotor cortices; thalamus; and midbrain. The aim of this study is to investigate graph theory-based functional network abnormalities at voxel-wise level in ALS patients on a whole brain scale. Forty-three ALS patients and 44 age- and sex-matched healthy volunteers were enrolled. The voxel-wise network degree centrality (DC), a commonly employed graph-based measure of network organization, was used to characterize the alteration of whole brain functional network. Compared with the controls, the ALS patients showed significant increase of DC in the left cerebellum posterior lobes, bilateral cerebellum crus, bilateral occipital poles, right orbital frontal lobe, and bilateral prefrontal lobes; significant decrease of DC in the bilateral primary motor cortex, bilateral sensory motor region, right prefrontal lobe, left bilateral precuneus, bilateral lateral temporal lobes, left cingulate cortex, and bilateral visual processing cortex. The DC's z-scores of right inferior occipital gyrus were significant negative correlated with the ALSFRS-r scores. Our findings confirm that the regions with abnormal network DC in ALS patients were located in multiple brain regions including primary motor, somatosensory and extra-motor areas, supporting the concept that ALS is a multisystem disorder. Specifically, our study found that DC in the visual areas was altered and ALS patients with higher DC in right inferior occipital gyrus have more severity of disease. The result demonstrated that the altered DC value in this region can probably be used to assess severity of ALS.
Keywords: amyotrophic lateral sclerosis, degree centrality, graph theory-based network, resting state, magnetic resonance imaging
Introduction
Amyotrophic lateral sclerosis (ALS) is a rare degenerative disorder selectively affecting upper and lower motor neurons, culminating in respiratory insufficiency and death after 3–5 years (Beghi et al., 2006). The cause of ALS remains unknown (Orrell, 2007). Functional MRI (fMRI) has provided an important tool to study ALS–related cortical function and reorganizations. In small samples of patients with ALS, the fMRI tasks of limb movements have demonstrated that regional modifications of cerebral activation involved extensive cortical regions including the primary motor cortex, supplementary motor areas, the premotor cortex, the motor learning areas (basal ganglia and cerebellum), and extra-motor areas (e.g., the temporal and inferior parietal cortices; Chiò et al., 2014). However, the task fMRI in ALS may have a large variability in task performance because of the disease induced peripheral weakness (Pradat and El Mendili, 2014). The resting-state functional MRI (rsfMRI) techniques avoid potential performance because of being independent of task demand. Several rsfMRI studies of ALS reported significantly decreased functional connectivity within the sensorimotor network (Mohammadi et al., 2009; Jelsone-Swain et al., 2010; Tedeschi et al., 2012; Fekete et al., 2013; Zhou et al., 2013; Chiò et al., 2014) and in brain networks related to cognition and behavior, in keeping with the altered motor and extramotor structural connectivity. Other studies have identified regions of increased functional connectivity, including somatosensory and extra-motor areas (Verstraete et al., 2010; Agosta et al., 2011, 2013; Douaud et al., 2011; Luo et al., 2012; Fekete et al., 2013; Zhou et al., 2013; Chiò et al., 2014; Zhou F. et al., 2014). As these studies are based on networkwise functional connectivity analysis using seed-based correlation analysis or independent component analysis, none of these studies fully characterize the brain's functional connectome of ALS.
Recently, the graph theory-based network analysis was applied to characterize functional connectivity within the whole-brain functional connectome (Rubinov and Sporns, 2010; Telesford et al., 2011). The voxel-wise degree centrality (DC), a class of graph theory-based network measures assessing functional importance degree, has been widely used to detect changes in resting-state functional networks (Cole et al., 2010; Wang et al., 2010; Fransson et al., 2011; Lord et al., 2011; Di Martino et al., 2013; Kullmann et al., 2014; Zhou Y. et al., 2014). When a node has numerous direct connections to other nodes, it will have a high DC. It represents the most local and directly quantifiable centrality measure. Different from seed-based approaches or independent component analysis, this graph-based measure of network organization can characterize the functional relationships of a given node(voxel) within the entire connectivity matrix of full-brain functional connectome rather than represent the relationships with specific nodes or networks (Tomasi and Volkow, 2011; Zuo et al., 2012). As such, DC analysis allow us to capture the complexity of the functional connectome (Zuo et al., 2012). Furthermore, the exploration of voxel-wise DC allowed us to identify the brain regions that may be involved by ALS without requiring a priori selection of nodes or networks of interest (Zhou Y. et al., 2014).
In this study, we explored the full-brain functional networks using rsfMRI data obtained in 43 sporadic ALS patients and 44 health control (HC). The voxel-wise network DC was analyzed. We hypothesize that ALS would alter the DC of multiple brain regions within the entire connectome compared with HC.
Materials and methods
Participants
Forty-three sporadic ALS patients (30 definite, 13 probable) were recruited consecutively from the Department of Neurology at Southwest Hospital in Chongqing and were diagnosed according to the El Escorial criteria (Brooks et al., 2000). Inclusion criteria: a diagnosis of definite or probable ALS and right-handedness. Exclusion criteria were as follows: (1) family history of motor neuron diseases; (2) clinical diagnosis of frontotemporal dementia (Neary et al., 1998); (3) history of brain injury, epilepsy, or a neurologic disease; (4) significant respiratory failure (forced vital capacity below 70); and (5) cognitive impairment as determined by Montreal Cognitive Assessment (MoCA) score < 26 (Nasreddine et al., 2005). Disease severity was assessed using the ALS Functional Rating Scale-revised (ALSFRS-r; Cedarbaum et al., 1999). Forty-four age- and sex-matched healthy volunteers, with no history of neurological or psychiatric disorders and a normal neurological examination, were recruited from the local community served as HC. All the participants were right-handed investigated by Edinburgh inventory (Oldfield, 1971) and all MRI scans were visually inspected by a radiologist to rule out major neuropathology such as tumor, stroke, or advanced white matter disease.
The study was approved by the local ethical committee. All subjects provided written informed consent before enrollment.
MRI acquisition
The MRI data were acquired with a 3.0T Siemens Tim Trio whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany) in the Southwest Hospital, Chongqing, China. Whole-brain functional scans were collected in 36 axial slices by using an echo-planar imaging (EPI) sequence with the following settings: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 192 × 192 mm, slices = 36, in-plane matrix = 64 × 64, thickness = 3 mm, voxel size = 3.0 × 3.0 × 3.0 mm. For each subject, a total of 240 volumes were acquired, resulting in a total scan time of 480s. Three dimensional T1-weighted anatomical images were acquired in a sagittal orientation using the following volumetric 3D magnetization-prepared rapid gradient–echo (MP-RAGE) sequence (TR = 1900 ms, TE = 2.52 ms, flip angle = 9°, slice thickness = 1 mm, slices, 176, FOV = 256 × 256 mm, matrix size = 256 × 256 and voxel size = 1 × 1 × 1 mm) on each subject. All subjects were instructed simply to rest with their eyes closed, not to think of anything in particular, and not to fall asleep.
Data preprocessing
All preprocessing steps were performed using the Data Processing Assistant for Resting-State fMRI (DPARSF2.3, http://www.restfmri.net), which is based on the Statistical Parametric Mapping (SPM8) program (http://www.fil.ion.ucl.ac.uk/spm) and the Resting-State fMRI Data Analysis Toolkit (REST1.8, http://www.restfmri.net). Prior to preprocessing, the first 10 volumes were discarded to allow for signal stabilization due to instability of the initial MRI signal and adaptation of participants to the circumstance. The remaining volumes of each subject were corrected for the acquisition differences between slices. The resulting images were then realigned to correct for small movements that occurred between scans. Based on the recorded motion correction estimates, the subjects with more than 1.5 mm maximum displacement in any of the x, y, or z directions or more than 1.5° of angular rotation about any axis for any of the 230 volumes were excluded from the study. Based on these criteria, four ALS and five HCs were excluded from the analyses. Individual 3D T1-weighted structural images were co-registered to the mean of the realigned EPI images. The transformed structural images were then separated into white matter, gray matter, and cerebrospinal fluid. The Diffeomorphic Anatomical Registration Through Exponentiated Lie (DARTEL) algebra tool (Ashburner, 2007) was applied to compute the transformations from individual native space to MNI space.
As rsfMRI measures have been shown to be sensitive to micro-head motions (Yan et al., 2013a), the Friston 24-parameter model was applied to regress head motion effects out of the realigned data (Satterthwaite et al., 2013; Yan et al., 2013a). Considering measures of voxel-wise differences in motion in its derivation, we further regressed the Jenkinson's mean frame-wise displacement (FD) (a measure of the micro-head motion; Yan et al., 2013b) of each subject, as recommended in a previous study (Yan et al., 2013a).
To further reduce the effects of confounding factors, the signals from the white matter and cerebrospinal fluid, the mean time series of all voxels across the whole brain and linear and quadratic trends were removed from the data with linear regression (Yan et al., 2013b). The resulting maps were then registered into MNI space with 3 mm3 cubic voxels using the transformation information acquired from DARTEL. A temporal filter (0.01–0.08 Hz) was applied to reduce low frequency drifts and high frequency physiological noise.
Degree centrality calculation
To exclude artifactual correlations from non-gray matter voxels, the voxel-wise centrality analyses were restricted to a predefined gray matter mask that included tissue with gray matter probabilities more than 20% as previously described (Zuo et al., 2012; Zhou Y. et al., 2014). The gray matter tissue probability template was published as a part of tissue priors in SPM8. Within the study mask, individual network centrality maps were created in a voxel-wise style. First, the preprocessed functional data sets were subjected to voxel-based whole-brain correlation analysis. The time course of each voxel within the gray matter mask from each participant was correlated with the time course of every other voxel, which generated a correlation matrix. An undirected adjacency matrix was then obtained by thresholding each correlation at r > 0.25 (Zuo et al., 2012; Yan et al., 2013a,b; Zhou Y. et al., 2014). Then, the DC was computed as the sum of the weights of the significant correlations (weighted) or the number of significant connections (binarized) for each voxel (Zuo et al., 2012; Zhou Y. et al., 2014). Finally, by subtracting the mean DC across the entire brain and then dividing by the standard deviation of the whole-brain DC, these individual-level voxel-wise DC maps were standardized into a z-score (Zuo et al., 2012; Yan et al., 2013b). A smoothing kernel of 4 mm was applied.
Statistical analysis
Two samples t-tests were performed to examine the differences between the DC measures of ALS group and HC group by REST1.8 (Song et al., 2011). Statistical significance was set at a voxel-wise p < 0.01 in conjunction with cluster wise AlphaSim (rmm = 5, clusters = 18) to correct for multiple comparisons. In AlphaSim, Monte Carlo permutation simulations are used to estimate the null distribution (Cox and Hyde, 1997; Jadach et al., 2001). Generally, the voxel-wise intensity threshold was set at p < 0.01 and a cluster-level threshold of 405 mm3 (which was 18 voxels) was calculated using the Monte Carlo simulation in AFNI (10,000 iterations, 61 × 73 × 61 dimensions, 3 × 3 × 3 m3, 4-mm smoothness), in which case the probability of a type I error was less than 0.01.
At last, pearson correlations (two-sided) were calculated between ALSFRS-r score and z-score of altered DC's areas in ALS group with each patient's age and gender as covariates.
Results
The demographic and clinical characteristics of participants analyses are summarized in Table 1. There was no significant difference in age and gender between the ALS group and HC group.
Table 1.
Demographic Data of the participants.
| Group | ALSa | Controls | p valuec |
|---|---|---|---|
| Age (years) | 49.05 ± 8.07529–71) | 51.00 ± 10.576 (24–65) | 0.386 |
| Male: female | 26:13 | 21:18 | 0.247 |
| El Escorial criteria (probable/definite) | 12/27 | – | – |
| Disease duration (months) | 17.77 ± 13.0012–71) | – | – |
| ALSFRS-Ra | 36.18 ± 6.472 (16–44) | – | – |
| Disease progression rateb | 0.9210 ± 0.70944 (0.17–3.14) | – | – |
ALS, amyotrophic lateral sclerosis; ALSFRS_R, ALS Functional Rating Scale_Revised.
Disease progression rate = (48–ALSFRS_R)/Disease duration.
Statistical significance was set at p < 0.05.
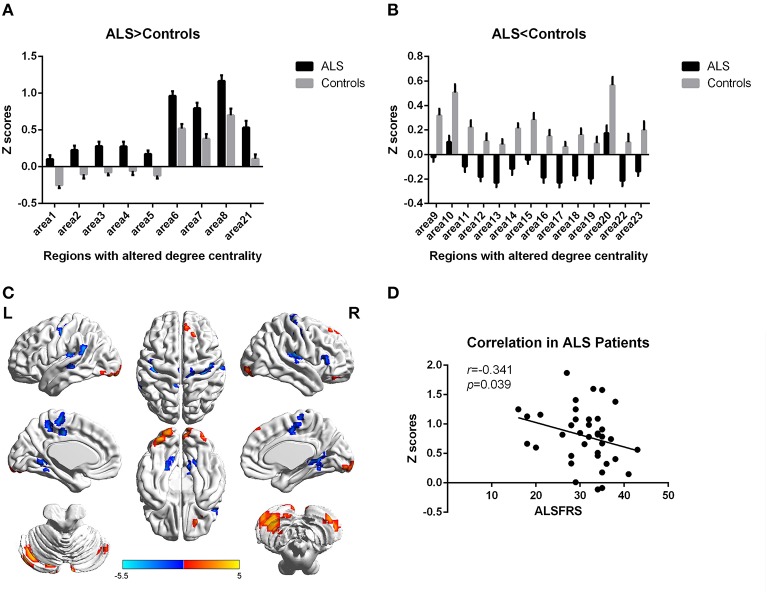
In the present work, centrality analyses were performed for both binary and weighted graphs representing the functional connectome. Compared to the HC group in the weighted DC, significant increases of DC in ALS group were found in the left cerebellum posterior lobes, bilateral cerebellum crus, bilateral occipital poles (Brodmann area(BA) 18/19), right orbital frontal lobe(BA11) and bilateral prefrontal lobes (BA8/9; Figures 1A,C; Table 2); significant decreases of DC in ALS group were found in the bilateral primary motor cortex(BA4/6), bilateral sensory motor region(BA3/5), right prefrontal lobe(BA45), left precuneus(BA5), bilateral lateral temporal lobes(BA48), left middle cingulate cortex(BA23), and bilateral visual processing cortex(BA17/18/37; Figures 1B,C; Table 3). The findings obtained from the binarized networks were similar to those obtained from the weighted networks (Figures 2A–C; Tables 4, 5). Correlation analysis shows that DC's z-scores of Right inferior Occipital gyrus (area7, BA 18) is negative correlated with the ALSFRS-r scores in both the binarized and weighted networks of ALS group (Figures 1D, 2D).
Figure 1.
Regions with altered degree centrality in ALS patients compared with healthy controls in weighted networks. (A,B) represents the regions with increase/decrease degree centrality in ALS patients in bar graphs (group mean Z scores and standard errors of the mean). The red areas in (C) show the regions with increased degree centrality, and the blue areas show the regions with decreased degree centrality. The color bar shows the T values. (D) shows the negative correlation between DC's z-scores in weighted networks of Right inferior Occipital gyrus (area7, BA 18) and the ALSFRS-r scores in ALS group with the age and gender as covariates.
Table 2.
Regions with increase degree centrality in patients with ALS compared with healthy controls in weighted networks.
| Areas | Voxel sizea | Voxels structure (BA)b | Peak MNI coordinateb | Peak intensity (T value) | Peak MNI coordinate region | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Area 1 | 190 | –27 | –69 | –48 | 4.3181 | Left Cerebellum Posterior Lobe7 | |
| Area 2 | 97 | –3 | –87 | –36 | 4.6095 | Left Cerebellum Crus 2 | |
| Area 3 | 38 | 33 | –69 | –42 | 3.4569 | Right Cerebellum Crus 2 | |
| Area 4 | 19 | –15 | –75 | –27 | 3.6728 | Left CerebellumCrus 1 | |
| Area 5 | 107 | BA18/19 | –33 | –87 | –18 | 4.044 | Left Lingual gyrus |
| Area 6 | 35 | BA11 | 24 | 42 | –21 | 3.4221 | Right Middle Orbital Frontal gyrus |
| Area 7 | 22 | BA18/19 | 36 | –96 | –9 | 3.6865 | Right inferior Occipital gyrus |
| Area 9 | 19 | BA18 | 9 | –94 | –12 | 3.087 | Right Lingual gyrus |
| Area 19 | 49 | BA8/9 | 12 | 45 | 54 | 3.8211 | Superior Frontal gyrus |
Statistical significance was set at a voxel-wise p < 0.01, in conjunction with cluster wise AlphaSim (rmm = 5, clusters = 18) to correct for multiple comparisons.
BA, Brodmann area; MNI, Montreal Neurological Institute.
Table 3.
Regions with decrease degree centrality in patients with ALS compared with healthy controls in weighted networks.
| Areas | Voxel sizea | Voxels structure (BA)b | Peak MNI coordinateb | Peak intensity (T value) | Peak MNI coordinate region | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Area 8 | 49 | BA18/37 | –21 | –51 | –3 | –4.3451 | Left Lingual gyrus, extent to fusiform gyrus |
| Area 10 | 89 | BA17/18/37 | 18 | –48 | 3 | –4.4131 | Right Calcarine gyrus, extent to Lingual gyrus, and fusiform gyrus |
| Area 11 | 21 | BA21 | –54 | –27 | –3 | –4.3048 | Left Middle Temporal |
| Area 12 | 18 | BA45 | 60 | 27 | 6 | –3.5382 | Right Triangular Part of inferior Frontal gyrus |
| Area 13 | 119 | BA48 | 33 | –18 | 21 | –4.7377 | Right Insula gyrus |
| Area 14 | 47 | BA48 | –36 | –27 | 12 | –4.4054 | Left Heschl gyrus |
| Area 15 | 25 | BA48 | –66 | –48 | 24 | –4.2531 | Left SupraMarginal gyrus |
| Area 16 | 175 | BA3/4 | 42 | –12 | 27 | –4.7412 | Right Precentral gyrus, extent to Postcentral gyrus |
| Area 17 | 32 | BA23 | –9 | –21 | 45 | –3.9378 | Left Middle Cingulum gyrus |
| Area 18 | 69 | BA3/6 | –45 | –15 | 51 | –5.1545 | Left Postcentral gyrus |
| Area 20 | 36 | BA5 | –9 | –39 | 60 | –4.3082 | Left Precuneus gyrus |
| Area 21 | 24 | BA4 | –6 | –27 | 60 | –3.8403 | Left Paracentral gyrus |
| Area 22 | 37 | BA4 | 15 | –21 | 72 | –3.7799 | Right Precentral gyrus |
Statistical significance was set at a voxel-wise p < 0.01, in conjunction with cluster wise AlphaSim (rmm = 5, clusters = 18) to correct for multiple comparisons.
BA, Brodmann area; MNI, Montreal Neurological Institute.
Figure 2.
Regions with altered degree centrality in ALS patients compared with healthy controls in binarized networks. (A,B) represents the regions with increase/decrease degree centrality in ALS patients in bar graphs (group mean Z scores and standard errors of the mean). The red areas in (C) show the regions with increased degree centrality, and the blue areas show the regions with decreased degree centrality. The color bar shows the T values. (D) shows the negative correlation between DC's z-scores in binarized networks of Right inferior Occipital gyrus (area7, BA 18) and the ALSFRS-r scores in ALS group with the age and gender as covariates.
Table 4.
Regions with increase degree centrality in patients with ALS compared with healthy controls in the binarizednetworks.
| Areas | Voxel sizea | Voxels structure (BA)b | Peak MNI coordinateb | Peak intensity (T value) | Peak MNI coordinate region | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Area 1 | 194 | –27 | –69 | –48 | 4.4222 | Left Cerebellum Posterior Lobe7 | |
| Area 2 | 41 | 33 | –69 | –42 | 3.5008 | RightCerebellumCrus | |
| Area 3 | 108 | –3 | –87 | –36 | 4.6621 | Left CerebellumCrus 2 | |
| Area 4 | 18 | –15 | –75 | –27 | 3.679 | Left CerebellumCrus 1 | |
| Area 5 | 37 | BA11 | 24 | 42 | –21 | 3.8082 | Right Middle Orbital Frontal gyrus |
| Area 6 | 85 | BA18/19 | –24 | –96 | –18 | 3.997 | Left Lingual gyrus |
| Area 7 | 25 | BA18 | 36 | –96 | –9 | 3.7465 | Right inferior Occipital gyrus |
| Area 8 | 21 | BA18 | 12 | –102 | –6 | 3.0777 | Right Lingual gyrus |
| Area 21 | 38 | BA8/9 | 12 | 45 | 54 | 3.7879 | Superior Frontal gyrus |
Statistical significance was set at a voxel-wise p < 0.01, in conjunction with cluster wise AlphaSim (rmm = 5, clusters = 18) to correct for multiple comparisons.
BA, Brodmann area; MNI, Montreal Neurological Institute.
Table 5.
Regions with decrease degree centrality in patients with ALS compared with healthy controls in the binarized networks.
| Areas | Voxel sizea | Voxels structure (BA)b | Peak MNI coordinateb | Peak intensity (T value) | Peak MNI coordinate region | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Area 9 | 42 | BA18/37 | –21 | –51 | –3 | –4.3062 | Left Lingual gyrus, extent to fusiform gyrus |
| Area 10 | 81 | BA17/18/37 | 18 | –48 | 3 | –4.3412 | Right Calcarine gyrus, extent to Lingual gyrus, and fusiform gyrus |
| Area 11 | 19 | BA22 | –54 | –27 | –3 | –4.2576 | Left Middle Temporal |
| Area 12 | 20 | BA45 | 60 | 27 | 6 | –3.6089 | Right Triangular Part of inferior Frontal gyrus |
| Area 13 | 115 | BA48 | 33 | –18 | 21 | –4.757 | Right Insula gyrus |
| Area 14 | 45 | BA48 | –36 | –27 | 12 | –4.3269 | Left Heschl gyrus |
| Area 15 | 22 | BA48 | –66 | –48 | 24 | –4.2694 | Left SupraMarginal gyrus |
| Area 16 | 166 | BA3/4/6 | 42 | –12 | 27 | –4.5985 | Right Precentral gyrus, extent to Postcentral gyrus |
| Area 17 | 42 | BA23 | –9 | –21 | 45 | –4.1167 | Left Middle Cingulum gyrus |
| Area 18 | 67 | BA3/4/6 | –45 | –15 | 51 | –5.2347 | Left Postcentral gyrus extent to Precentral gyrus |
| Area 19 | 19 | BA4/23 | 6 | –18 | 57 | –3.7851 | Right Supplement Motor Area, extent to Precentral gyrus |
| Area 20 | 39 | BA5 | –9 | –39 | 60 | –4.3001 | Left Precuneus gyrus |
| Area 22 | 24 | BA4 | –6 | –27 | 60 | –3.8749 | Left Paracentral gyrus |
| Area 23 | 37 | BA4 | 15 | –21 | 72 | –3.8176 | Right Precentral gyrus |
Statistical significance was set at a voxel-wise p < 0.01, in conjunction with cluster wise AlphaSim (rmm = 5, clusters = 18) to correct for multiple comparisons.
BA, Brodmann area; MNI, Montreal Neurological Institute.
Discussion
Intrinsic functional connectivity provides a powerful and unique tool for the examination of the organization of the human brain (Buckner et al., 2013). To our knowledge, this is the first study to conduct a voxel-based analysis of brain functional network during the resting state on a whole brain scale in ALS patients with DC measurement, and our results revealed that ALS patients showed abnormal network DC in multiple brain regions. Our results suggest that the DC measurement of full-brain functional networks could be useful to characterize the pathophysiology of ALS. Voxel-wise whole-brain analysis of functional connectivity, as is conducted in our study, allows for unbiased mapping of ALS-associated functional abnormalities which offers advantages over the region-based analysis of functional connectivity requiring a priori spatial demarcations (Zhou Y. et al., 2014). Furthermore, compared with previous functional connectivity analysis using seed-based correlation analysis or independent component analysis, the voxel-based network DC measurement directly and quantifiably reflects the change between multiple brain regions system and the whole brain functional network connectome.
Specifically, our study found that DC in the visual areas was altered in ALS patients: significant decreases DC in the bilateral anterior visual processing regions, significant increases DC in the bilateral caudate occipital poles. Previous study by detecting spontaneous low-frequency fluctuations (LFF) of blood oxygen level–dependent signals of rfMRI showed decreased ALFF in the visual processing areas: the inferior occipital lobe and fusiform gyri (Luo et al., 2012). This result and our findings suggest a disorder of visual process, which is in accordance with previous task-related fMRI studies (Lulé et al., 2007; Wang et al., 2013) and electrophysiological studies(Münte et al., 1998, 1999). A delayed and decreased response to visual stimuli was observed in sensory processing cortical areas in ALS patients by electrophysiological studies (Münte et al., 1998, 1999), and a previous longitudinal study identified progressive reduced activation of extrastriate regions over the duration of ALS (Lulé et al., 2007). Combining fMRI with structural MRI, investigators demonstrated the existence of a decreased response in secondary visual areas in ALS during visual stimulation, and significantly less white matter fiber tracts projecting to visual areas (Lulé et al., 2010). The decreases DC in visual processing areas strongly suggest that abnormalities in visual processing areas may be aroused by the demyelination of nerve fibers in the optic tract (Lulé et al., 2010). The increased DC in caudate visual areas (occipital pole) probably reflects a compensatory response due to disorder of visual process. Moreover, Correlation analysis shows that the DC's z-scores of right inferior occipital gyrus (BA18) was negative correlated with the ALSFRS-r scores in both the binarized and weighted networks of ALS group after eliminating the influence of age and gender, showing that ALS patient with higher DC in the area have more severity of disease. It demonstrated that increasing DC in right inferior occipital gyrus can probably be used to assess severity of ALS.
Our finding showed the decreased DC in the primary motor cortex and the sensory motor cortex, which represents that the sum of this region's functional connectivity with the entire connectome is reduced. The primary motor cortex is the hallmark area of impairment in ALS. Surface-based morphometry has showed cortical thinning of the motor cortex (Verstraete et al., 2010; Agosta et al., 2012; Mezzapesa et al., 2013). Previous rsfMRI studies of ALS reported significantly decreased functional connectivity within the sensorimotor network (Mohammadi et al., 2009; Jelsone-Swain et al., 2010; Tedeschi et al., 2012; Fekete et al., 2013; Zhou et al., 2013), which are in keeping with the altered motor and extra-motor functional connectivity (Luo et al., 2012; Agosta et al., 2013). In addition, neuronal loss in the motor cortex has also been demonstrated in previous PET studies and 1H-magnetic resonance spectroscopy study (Mitsumoto et al., 2007; Lombardo et al., 2009; Han and Ma, 2010; Pyra et al., 2010; Sudharshan et al., 2011; Chiò et al., 2014). Therefore, our findings are consistent with these neuroimaging studies and the ALS pathological feature.
In addition, our study also shows significant increases DC in the left cerebellum posterior lobes, bilateral cerebellum crus in ALS. Several neuropathological studies (Wang et al., 2012; Yue et al., 2015) and neuroimaging studies using voxel-based morphometry, diffusion-tensor imaging and functional MRI (Prell and Grosskreutz, 2013; Meoded et al., 2015) have shown evidence for involvement of the cerebellum in ALS. Our finding support the perspectives that recruitment of the cerebellum probably represents a compensatory response due to declining motor function and a loss of cortical inhibitory neuronal influence. However, this study didn't reveal significant correlation between ALSFR-s and altered DC of areas in the primary motor area, the sensory motor area and cerebellum, which may be owing to inclusion of patients with clinically homogeneous.
Moreover, our findings in the limbic system of the ALS patients, including the increased DC in right orbital frontal lobe and decreased DC in the temporal lobe probably reflect the dysfunctions of the emotional regulation. The depression, anxiety, apathy, personality disorders, and scarce impulse control have been described in up to 60% of ALS patients (An et al., 2013; Yuan et al., 2013; Xu et al., 2014). The previous fMRI studies about ALS patients in a series of brain regions broadly related to emotional functions have reported both reduced and enhanced responses (Lulé et al., 2007; Agosta et al., 2013; Passamonti et al., 2013; Wang et al., 2013; Chiò et al., 2014). In the other study, ALS patients displayed the dysfunctions of the limbic system in ALS patients and the interplay between emotional and motor control brain areas (Passamonti et al., 2013). These findings in the limbic system demonstrate that emotional dysregulation may underlie the inappropriate reactions to emotional stimuli and the abnormalities in social and behavior functioning in ALS patients (Sui et al., 2013; Vargas et al., 2013).
In the present study, we found increased DC in the bilateral superior frontal gyrus and right orbital frontal lobe, and decreased DC in left middle cingulate cortex and the left precuneus, which are crucial nodes of the default mode network (DMN; Buckner et al., 2008). Some researchers detecting an impaired DMN in both the posterior cingulate and inferior parietal cortices and the anterior cingulate and medial prefrontal cortices in ALS patients (Mohammadi et al., 2009), while others detected no such observations (Tedeschi et al., 2012). All these discordance across studies may be owing to methodological differences in the fMRI analysis or inclusion of patients with different clinical and neuropsychological features (Agosta et al., 2013). Future studies with neuropsychological assessments are needed to evaluate whether an association exists between abnormal network DC and behavioral or neuropsychology disturbances in ALS.
There are several limitations in this study. First, this study has a major limitation related to the small sample size and unbalanced characteristics of patients. Different onset and involvement of upper motor neuron, bulbar motor neurons and spinal motor neurons at an early stage in ALS represents different endophenotypes of the disorder, and there is a need to recruit a larger group of ALS patients and clinically homogeneous group for long-term longitudinal observation in future studies. Identification of subgroups depending on the onset clinical symptoms would be useful to discriminate putative central differentiation patterns of altered networks. Second, lack of objective assessment of behavior or neuropsychology in ALS patients hampers our interpretations of the results. Further investigations should be focused on the relationship between neuropsychological deficits and abnormal network DC in the extra-motor areas.
In conclusion, the present work shows the importance of DC in exploring the whole-brain functional network reorganization in ALS, whose neurobiological mechanisms are only partially known. Overall, our findings confirm that the abnormal network DC in ALS patients was located in multiple brain regions including the primary motor, somatosensory, and extra-motor areas, supporting the concept that ALS is a multi-system disorder. Specifically, our study shows an interesting finding that DC in the visual areas was altered in patients. ALS patients with higher DC in right inferior occipital gyrus have more severity of disease and the altered DC value in right inferior occipital gyrus can probably be used to assess severity of ALS.
Author contributions
CZ: Project conception and execution; writing of the first draft. XH: Project conception, article review and critique. ML: MRI data acquisitions. XY: MRI data analysis. LC: Participants recruitment and clinical data acquisition. JH: Participants recruitment and clinical data acquisition. JZ: Project conception, article review and critique. JW: Project conception, article review and critique.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 81200882), Chongqing Provincial Natural Science Fund (Grant No. CSTC2011jjA0737, CSTC2012jjA10086).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agosta F., Canu E., Valsasina P., Riva N., Prelle A., Comi G., et al. (2013). Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging 34, 419–427. 10.1016/j.neurobiolaging.2012.04.015 [DOI] [PubMed] [Google Scholar]
- Agosta F., Valsasina P., Absinta M., Riva N., Sala S., Prelle A., et al. (2011). Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb. Cortex 21, 2291–2298. 10.1093/cercor/bhr002 [DOI] [PubMed] [Google Scholar]
- Agosta F., Valsasina P., Riva N., Copetti M., Messina M. J., Prelle A., et al. (2012). The cortical signature of amyotrophic lateral sclerosis. PLoS ONE 7:e42816. 10.1371/journal.pone.0042816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L., Cao Q. J., Sui M. Q., Sun L., Zou Q. H., Zang Y. F., et al. (2013). Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fMRI study. Neurosci. Bull. 29, 603–613. 10.1007/s12264-013-1353-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Beghi E., Logroscino G., Chiò A., Hardiman O., Mitchell D., Swingler R., et al. (2006). The epidemiology of ALS and the role of population-based registries. Biochim. Biophys. Acta 1762, 1150–1157. 10.1016/j.bbadis.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Miller R. G., Swash M., Munsat T. L. (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 1, 293–299. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., Schacter D. L. (2008). The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Krienen F. M., Yeo B. T. (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 16, 832–837. 10.1038/nn.3423 [DOI] [PubMed] [Google Scholar]
- Cedarbaum J. M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., et al. (1999). The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (Phase III). J. Neurol. Sci. 169, 13–21. 10.1016/s0022-510x(99)00210-5 [DOI] [PubMed] [Google Scholar]
- Chiò A., Pagani M., Agosta F., Calvo A., Cistaro A., Filippi M. (2014). Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol. 13, 1228–1240. 10.1016/S1474-4422(14)70167-X [DOI] [PubMed] [Google Scholar]
- Cole M. W., Pathak S., Schneider W. (2010). Identifying the brain's most globally connected regions. Neuroimage 49, 3132–3148. 10.1016/j.neuroimage.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Cox R. W., Hyde J. S. (1997). Software tools for analysis and visualization of fMRI data. NMR Biomed. 10, 171–178. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Zuo X. N., Kelly C., Grzadzinski R., Mennes M., Schvarcz A., et al. (2013). Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry 74, 623–632. 10.1016/j.biopsych.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Filippini N., Knight S., Talbot K., Turner M. R. (2011). Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain 134, 3470–3479. 10.1093/brain/awr279 [DOI] [PubMed] [Google Scholar]
- Fekete T., Zach N., Mujica-Parodi L. R., Turner M. R. (2013). Multiple kernel learning captures a systems-level functional connectivity biomarker signature in amyotrophic lateral sclerosis. PLoS ONE 8:e85190. 10.1371/journal.pone.0085190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. (2011). The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex 21, 145–154. 10.1093/cercor/bhq071 [DOI] [PubMed] [Google Scholar]
- Han J., Ma L. (2010). Study of the features of proton MR spectroscopy ((1)H-MRS) on amyotrophic lateral sclerosis. J. Magn. Reson. Imaging 31, 305–308. 10.1002/jmri.22053 [DOI] [PubMed] [Google Scholar]
- Jadach S., Ward B., Was Z. (2001). Coherent exclusive exponentiation for precision Monte Carlo calculations. Phys. Rev. D 63:113009 10.1103/PhysRevD.63.113009 [DOI] [Google Scholar]
- Jelsone-Swain L. M., Fling B. W., Seidler R. D., Hovatter R., Gruis K., Welsh R. C. (2010). Reduced interhemispheric functional connectivity in the motor cortex during rest in Limb-Onset amyotrophic lateral sclerosis. Front. Syst. Neurosci. 4:158. 10.3389/fnsys.2010.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Giel K. E., Teufel M., Thiel A., Zipfel S., Preissl H. (2014). Aberrant network integrity of the inferior frontal cortex in women with anorexia nervosa. Neuroimage Clin. 4, 615–622. 10.1016/j.nicl.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F., Frijia F., Bongioanni P., Canapicchi R., Minichilli F., Bianchi F., et al. (2009). Diffusion tensor MRI and MR spectroscopy in long lasting upper motor neuron involvement in amyotrophic lateral sclerosis. Arch. Ital. Biol. 147, 69–82. [PubMed] [Google Scholar]
- Lord L. D., Allen P., Expert P., Howes O., Lambiotte R., McGuire P., et al. (2011). Characterization of the anterior cingulate's role in the at-risk mental state using graph theory. Neuroimage 56, 1531–1539. 10.1016/j.neuroimage.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Lulé D., Diekmann V., Anders S., Kassubek J., Kübler A., Ludolph A. C., et al. (2007). Brain responses to emotional stimuli in patients with amyotrophic lateral sclerosis (ALS). J. Neurol. 254, 519–527. 10.1007/s00415-006-0409-3 [DOI] [PubMed] [Google Scholar]
- Lulé D., Diekmann V., Müller H. P., Kassubek J., Ludolph A. C., Birbaumer N. (2010). Neuroimaging of multimodal sensory stimulation in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 899–906. 10.1136/jnnp.2009.192260 [DOI] [PubMed] [Google Scholar]
- Luo C., Chen Q., Huang R., Chen X., Chen K., Huang X., et al. (2012). Patterns of spontaneous brain activity in amyotrophic lateral sclerosis: a resting-state FMRI study. PLoS ONE 7:e45470. 10.1371/journal.pone.0045470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meoded A., Morrissette A. E., Katipally R., Schanz O., Gotts S. J., Floeter M. K. (2015). Cerebro-cerebellar connectivity is increased in primary lateral sclerosis. Neuroimage Clin. 7, 288–296. 10.1016/j.nicl.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzapesa D. M., D'Errico E., Tortelli R., Distaso E., Cortese R., Tursi M., et al. (2013). Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PLoS ONE 8:e80748. 10.1371/journal.pone.0080748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto H., Ulug A. M., Pullman S. L., Gooch C. L., Chan S., Tang M. X., et al. (2007). Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 68, 1402–1410. 10.1212/01.wnl.0000260065.57832.87 [DOI] [PubMed] [Google Scholar]
- Mohammadi B., Kollewe K., Samii A., Krampfl K., Dengler R., Münte T. F. (2009). Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 217, 147–153. 10.1016/j.expneurol.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Münte T. F., Tröger M. C., Nusser I., Wieringa B. M., Johannes S., Matzke M., et al. (1998). Alteration of early components of the visual evoked potential in amyotrophic lateral sclerosis. J. Neurol. 245, 206–210. 10.1007/s004150050206 [DOI] [PubMed] [Google Scholar]
- Münte T. F., Tröger M. C., Nusser I., Wieringa B. M., Matzke M., Johannes S., et al. (1999). Abnormalities of visual search behaviour in ALS patients detected with event-related brain potentials. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 21–27. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., et al. (2005). The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J. S., Gustafson L., Passant U., Stuss D., Black S., et al. (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Orrell R. W. (2007). Understanding the causes of amyotrophic lateral sclerosis. N. Engl. J. Med. 357, 822–823. 10.1056/NEJMe078146 [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fera F., Tessitore A., Russo A., Cerasa A., Gioia C. M., et al. (2013). Dysfunctions within limbic-motor networks in amyotrophic lateral sclerosis. Neurobiol. Aging 34, 2499–2509. 10.1016/j.neurobiolaging.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Pradat P. F., El Mendili M. M. (2014). Neuroimaging to investigate multisystem involvement and provide biomarkers in amyotrophic lateral sclerosis. Biomed Res. Int. 2014:467560. 10.1155/2014/467560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell T., Grosskreutz J. (2013). The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 507–515. 10.3109/21678421.2013.812661 [DOI] [PubMed] [Google Scholar]
- Pyra T., Hui B., Hanstock C., Concha L., Wong J. C., Beaulieu C., et al. (2010). Combined structural and neurochemical evaluation of the corticospinal tract in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 11, 157–165. 10.3109/17482960902756473 [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Satterthwaite T. D., Elliott M. A., Gerraty R. T., Ruparel K., Loughead J., Calkins M. E., et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. W., Dong Z. Y., Long X. Y., Li S. F., Zuo X. N., Zhu C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 6:e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharshan N., Hanstock C., Hui B., Pyra T., Johnston W., Kalra S. (2011). Degeneration of the mid-cingulate cortex in amyotrophic lateral sclerosis detected in vivo with MR spectroscopy. AJNR Am. J. Neuroradiol. 32, 403–407. 10.3174/ajnr.A2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., He H., Yu Q., Chen J., Rogers J., Pearlson G. D., et al. (2013). Combination of resting state fMRI, DTI, and sMRI data to discriminate schizophrenia by N-way MCCA + jICA. Front. Hum. Neurosci. 7:235. 10.3389/fnhum.2013.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi G., Trojsi F., Tessitore A., Corbo D., Sagnelli A., Paccone A., et al. (2012). Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol. Aging 33, 886–898. 10.1016/j.neurobiolaging.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Telesford Q. K., Simpson S. L., Burdette J. H., Hayasaka S., Laurienti P. J. (2011). The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect. 1, 295–308. 10.1089/brain.2011.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N. D. (2011). Functional connectivity hubs in the human brain. Neuroimage 57, 908–917. 10.1016/j.neuroimage.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas C., López-Jaramillo C., Vieta E. (2013). A systematic literature review of resting state network–functional MRI in bipolar disorder. J. Affect. Disord. 150, 727–735. 10.1016/j.jad.2013.05.083 [DOI] [PubMed] [Google Scholar]
- Verstraete E., van den Heuvel M. P., Veldink J. H., Blanken N., Mandl R. C., Hulshoff Pol H. E., et al. (2010). Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS ONE 5:e13664. 10.1371/journal.pone.0013664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zuo X., He Y. (2010). Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 4:16. 10.3389/fnsys.2010.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Dai W., Su Y., Wang G., Tan Y., Jin Z., et al. (2012). Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS ONE 7:e48658. 10.1371/journal.pone.0048658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Han Z., He Y., Caramazza A., Song L., Bi Y. (2013). Where color rests: spontaneous brain activity of bilateral fusiform and lingual regions predicts object color knowledge performance. Neuroimage 76, 252–263. 10.1016/j.neuroimage.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Xu K., Liu H., Li H., Tang Y., Womer F., Jiang X., et al. (2014). Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. J. Affect. Disord. 152–154, 237–242. 10.1016/j.jad.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Yan C. G., Cheung B., Kelly C., Colcombe S., Craddock R. C., Di Martino A., et al. (2013a). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. G., Craddock R. C., Zuo X. N., Zang Y. F., Milham M. P. (2013b). Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. Neuroimage 80, 246–262. 10.1016/j.neuroimage.2013.04.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Jin C., Cheng P., Yang X., Dong T., Bi Y., et al. (2013). Amplitude of low frequency fluctuation abnormalities in adolescents with online gaming addiction. PLoS ONE 8:e78708. 10.1371/journal.pone.0078708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Jia X., Hou Z., Zang Y., Yuan Y. (2015). Frequency-dependent amplitude alterations of resting-state spontaneous fluctuations in late-onset depression. Biomed Res. Int. 2015:505479. 10.1155/2015/505479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Gong H., Li F., Zhuang Y., Zang Y., Xu R., et al. (2013). Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. Neuroreport 24, 657–662. 10.1097/WNR.0b013e328363148c [DOI] [PubMed] [Google Scholar]
- Zhou F., Xu R., Dowd E., Zang Y., Gong H., Wang Z. (2014). Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neurosci. Lett. 558, 192–196. 10.1016/j.neulet.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang Y., Rao L. L., Liang Z. Y., Chen X. P., Zheng D., et al. (2014). Disrutpted resting-state functional architecture of the brain after 45-day simulated microgravity. Front. Behav. Neurosci. 8:200. 10.3389/fnbeh.2014.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X. N., Ehmke R., Mennes M., Imperati D., Castellanos F. X., Sporns O., et al. (2012). Network centrality in the human functional connectome. Cereb. Cortex 22, 1862–1875. 10.1093/cercor/bhr269 [DOI] [PubMed] [Google Scholar]