Abstract
Studies of subsurface microorganisms have yielded few environmentally relevant isolates for laboratory studies. In order to address this lack of cultivated microorganisms, we initiated several enrichments on sediment and underlying basalt samples from North Pond, a sediment basin ringed by basalt outcrops underlying an oligotrophic water-column west of the Mid-Atlantic Ridge at 22°N. In contrast to anoxic enrichments, growth was observed in aerobic, heterotrophic enrichments from sediment of IODP Hole U1382B at 4 and 68 m below seafloor (mbsf). These sediment depths, respectively, correspond to the fringes of oxygen penetration from overlying seawater in the top of the sediment column and upward migration of oxygen from oxic seawater from the basalt aquifer below the sediment. Here we report the enrichment, isolation, initial characterization and genomes of three isolated aerobic heterotrophs from North Pond sediments; an Arthrobacter species from 4 mbsf, and Paracoccus and Pseudomonas species from 68 mbsf. These cultivated bacteria are represented in the amplicon 16S rRNA gene libraries created from whole sediments, albeit at low (up to 2%) relative abundance. We provide genomic evidence from our isolates demonstrating that the Arthrobacter and Pseudomonas isolates have the potential to respire nitrate and oxygen, though dissimilatory nitrate reduction could not be confirmed in laboratory cultures. The cultures from this study represent members of abundant phyla, as determined by amplicon sequencing of environmental DNA extracts, and allow for further studies into geochemical factors impacting life in the deep subsurface.
Keywords: deep biosphere, bacteria, cultivation, genome, amplicon
Introduction
The marine environment harbors numerous uncultivated microbes with estimates of culturable marine bacteria around 0.01 to 0.1% of cells present (Kogure et al., 1979; Connon and Giovannoni, 2002). The “great plate count anomaly” (Staley and Konopka, 1985) extends into the marine deep biosphere, yet there is still a need for cultivation-based research to better fill critical knowledge gaps, transforming our understanding of the biogeochemical impacts in the deep biosphere (Parkes et al., 2009; Fichtel et al., 2012; Lomstein et al., 2012; Lever et al., 2013; Ciobanu et al., 2014). Cultivated microbes from the deep marine subsurface have often come from anoxic sediments, including anaerobes such as Desulfovibrio profundus (Bale et al., 1997) and Methanoculleus submarinus (Mikucki et al., 2003). Facultative anaerobes (Shewanella profunda, Toffin et al., 2004) and numerous aerobes have been retrieved from anaerobic sediments (D’Hondt et al., 2004; Biddle et al., 2005). Overall, most isolates from the deep biosphere have poor environmental context to date, and few isolated aerobes have originated from aerobic sediments.
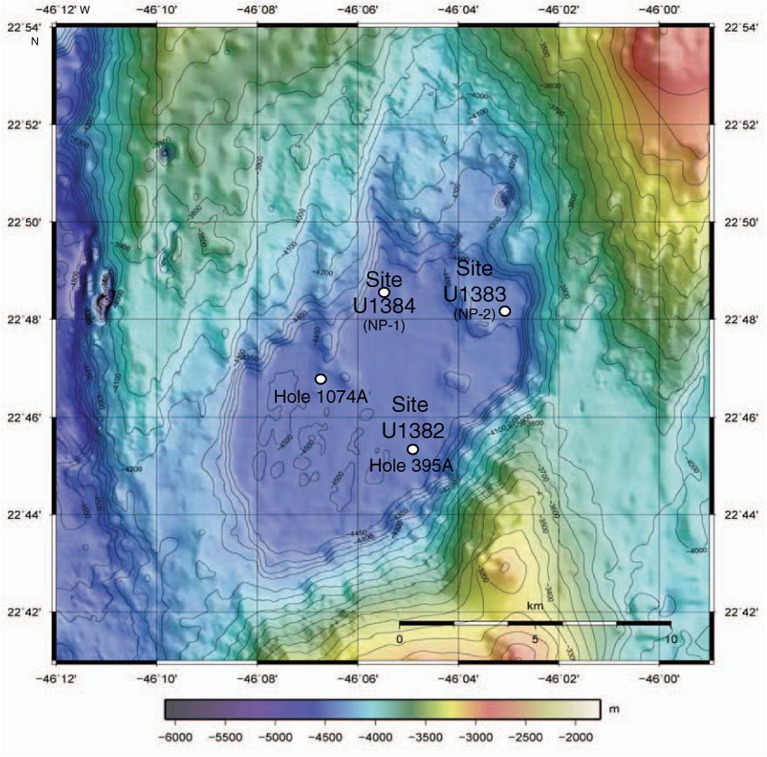
The collection of deep biosphere sediment and basalt via drilling during International Ocean Drilling Program (IODP) Expedition 336 to North Pond offered an opportunity to initiate cultivation studies across several subsurface environments: young aerobic sediments, older anoxic sediments, still older aerobic sediments, and 7-million-year-old ridge flank basalt (Expedition 336 Scientists, 2012). North Pond is a sediment-filled basin between exposed basalt outcrops, where temperature differences exist between warmer out-crops closer to tectonic activity at the Mid-Atlantic Ridge and colder outcrops further away at the southwest section of the basin (Langseth et al., 1992; Edwards et al., 2012). In the colder, southwest corner of the basin, Site U1382 was drilled to a depth of 210 m below seafloor (mbsf), including 90 m of sediment from Hole U1382B and 120 m of basement from Hole U1382A (Expedition 336 Scientists, 2012; Figure 1).
FIGURE 1.
Bathymetric map of North Pond in the Atlantic Ocean, showing drill sites (from Expedition 336 Scientists, 2012). Isolates in this paper are from Site U1382, Hole U1382B and enrichments were also started from Hole U1382A, Site U1383 (Holes U1383C and U1383E) and Site U1384 (Hole 1384A; Supplementary Table S1). Sites from IODP Expedition 336 are shown (Site #, NP #), as are holes previously drilled in the area (DSDP Hole 395A, ODP Hole 1074A).
At Site U1382, oxygen penetrated the sediment to approximately 4 mbsf and returned at approximately 68 mbsf due to flow from the underlying basalt (Orcutt et al., 2013). In addition to oxygen, other electron acceptors such as nitrate are reported (Edwards et al., 2012; Wankel et al., 2015). While electron acceptor concentrations are similar at these depths, the buried organic carbon at 68 mbsf is over 6 million years older than at 4 mbsf and has presumably undergone some level of remineralization given that oxygen is used up in the middle of the sediment column (Burdige, 2007). Currently, our knowledge of oxygen- and nitrogen-cycling heterotrophy in North Pond sediments are based on modeling of nitrogen isotopes and the examination of sediment slurries with labeled carbon substrates (Picard and Ferdelman, 2011; Wankel et al., 2015). Physical characteristics of the sediment that enhance the delivery of nutrient/electron-acceptor rich pore-water are more important than indigenous carbon substrates for constraining heterotrophy (Picard and Ferdelman, 2011). Active nitrogen cycling in North Pond sediments does not follow canonical patterns of redox separation between consumption and regeneration of nitrate, with overlapping zones of aerobic (nitrification) and anaerobic (denitrification) processes (Wankel et al., 2015). North Pond sediments are likely to be representative of a majority of the global seafloor, as oligotrophic water columns such as those overlying North Pond comprise a majority of the global ocean (D’Hondt et al., 2009).
Here we report the enrichment, isolation, initial characterizations and genomes of three isolated aerobic heterotrophs from North Pond sediments; an Arthrobacter species from 4 mbsf, and Paracoccus and Pseudomonas species from 68 mbsf. Dissimilatory nitrate reduction could not be induced under laboratory conditions of all three isolates, however, genomic data suggests a metabolic capability for denitrification in the Arthrobacter isolate from 4 mbsf and Pseudomonas isolate from 68 mbsf at U1382B, depths where we expect this metabolism to be occurring (Wankel et al., 2015). Based on amplicon sequencing abundances, these isolates are rare members of abundant phyla in the environment. To our knowledge, these are the first aerobic isolates reported from oxygenated deep marine sediments.
Materials and Methods
Sampling
Samples were acquired from the scientific drilling vessel JOIDES Resolution during IODP Expedition 336 in November, 2011 (Figure 1). Samples were taken from Holes U1382A, U1382B, U1383C, U1383E, and U1384A (Supplementary Table S1). Whole round core sections were sampled at 10 m intervals when available. Samples for DNA extraction were immediately stored at -80°C and kept at this temperature for the remainder of the cruise and during shipping to shore-based laboratory. Samples for cultivation were stored at 4°C for no longer than 2 h before being inoculated into enrichment media. Enrichment media was prepared from literature-sourced recipes to attempt to cultivate aerobic heterotrophs (GYPS; Burgaud et al., 2009), sulfate reducers (Pfennig et al., 1981), methanogens (Wolfe et al., 2011), sulfide oxiders (Nelson et al., 1986), iron reducers (Wrighton et al., 2011) iron oxidizers/nitrate reducers (Straub and Buchholz-Cleven, 1998), Mn oxiders (Krumbein and Altmann, 1973), and Mn reducers (Burdige and Nealson, 1985) from both sediments and basalts. Numerous enrichments were prepared across different samples and depths with negative controls of uninoculated broth (Supplementary Table S1). However, confirmed growth was only observed in the aerobic heterotroph enrichments from sediments taken by Advanced Piston Coring (APC) at Site U1382B at 22°45.353′N, 46°04.891′W; as such, this is the only enrichment we will report in detail.
Culture Media, Growth Conditions, and Growth Measurements
Original GYPS media for aerobic heterotrophs was prepared in 1 L batches onboard the JOIDES Resolution (JR) and consisted of the following; 1 g each of glucose, peptone, starch, and yeast extract, 1 L filtered/autoclaved seawater (Burgaud et al., 2009). Media was then autoclaved for 1 h. After autoclaving and allowing 30 min for cooling, 5 mL Wolfe’s vitamins and 5 mL Wolfe’s minerals was added and 40 mL was aseptically dispensed in autoclaved 60 ml glass vials and capped with rubber septa, which were removed during sample addition and every week after to reoxygenate the media. This dispensing, and all sediment additions, were performed in the sterile flow hood aboard the JR. The top 3–5 mm of sediment from each whole round core section was removed with a flame-sterilized spatula to reduce any potential drilling contamination and 3 cubic centimeter samples were taken from the center of the core using sterilized cut-off syringes. Care was taken to sample from the interior of the core to avoid sampling sediment contaminated with seawater during the drilling process. This drilling-induced seawater contamination was monitored using the fluorescent microsphere method, as described previously (Smith et al., 2000). Microsphere data for sediment horizons from which isolates derived is <1 microsphere per field of view for interior core samples, indicating a very low risk that isolates originated from seawater (Expedition 336 Scientists, 2012). Basalt whole-round core samples were broken into smaller pieces with a flame-sterilized chisel in a flame-sterilized steel rock processing box. After processing, 2–3 g of basalt chips were used as inoculum in enrichment media. The sediment and basalt subsamples were immediately inoculated into enrichment vials aerobically and the vials were capped with rubber septa and stored at 4°C. Growth was observed by turbidity of media from sediment samples from 4 and 68 mbsf (section 1 and 8H) within 2 days and transfers into new media were done every 5 days for the remainder of the cruise. At the end of the cruise, culture vessels were crimp-sealed for shipping to the Biddle Lab at 4°C. Once on shore, enriched heterotrophs were plated on Difco Marine Agar 2216 plates and placed at 10°C under aerobic conditions. Individual colonies were picked and streaked on new plates in five successive rounds of isolation at 10°C. Culture media for anaerobic growth on was composed of the following, in 500 mL dH2O; 0.75 g of KH2PO4, 0.15 g of NH4Cl, 1 g of KNO3, 5 mM (NH4)2Fe(SO4)2.6H2O, 0.5 mg resazurin, 4% w/v Sigma sea salts, 10 mM glucose, 1 g peptone (Samuelsson, 1985). Culture media for Mn(II) oxidation was prepared as described previously (Krumbein and Altmann, 1973), in 1 L; 75% natural seawater, 25% dH2O, 20 mM HEPES, 2 g peptone, 0.5 g yeast extract, 1 mM MnCl2 (Templeton et al., 2005). Transfers were performed by growing isolates to late log phase in Difco Marine Broth 2216C at 10°C and 100 μl of this culture was used as inoculum to 40 mL of and 40 mL of Mn(II) oxidizing media (Krumbein and Altmann, 1973) in 60 ml glass vials, capped with rubber septa. Cultivation on nitrate and manganese media was attempted three times for each isolate. Growth temperature tests were initially performed at 4, 12, 22, 37, 42, and 50°C on solid Difco Marine 2216C agar media. Due to equipment limitations on shaking incubators, we only measured growth rates at temperatures starting at 12°C. For growth rate measurements, cells were grown in liquid Difco Marine Broth with shaking and 500 μl timepoints were taken to measure optical density in reference to uninoculated media at 600 nm wavelength on a Nanodrop 2000c (ThermoFisher Scientific). Glycerol stocks of each isolate were prepared and frozen at -80°C for long term archiving. They are available to researchers upon request.
DNA Extractions and Sequencing
Isolates were grown to late stationary phase in 5 ml of media. Cultures were centrifuged for 10 min at 4000 rpm to generate a cell pellet. The cell pellet for each isolate was re-suspended in 500 μl dH2O and DNA was extracted using the UltraClean Microbial DNA Isolation Kit from MoBIO Laboratories, Inc. (Carlsbad, CA, USA). Full-length 16S rRNA amplicons were generated with bacterial primers 8F/1492R (Eden et al., 1991) with the following PCR protocol; 94°C for 2 min, (94°C for 30 s, 59°C for 1 min, 68°C for 2 min) × 30 cycles, 68°C for 5 min, then cloned with the TOPO TA Cloning Kit for Sequencing (Life Technologies Inc., Grand Island, NY, USA). Ten clones per isolate were picked and sent for Sanger sequencing at GeneWiz Inc., (South Plainfield, NJ, USA) to confirm culture purity. Full length 16S rRNA DNAs are deposited under NCBI accession numbers KU707917–KU707919. Genomic DNA was sent to the United States Department of Energy’s Joint Genome Institute for full genome sequencing. Isolate genomes and raw sequencing data are available from the GOLD database via accession numbers: Pseudomonas stutzeri NP_8HT: Gp0114906, Arthrobacter subterraneus NP_1H: Gp0115197, Paracoccus sp. NP_8HO: Gp0114905 and under NCBI project ID: 303658. For total microbial community analysis at both depths (4 and 68 mbsf), 10 g of sediment from each depth was processed with the PowerMax Soil DNA Isolation Kit from MoBio Laboratories, Inc. (Carlsbad, CA, USA). Total bacterial community was amplified from 2 ng template DNA using 16S rRNA primers 27F/338R via PCR protocol: 95°C for 2 min, (95°C for 30 s, 60°C for 1 min, 72°C for 2 min) × 35 cycles, 72°C for 5 min. An extraction blank was processed with the samples. All PCR products, including the one from the extraction blank, were sent for Illumina library prep and sequencing at Next Generation Sequencing Services (Shallowater, TX, USA). This involved a nested PCR approach, resulting in a minimal number of cycles occurring after initial PCR amplification, which may slightly reduce the diversity of the environmental samples. Amplicon sequencing data is available under NCBI accession numbers SRS1277883–SRS1277884.
Sequence Data Processing and Analysis
The raw data from 16S rRNA gene amplicon Illumina reads were converted to a fastq file in QIIME version 1.8 (Caporaso et al., 2010) with the convert_fastaqual_fastq.py script. Primers and barcodes were extracted with the extract_barcodes.py script and sequences were de-multiplexed via split_libraries_fastq.py and an in-house perl script. 1,846 operational taxonomic unit (OTUs) were shared between the extraction blank and the section 1H sample. 1,446 OTUs were shared between the extraction blank and the section 8H sample. The most abundant taxa associated with these OTUs were from families often observed as common kit contaminants, such as Propionibacteriaceae, Enterobacteriaceae, Ralstoniaceae, and Bradyrhizobiaceae (Salter et al., 2014). Several proteobacterial OTUs, including Pseudomonas and Paracoccus genera, were observed in the extraction blank. No Arthrobacter-related OTUs were observed in the blank. All OTUs shared between samples and the extraction blank were removed before further analysis. Sequences were then uploaded to the SILVA NGS pipeline1 (Quast et al., 2013) where default quality-trimming parameters were used, sequences were aligned with the SINA aligner (Pruesse et al., 2012), and OTU clustering was performed at 98% sequence identity. Taxonomy was called against the SILVA database (Quast et al., 2013). OTUs identified in the extraction blank sample that were also seen in sediment samples were removed from further analysis. The resulting OTU table was used for creation of taxonomy figures. Full-length 16S rRNA gene amplicon data was trimmed in Sequencher v.5.0.1 (GreenCodes Corp., Ann Arbor, MI, USA), sequences aligned with the online SINA aligner2 and manually refined within ARB (Ludwig et al., 2004). Phylogenetic analysis was performed using ARB via a neighbor-joining method with 1000 bootstrap replicates on the highest quality clone sequence from each isolate. Genomes were quality trimmed using Nesoni3. We then assembled these genomes using SPAdes 3.1.1 in multi cell mode (Bankevich et al., 2012). The – – careful flag was used to reduce the number of mismatches and short indwells and to run error correction in the assembly. The assembly iteratively used kmers 21, 33, 55, and 77. PHRED quality offset for the input reads was set at 33. During assembly and initial annotation, we found that the raw data for the Pseudomonas isolate also included the Paracoccus genome. Therefore, after assembly by SPAdes, we separated the genomes using MaxBin (Wu et al., 2014), evaluated bin validity with VizBin (Laczny et al., 2015) and CheckM (Parks et al., 2015) and deposited the new genomes in the integrated microbial genomes (IMG) database (Markowitz et al., 2012; Accession numbers 74356, 79457, and 79456; Table 1). Genomes were annotated by Prokka (Seeman, 2014). Genes for nitrogen cycling were extracted from the Prokka output and annotations were double checked via manual BLAST analysis against the NCBI nr database4 and the Interpro protein domain database5. Additional genes found via IMG annotation were also added. Proteins annotated as active subunits of significant enzymes were aligned with close relatives of the nr database via MUSCLE (Edgar, 2004) and visually inspected and trimmed with Jalview software (Waterhouse et al., 2009). Trees were made using maximum likelihood in FastTree (Price et al., 2009). Carbohydrate active enzymes were identified by comparison with the Carbohydrate Active Enzymes database6 (Cantarel et al., 2009) via pFAM scan (Mistry et al., 2007).
Table 1.
Genome statistics of three heterotrophic aerobic isolates from sediment of Hole U1382B collected during IODP Expedition 336.
| Organism | Sediment depth (mbsf) | Genome size (Mb) | Contigs∗ | Number of predicted genes | %GC | % completeness∗ |
|---|---|---|---|---|---|---|
| Arthrobacter sp. NP1H | 4 | 3.90 | 99 | 3674 | 65 | 99.1 |
| Pseudomonas sp. NP8HT | 68 | 4.74 | 24 | 4353 | 60 | 100.0 |
| Paraccocus sp. NP8HO | 68 | 3.32 | 21 | 3193 | 68 | 99.7 |
∗Reported statistics are based on contigs >500 bp, completeness from CheckM calculation.
Results and Discussion
Isolation and Phylogeny of Heterotrophs
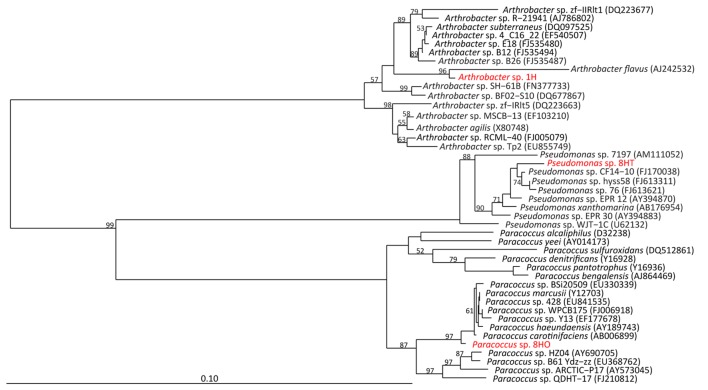
Only the heterotrophic enrichments yielded observable growth from these deep samples, despite the media being quite high in carbon compared to the in situ environment (Edwards et al., 2012). A single colony morphotype was observed on Difco marine agar media during successive rounds of isolation from the 4 mbsf sample (section 1H); a light tan color with moderate EPS production. Two separate morphologies were identified from the 68 mbsf sample (section 8H). The first colony morphotype was light tan colored with moderate EPS production. The second was a bright orange color colonies with low EPS production. Full-length 16S rRNA gene sequencing indicates the section 1H isolate as an Arthrobacter species from the Micrococcaceae within the Actinobacteria, the orange 8H isolate as a Paracoccus species from the Rhodobacteraceae within the Alphaproteobacteria, and the tan 8H isolate as a Pseudomonas species from within the Gammaproteobacteria (Figure 2). The isolates were all related to species found previously in the subsurface and sediments.
FIGURE 2.
Neighbor-joining tree of full length 16S rRNA gene sequences of isolates (red) to nearest database neighbors (black), based on 1000 bootstrap replicates. Bootstrap values >50% are shown.
The section 1H Arthrobacter sp. isolate was closely related to Arthrobacter species retrieved from deep subsurface water in South Korea (A. subterraneus) and Himalayan ice (Arthrobacter sp. zf-IIRIt1), as well as marine sediment off Svalbard, Norway (Arthrobacter sp. SH-61B; Figure 2). The closest relative (96.7% 16S rRNA gene identity) is A. flavus, a psychrophile isolated from a cyanobacterial mat in a pond in the McMurdo Dry Valley, Antarctica (Reddy et al., 2000). This isolate is the most novel based on distance from known relatives, in terms of 16S rRNA identity of the three we cultivated. It had a typical rod/coccus morphology depending on growth stage, with diameters ranging from 0.9 to 2 μm.
Clone A45 from the orange 8H isolate branched close to Paracoccus species previously isolated from Korean salt marsh sediments (Paracoccus sp. HZ04), Chinese salt deposits (Paracoccus sp. Y13), and deep sediments of the Arctic Ocean (Paracoccus sp. BSi20509). It is most closely related (99.2% 16S rRNA gene identity) to Paracoccus carotinifaciens, an aerobic, orange-pigmented, Gram-negative, rod-shaped microbe originally isolated from soil (Tsubokura et al., 1999; Figure 2). Our isolate stained gram-negative and exhibited a primarily coccoid shape (average diameter of 0.85 μm) though some rod shapes were observed, indicating a potential for growth related morphological changes.
Clone A25 from the tan 8H isolate branched close to Pseudomonas species previously isolated from South China Sea sediments (Pseudomonas sp. CF14-10), sediments of the southwest Pacific Ocean (Pseudomonas sp. 76, Pseudomonas sp. hyss58), and its closest relative was isolated from East Pacific Rise hydrothermal sediments, Pseudomonas sp. EPR 12 (Vetriani et al., 2005; 99% 16S rRNA gene identity; Figure 2). The isolate was rod-shaped and approximately 0.9 to 1.5 μm long.
Amplicon Sequencing from Enviromental Samples
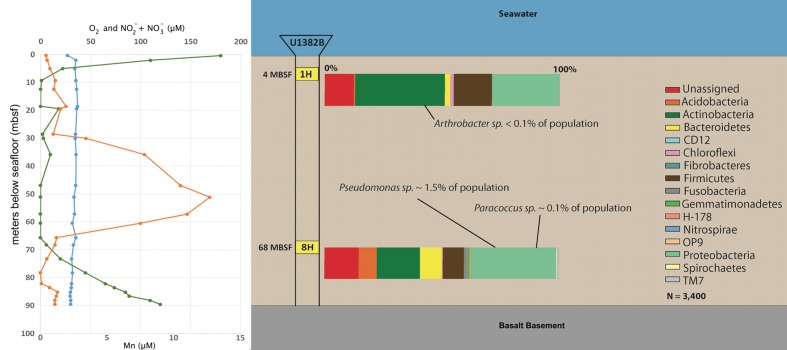
To determine relative abundance of our isolates in the context of the greater microbial community from which they were collected from, we generated 16S rRNA gene amplicon libraries from frozen samples of sections 1 and 8H (Figure 3). Our enrichments had generated isolates from the Actinobacteria in the shallow sample (1H) and Proteobacteria from the deeper sample (8H). Consistent with the enrichments, the Actinobacteria and Proteobacteria were the dominant phyla represented in 1 and 8H microbial communities. Actinobacteria, which includes Arthrobacter, were 36.7 and 14.8% of the population at 1 and 8H, respectively. Proteobacteria were 29.1 and 29.4% of the population at 1 and 8H, respectively. However, the isolates were in extremely low abundance with Arthrobacter sp. 1H comprising <0.1% of the population at section 1H (Table 2). At section 8H, Paracoccus sp. 8HO was 0.1% of the population and Pseudomonas sp. 8HT was 1.5% (Figure 3; Table 2). Since the primers used in this analysis were much less discriminatory than those used to verify the identity of the isolates (310 and 1484 bp PCR products, respectively, whereas full length 16S rRNA gene primers were used for isolate identification), and this involved a nesting priming approach, the abundances could increase if longer sequences were clustered for the environmental sample. Considering the relationship to other subsurface and sediment relatives that were also isolated, these organisms may not be numerous in these environments, but widely disbursed across these environments and respond well to cultivation. The same OTUs are found at different depths (Table 2), which correspond to different ages of sediment. Notably, the Arthrobacter is not found at 68 mbsf, which corresponds with the overall decrease of members of Actinobacterium phylum at depth (Figure 3). We presume that organisms are deposited at the surface of the sediment column, and that the Actinobacter may not survive the lowest oxygen portion of the sediment column as well as the Proteobacteria, which results in the abundance shift at depth (Table 2; Figure 3).
FIGURE 3.
Down-core O2 (green), Mn (orange), and NO3 + NO2 (blue) concentrations (from Expedition Expedition 336 Scientists, 2012 and Orcutt et al., 2013) and 16S rRNA gene taxonomic profile, by phyla, for bacteria in frozen sediments of sections 1H (4 mbsf) and 8H (68 mbsf). Each amplicon sample was rarified to 3400 sequences for analysis. Notations regarding the percentage of isolate signatures are listed (Table 2).
Table 2.
Abundances of isolate small subunit ribosomal (rRNA) signatures in situ.
| Depth (mbsf) | Arthrobacter isolate | Pseudomonas isolate | Paracoccus isolate |
|---|---|---|---|
| 4 | 3 (0.001) | 67 (0.025) | 6 (0.002) |
| 68 | 0 | 52 (0.02) | 3 (0.001) |
First number is amount of sequences detected. Percent abundance is indicated in parentheses.
Cultivation Responses of Cultivated Bacteria
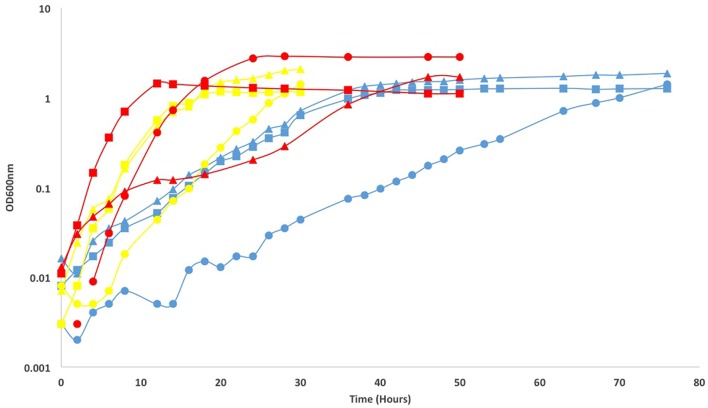
The response of the isolates to varying temperatures (4, 12, 22, 37, 42, and 50°C) was measured on Difco marine broth agar, using organic carbon as an electron donor and O2 as an electron acceptor. For all three isolates, growth was very slow but detected at 4°C. No growth was detected at 50°C. Slow growth at 42°C was only seen for the Pseudomonas sp. and Arthrobacter sp.. Growth rates were measured for three temperatures using equivalent methods across temperatures (12, 22, and 37°C). The Arthrobacter sp. had the highest growth rate at 37°C (0.36 h-1), as did the Pseudomonas sp. (0.48 h-1). The Paracoccus isolate had the highest growth rate at 22°C (0.24 h-1; Figure 4). Growth across a wide range of temperatures has been seen for previous isolates of deep subsurface heterotrophs (Biddle et al., 2005). Additionally, we saw low temperatures induce the production of extra polymeric substances, or EPS, which can be seen in the growth curves that do not plateau (Figure 4). This response may be induced in situ, which may allow greater survival in an environment such as North Pond, as the development of EPS around a cell can allow for carbon resources to be consumed in the oligotrophic conditions of a sediment core, and also allow for maintained water availability as the deeper sediments are under greater pressure and typically have less pore fluid than shallow ones (Marx et al., 2009).
FIGURE 4.
Growth curves and calculated growth rates in parentheses (h-1) of North Pond isolates Paracoccus sp. 8HO (triangles), Arthrobacter sp. 1H (circles) and Pseudomonas sp. 9HT(squares) at 12°C (blue), 22°C (yellow), and 37°C (red). Paracoccus sp. 8HO growth rates were 0.12, 0.24, and 0.06, per hour, at 12, 22, and 37°C, respectively. Arthrobacter sp. 1H growth rates were 0.06, 0.24, 0.36 and Pseudomonas sp. 8HT growth rates were 0.06, 0.24, and 0.48. Curves without a plateau, seen in Paracoccus sp. 8HO at 12°C may be caused by a build up in EPS material, not cell division, since cultures became sticky with increasing time, resulting in difficulty pipetting. This was not confirmed by direct cell counts.
Given the reasonably high pore water concentrations (up to 35 μM) at 4 and 68 mbsf at U1382B and the very low O2 concentration (under 20 μM) at these depths (Figure 3, Expedition 336 Scientists, 2012; Orcutt et al., 2013), we tested growth of isolates in anaerobic, heterotrophic, nitrate-reducing media. Also tested was fermentation capability, with the same media as for nitrate-reduction, without nitrate amendment. No growth was observed in either anaerobic media. Our findings are not consistent with the documented nitrate-reducing capabilities of all three isolate genera (Alefounder et al., 1983; Carlson and Ingraham, 1983; Eschbach et al., 2003). An anomalous sharp peak in Mn(II) was measured in porewaters of sediments from just below 50 mbsf at Hole U1382B (Figure 3). Given that the Pseudomonas and Paracoccus isolates originated near the Mn(II) peak at U1382B, we also evaluated our isolates for the capacity to aerobically oxidize Mn(II). Members of the Actinobacteria and Pseudomonas are known to oxidize Mn(II) (Okazaki et al., 1997; Tebo et al., 2005). Growth was observed from all three isolates, but no Mn-oxides were visually precipitated, indicating a lack of Mn-oxidation capability. These tests were performed after the isolates had been in culture for over a year and thus it is possible that Mn-oxidation capacity was lost as previously reported (Gregory and Staley, 1982).
Genome Sequencing of Cultivated Bacteria
Isolates genomes were sequenced to validate our physiological data and compare these subsurface isolates to surface relatives. The Arthrobacter draft genome assembled into 99 contigs, with a total genome size estimated at 3.9 Mb with a GC content of 65% (Table 1). The Pseudomonas isolate genome assembled into 24 contigs, with an estimated genome size of 4.7 Mb and a GC content of 60% (Table 1). The Paracoccus genome assembled into 21 contigs, with an estimated genome size of 3.3 Mb and a GC content of 68% (Table 1). All genomes have over 99% completeness, based on the detection of marker genes, and are representative of only one organism. Based on average nucleotide identity (ANI) of equal to or greater than 90% ANI with other genomes, two of these genomes formed cliques with other marine and extreme environment isolates. The Paracoccus genome formed a genome clique with Paracoccus sp. 361 (Gp0119386) from the Baltic Sea; the Arthrobacter formed a clique with Arthrobacter sp. 35/47 (Ga0012047) from Antarctic soils. The Pseudomonas isolate did not form any cliques, which is typical for this group of microbes, despite the high number of closely related genomes based on ribosomal sequences (25 complete or draft genomes in IMG; Markowitz et al., 2012; database accessed on December 28, 2015).
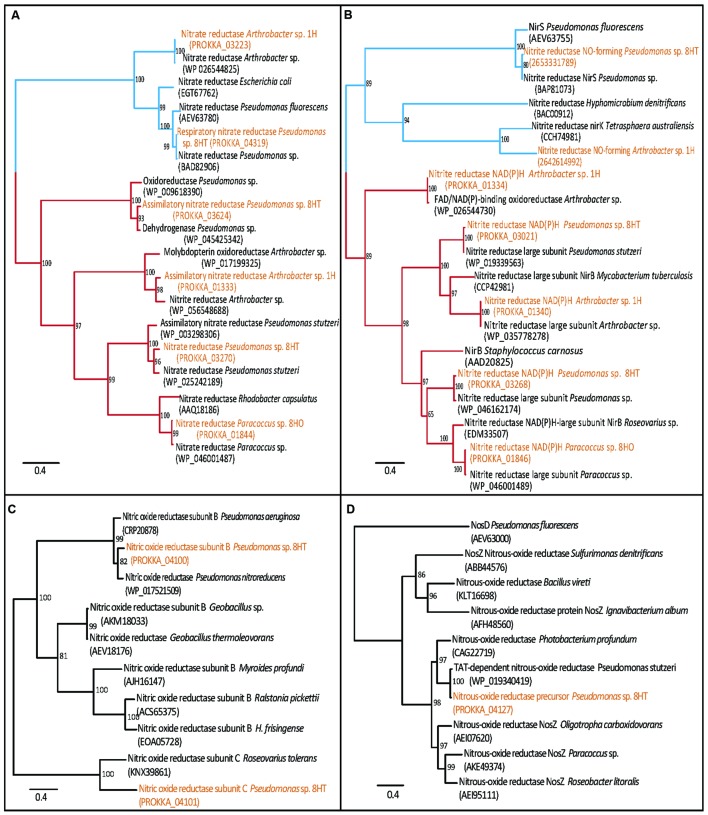
The Arthrobacter sp. 1H isolate from 4 mbsf possessed a denitrifying nitrate reductase gene cluster (Supplementary Table S2; Figure 5). Specifically, narG, narH, narJ, and narI were all detected, suggesting a capability for the dissimilatory reduction of nitrate () to nitrite (NO2-). This genome also possesses the genes for nitrite reductase (NADH) small and large subunits (nirD and nirB, respectively), completing the dissimilatory reduction pathway to ammonia. The nitrite reductase NO-forming enzyme (nirK) is also encoded within the genome, which performs the second step in the denitrification pathway to nitrogen. The ability to reduce nitrate is not widespread in the Arthrobacter genus, with only 8 of the 75 available full to draft genomes available in IMG encoding similar genes, including this North Pond isolate (Markowitz et al., 2012; database accessed on December 28, 2015). Previous detection of nitrate reduction in Arthrobacter noted the difficulties of getting the cells to activate the denitrification pathway in culture, and our cultivation setup may not have enabled the shift of the cells into denitrification (Eschbach et al., 2003). Besides the ability of dissimilatory reduction of nitrate, the Arthrobacter isolate also encodes genes for the assimilatory nitrate reductase enzymes (the catalytic nasA and electron transfer nasB subunits). Alcohol dehydrogenase and lactate dehydrogenase were present, but we were unable to map the entire mixed acid fermentation previously proposed for A. globiformis (Eschbach et al., 2003). In general, the genome showed a high degree of auxotrophy, unable to biosynthesize 6 amino acids and coenzyme A, which may explain why this organism in particular was the one isolated from the enrichment procedure in rich media. No genes for motility or urease were observed.
FIGURE 5.
Maximum-likelihood trees of nitrogen cycle proteins present in genomes, with dissimilatory branches in blue and assimilatory branches in red. (A) Nitrate reductase; showing Arthrobacter sp. 1H and Pseudomonas sp. 8HT have dissimilatory nitrate reductase, narG, and also assimilatory types (Pseudomonas sp. 8HT has two separate types). Paracoccus sp. 8HO and Arthrobacter sp. 1H each have one type of assimilatory nitrate reductase. (B) Nitrite reductase; showing all isolates have assimilatory type nitrite reductases. (C) Nitric oxide reductase; only the Pseudomonas sp 8HT isolate contains one, subunits (B) and (C) are shown (D) Nitrous oxide reductase; only found in Pseudomonas sp. 8HT. Proteins found in the genomes are highlighted in orange. Confidence values are located at the branch nodes.
The Paracoccus sp. 8HO genome contained multiple genes involved with nitrate processing including nasA, nasE, nasD and regulation genes such as nirQ (Supplementary Table S2). It contains the genes for nitrite reductase (NADH) small and large subunits (nirD and nirB, respectively) to ammonia. Although P. denitrificans is the canonical organism for the study of denitrification in this group and many other Paracoccus species can denitrify via the nap pathway, we did not find good genomic evidence of a dissimilatory nitrate reduction pathway, and interpret the nitrate reduction genes seen as playing a mostly assimilatory role (Supplementary Table S2; Figure 5; Alefounder et al., 1983). Considering our inability to activate dentrification activity in culture, along with the genomic evidence, we presume this organism is not capable of conserving energy through nitrate reduction. This genome was also auxotrophic for eight amino acids, perhaps explaining its success in complex media cultivation and contained genes for α, β, γ urease subunits and formate dehydrogenase, suggesting that ammonia recycling and potential for anaerobic activity exist. This genome contained 44 genes from KEGG pathways for cell motility, including genes for flagellar assembly and chemotaxis (Che A, B, D, W, X, Y). Motility requires a high level of electron flow for its operation, and while motility has been suggested in the subsurface (Orsi et al., 2013), it is unknown if the system would operate in this organism in situ.
The genome of the Pseudomonas sp. 8HT isolate from 68 mbsf contained the nitrate-reducing genes seen in Paracoccus sp. 8HO, as well as those genes necessary for reduction of NO to N2O (norE, norF, norC, norB, norQ, and norD), and N2O to N2 (nosR, nosZ, nosD, nosF, nosY, and nosL) and periplasmic nitrate reductase genes napA and napB (Supplementary Table S2; Figure 5). P. stutzeri is a well-known denitrifying bacterium, and this isolate genome contains the transcriptional regulator, NirQ, suggesting activity should be able to be stimulated in proper growth conditions (Supplementary Table S2; Berks et al., 1995). We anticipate that further lab work would be able to induce the expression of these genes in laboratory culture, but for now, the presented genomic information is the only evidence of potential dissimilatory nitrate reduction. Genes are present for β, γ urease subunits (not the catalytic α subunit), formate dehydrogenase and lactate dehydrogenase, suggesting as with Paracoccus that potential for ammonia recycling and anaerobic activity exist. The genome is also auxotrophic for 12 amino acids and coenzyme A biosynthesis, which may be why it grew in complex cultivation media. Over 117 genes were mapped to cellular motility pathways, including multiple copies of chemotaxis proteins and flagellar synthesis, as stated before, it is unknown if these would be used in situ, however, they could be advantageous for finding food in a limited physical environment.
We investigated the three genomes for genes known to be involved in manganese oxidation, considering its increase in this environment (Figure 3), searching for genes previously implicated in the process (Brouwers et al., 1999; Dick et al., 2006, 2008). There were no genes annotated as mnxG, and at first glance, none annotated as cumA. However, when we investigated for more general multicopper oxidase annotations, we did find a gene in the Pseudomonas isolate genome that was 96% similar to CumA-like oxidase, which is suggested to be the gene responsible for Mn oxidation in P. putida (Brouwers et al., 1999). Considering the negative result in the culture screening, we cannot confirm this gene confers the ability to oxidize manganese.
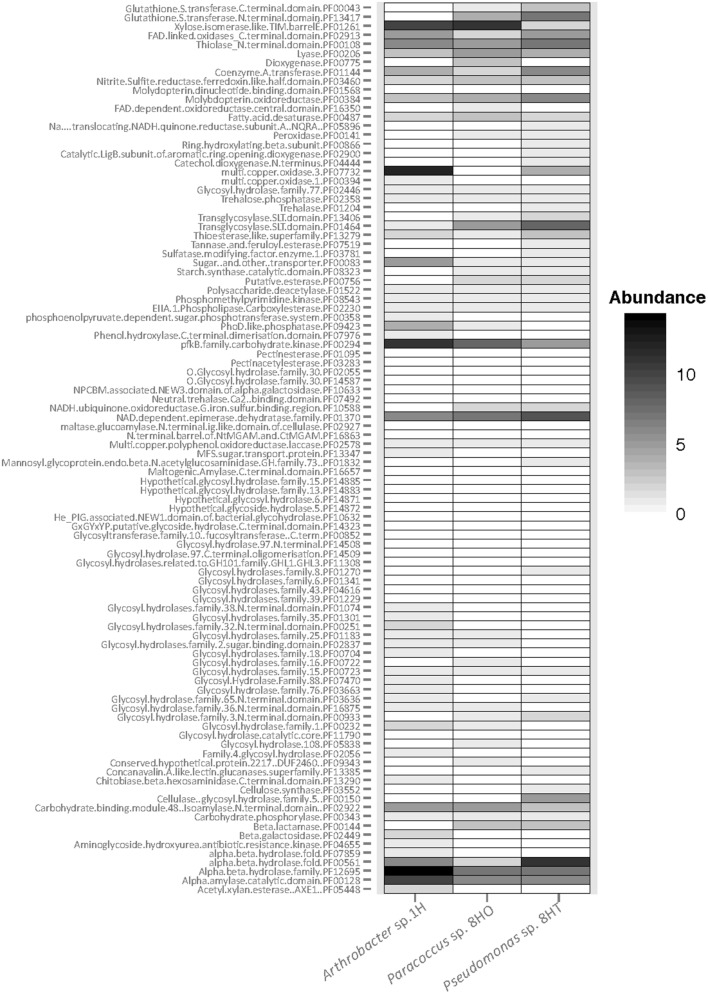
We examined all three isolates for carbohydrate-active enzymes (CAZy: Cantarel et al., 2009). As anticipated for aerobic heterotrophs, numerous genes were found, including 144 in Arthobacter sp. 1H, 140 in Pseudomonas sp. 8HT and 113 in Paracoccus sp. 8HO (Figure 6). The shallow isolate, Arthrobacter sp. 1H, contained abundant genes for glycosyl hydrolases and carbon processing compared to the deeper isolates. It had a higher number of α/β hydrolase (PF12695) and α amylase (PF00128) genes compared to the deeper isolates (Figure 6). Many genes are shared between all isolates, but only a few categories were present in abundance in the deeper isolates, Paracoccus and Pseudomonas and not the shallow Arthrobacter sp. (Figure 6). This includes β-lactamase (PF00144), a putative esterase (PF00756) and glutathione S-transferase (GST) in both C and N terminal domains (PF13417, PF00043), which has 10 total genes in Pseudomonas sp. 8HT and 5 in Paracoccus sp. 8HO, but none in Arthrobacter sp. 1H. These genes are noted for conferring the ability to survive oxidative stress (Vuilleumier and Pagni, 2002). We make the presumption that the sediment column is seeded from the overlying water, and when deposited, the Arthrobacter sp. 1H has a higher ability to utilize sugars. However, as the sediment column builds on top of it, it and the rest of its phylum will be pushed to lower depths of sediment, where they encounter the low oxygen zone that exists mid-sediment column (Figure 3). Our 16S rRNA gene amplicon sequencing of environmental DNA shows that there is a decrease in Actinobacteria and an increase in Proteobacteria when the sediment is reintroduced to oxygen diffusing up from flow above the basalt layer (Figure 3). The Proteobacteria have been noted to possess a greater abundance of GSTs than other bacterial phyla (Allocati et al., 2009), which could confer on them a greater ability to recover from the oxidative stress that can be caused from a reintroduction of oxygen after existence in a low or no-oxygen mid sediment column. This, of course, is speculation and should be investigated further using these laboratory cultures or environmental metagenomic data. Overall, we do not see clear indication in a differential ability to utilize carbon sources from these isolates dependent on depth of recovery, but we suggest the ability to withstand oxidative stress may be more crucial in survival downcore at this site.
FIGURE 6.
Abundance of carbohydrate-active enzymes by protein family (pfam) across the three isolate genomes: Arthrobacter sp. 1H, Paracoccus sp. 8HO, Pseudomonas sp. 8HT. Black indicates more abundant genes, white indicates absence of genes. Categories without matches are not shown.
Summary
The discrepancy between physiological characterization and genomic data hints at the difficulty of using single cultivation strategies. Given the presence of genes, it is likely that Arthrobacter and Pseudomonas isolates are capable of nitrate reduction in situ. This finding is particularly compelling since the geochemical profiles of their respective isolation depths suggested this metabolic versatility may be important in these low oxygen, nitrate containing porewaters. Recent work has indicated the importance of microbial nitrogen cycling in North Pond sediments (Wankel et al., 2015). In areas of low oxygen, high rates of denitrification were measured. Despite the presumed slow growth rate of organisms in these sediments, isotopic evidence was seen for the energetically costly pathways of nitrate assimilation, nitrification and dentrification (Wankel et al., 2015). The previous work postulates that greater regulation of these processes should be needed in an oligotrophic sedimentary setting such as North Pond. However, our study shows that recoverable isolates are quite similar to species in higher energy environments (Figures 2, 3, and 5), yet cultivation work suggests that metabolisms beyond aerobic heterotrophy are difficult to induce. The distribution of carbohydrate-active enzymes suggests that genes for oxidative stress may play a role in determining if cells can recover when reintroduced to oxygen in this sediment column (Figure 6). With genomes in hand, further investigations can be taken to examine additional metabolic controls and usage of carbon substrates by these isolates, which provide deep subsurface species on which future adaptation or metabolic tests may be performed.
Conclusion
We present the enrichment and isolation of three species of aerobic heterotrophs from the shallow and deep micro-aerobic zones of the U1382B sediment column. The three isolates were detected in very low relative abundance at their respective sediment depths according to culture-independent molecular analysis. This phenomenon of culturable microbes often being ‘rare’ community members is common across many environments (Shade et al., 2012), including the marine deep biosphere (Teske, 2006). However, these isolates, examined in pure culture and through genomics, have traits such as genes for nitrate reduction and denitrification, that would aid their survival in this environment where models have shown these activities to be important. These isolates can serve as type-species for investigations into adaptations for life in the deep biosphere.
Author Contributions
JR: performed research, analyzed data, wrote paper. RL-Z: analyzed data, wrote paper. KW: funded research, wrote paper. JB: funded research, analyzed data, wrote paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the co-chiefs, staff, and scientists aboard IODP Expedition 336, of which JR was a member. We thank Rebecca Daly for assistance with interfacing with JGI and Richard Wolfe for CaZy assistance. Research was supported by a postcruise award to JR and JB through the Consortium for Ocean Leadership. This is CDEBI publication 326.
Funding. JR was supported by CDEBI (OCE-0939564) and Schlanger Ocean Drilling graduate fellowships (SAF-12-05). RL-Z was funded by a CDEBI Postdoctoral Fellowship (OCE-0939564). Research was supported by a postcruise award to JR and JB through the Consortium for Ocean Leadership. A portion of this research was performed under the JGI-EMSL Collaborative Science Initiative awarded to Wrighton and used resources at the DOE Joint Genome Institute, which are DOE Office of Science User Facilities. JGI is sponsored by the Office of Biological and Environmental Research and operated under Contract Nos. DE-AC02-05CH11231.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00678
References
- Alefounder P. R., Greenfield A. J., McCarthy J. E., Ferguson S. J. (1983). Selection and organisation of denitrifying electron-transfer pathways in Paracoccus denitrificans. Biochim. Biophys. Acta (BBA)-Bioenergetics 724 20–39. 10.1016/0005-2728(83)90022-1 [DOI] [Google Scholar]
- Allocati N., Federici L., Masulli M., Di Illio C. (2009). Glutathione transferases in bacteria. FEBS J. 276 58–75. 10.1111/j.1742-4658.2008.06743.x [DOI] [PubMed] [Google Scholar]
- Bale S. J., Goodman K., Rochelle P. A., Marchesi J. R., Fry J. C., Weightman A. J., et al. (1997). Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int. J. Syst. Evol. Bacteriol. 47 515–521. 10.1099/00207713-47-2-515 [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its application to single-cell sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks B. C., Ferguson S. J., Moir J. W. B., Richardson D. J. (1995). Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232 97–173. 10.1016/0005-2728(95)00092-5 [DOI] [PubMed] [Google Scholar]
- Biddle J. F., House C. H., Brenchley J. E. (2005) “Enrichment and cultivation of microorganisms from sediment from the slope of the Peru Trench (ODP Site 1230),” in Proceedings of the ODP, Science Results, 201, eds Jørgensen, B. B., D’Hondt, S. L., Miller D. J. (College Station, TX: Ocean Drilling Program; ), 1–19. 10.2973/odp.proc.sr.201.107.2005 [DOI] [Google Scholar]
- Brouwers G. J., de Vrind J. P. M., Corstjens P. L. A. M., Cornelis P., Baysse C., de Vrind-de Jong E. W. (1999). cumA, a gene encoding a multicopper oxidase, is involved in Mn+2 oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 54 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdige D. J. (2007). Preservation of organic matter in marine sediments: controls, mechanisms and an imbalance in sediment organic carbon budgets? Chem. Rev. 107 467–485. 10.1021/cr050347q [DOI] [PubMed] [Google Scholar]
- Burdige D. J., Nealson K. H. (1985). Microbial manganese reduction by enrichment cultures from coastal marine sediments. Appl. Environ. Microbiol. 50 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaud G., Le Calvez T., Arzur D., Vandenkoornhuyse P., Barbier G. (2009). Diversity of culturable marine filamentous fungi from deep-sea hydrothermal vents. Environ. Microbiol. 11 1588–1600. 10.1111/j.1462-2920.2009.01886.x [DOI] [PubMed] [Google Scholar]
- Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009). The carbohydrate-active enzymes database (CAZy): an expert resources for glycogenomics. Nucleic Acids Res. 37 D233–D238. 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high- throughput community sequencing data. Nat. Methods 7 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Ingraham J. L. (1983). Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 45 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciobanu M. C., Burgaud G., Dufresne A., Breuker A., Rédou V., Maamar S. B., et al. (2014). Microorganisms persist at record depths in the subseafloor of the Canterbury Basin. ISME J. 8 1370–1380. 10.1038/ismej.2013.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connon S. A., Giovannoni S. J. (2002). High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl. Environ. Microbiol. 68 3875–3885. 10.1128/AEM.68.8.3878-3885.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hondt S., Jørgensen B. B., Miller D. J., Batzke A., Blake R., Cragg B. A., et al. (2004). Distributions of microbial activities in deep subseafloor sediments. Science 306 2216–2221. 10.1126/science.1101155 [DOI] [PubMed] [Google Scholar]
- D’Hondt S., Spivack A. J., Pockalny R., Ferdelman T. G., Fischer J. P., Kallmeyer J., et al. (2009). Subseafloor sedimentary life in the South Pacific Gyre. Proc. Natl. Acad. Sci. U.S.A. 106 11651–11656. 10.1073/pnas.0811793106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G. J., Lee Y. E., Tebo B. M. (2006). Manganese(II)-oxidizing Bacillus spores in guaymas basin hydrothermal sediments and plumes. Appl. Environ. Microbiol. 72 3184–3190. 10.1128/AEM.72.5.3184-3190.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick G. J., Podell S., Johnson H. A., Rivera-Espinoza Y., Bernier-Latmani R., McCarthy J. K., et al. (2008). Genomic insights into Mn(II) oxidation by the marine Alphaproteobacterium Aurantimonas sp. Strain DI85-9A1. Appl. Environ. Microbiol. 74 2646–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden P. A., Schmidt T. M., Blakemore R. P., Pace N. R. (1991). Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16S rRNA specific DNA. Int. J. Syst. Bacteriol. 41 324–325. 10.1099/00207713-41-2-324 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. J., Becker K., Colwell F. (2012). The deep, dark energy biosphere: intraterrestrial life on earth. Annu. Rev. Earth Planet. Sci. 40 551–568. 10.1146/annurev-earth-042711-105500 [DOI] [Google Scholar]
- Eschbach M., Möbitz H., Rompf A., Jahn D. (2003). Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol. Lett. 223 227–230. 10.1016/S0378-1097(03)00383-5 [DOI] [PubMed] [Google Scholar]
- Expedition 336 Scientists (2012). Mid-Atlantic Ridge Microbiology: Initiation of Long-Term Coupled Microbiological, Geochemical, and Hydrological Experimentation Within the Seafloor at North Pond, Western Flank of the Mid-Atlantic Ridge. IODP Prel. Rept. College Station, TX: Integrated Ocean Drilling Program, 336; 10.2204/iodp.pr.336.2012 [DOI] [Google Scholar]
- Fichtel K., Mathes F., Könneke M., Cypionka H., Engelen B. (2012). Isolation of sulfate-reducing bacteria from sediments above the deep- subseafloor aquifer. Front. Microbiol. 3:65 10.3389/fmicb.2012.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E., Staley J. T. (1982). Widespread distribution of ability to oxidize manganese among freshwater bacteria. Appl. Environ. Microbiol. 44 509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. (1979). A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 25 415–420. 10.1139/m79-063 [DOI] [PubMed] [Google Scholar]
- Krumbein W. E., Altmann H. J. (1973). A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol. Wiss. Meeresunters. 25 347–356. 10.1007/BF01611203 [DOI] [Google Scholar]
- Laczny C. C., Sternal T., Plugaru V., Gawron P., Atashpendar A., Margossian H. H., et al. (2015). VizBin – an application for reference-independent visualization and human-augmented binning of metagenomic data. Microbiome 3:1 10.1186/s40168-014-0066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langseth M. G., Becker K., Von Herzen R. P., Schultheiss P. (1992). Heat and fluid flux through sediment on the western flank of the mid-atlantic ridge: a hydrogeological study of north pond. Geophys. Res. Lett. 19 517–520. 10.1029/92GL00079 [DOI] [Google Scholar]
- Lever M. A., Rouxel O., Alt J. C., Shimizu N., Ono S., Coggon R. M., et al. (2013). Evidence for microbial carbon and sulfur cycling in deeply buried ridge flank basalt. Science 339 1305–1308. 10.1126/science.1229240 [DOI] [PubMed] [Google Scholar]
- Lomstein B. A., Langerhuus A. T., D’Hondt S., Jørgensen B. B., Spivack A. J. (2012). Endospore abundance, microbial growth and necromass turnover in deep subseafloor sediment. Nature 484 101–104. 10.1038/nature10905 [DOI] [PubMed] [Google Scholar]
- Ludwig W., Strunk O., Westram R., Richter L., Meier H., Yadhukumaret al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res. 32 1363–1371. 10.1093/nar/gkh293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz V. M., Chen I. M., Palaniappan K., Chu K., Szeto E., Grechkin Y., et al. (2012). IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 40 D115–D122. 10.1093/nar/gkr1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. G., Carpenter S. D., Deming J. W. (2009). Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 55 63–72. 10.1139/W08-130 [DOI] [PubMed] [Google Scholar]
- Mikucki J. A., Liu Y., Delwiche M., Colwell F. S., Boone D. R. (2003). Isolation of a methanogen from deep marine sediments that contain methane hydrates, and description of Methanocullus submarinus sp. nov. Appl. Environ. Microbiol. 69 3311–3316. 10.1128/AEM.69.6.3311-3316.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J., Bateman A., Finn R. D. (2007). Predicting active site residue annotations in the Pfam database. BMC Bioinformatics 8:298 10.1186/1471-2105-8-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Jørgensen B. B., Revsbech P. (1986). Growth pattern and yield of a chemoautotrophic Beggiatoa sp. in oxygen-sulfide microgradients. Appl. Environ. Microbiol. 52 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki M., Sugita T., Shimizu M., Ohode Y., Iwamoto K., De Vrind-De Jong E. W., et al. (1997). Partial purification and characterization of manganese oxidizing factors of Pseudomonas fluorescens GB-1. Appl. Environ. Microbiol. 63 4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt B. N., Wheat C. G., Rouxel O., Hulme S., Edwards K. J., Bach W. (2013). Oxygen consumption rates in subseafloor basaltic crust derived from a reaction transport model. Nat. Commun. 4:2539 10.1038/ncomms3539 [DOI] [PubMed] [Google Scholar]
- Orsi W. O., Christman G., Edgcomb V. E., Biddle J. F. (2013). Gene expression in the deep biosphere. Nature 499 205–208. 10.1038/nature12230 [DOI] [PubMed] [Google Scholar]
- Parkes R. J., Martin D., Amann H., Anders E., Holland M., Schultheiss P. J., et al. (2009). “Technology for high-pressure sampling and analysis of deep-sea sediments, associated gas hydrates, and deep- biosphere processes,” in Natural Gas Hydrates - Energy Resource Potential and Associated Geologic Hazards: AAPG Memoir 89, eds Collett T., Johnson A., Knapp C., Boswell R. (Tulsa, OK: American Association of Petroleum Geologists; ), 672–683. [Google Scholar]
- Parks D. H., Imelfort M., Skennerton C. T., Hugenholtz P., Tyson G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25 1043–1055. 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Widdel F., Trüper H. G. (1981). The Dissimilatory Sulfate-Reducing Bacteria. The Prokaryotes. Berlin: Springer, 926–940. [Google Scholar]
- Picard A., Ferdelman T. G. (2011). Linking microbial heterotrophic activity and sediment lithology in oxic, oligotrophic sub-seafloor sediments of the North Atlantic Ocean. Front. Microbiol. 2:263 10.3389/fmicb.2011.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., Arkin A. P. (2009). FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26 1641–1650. 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse E., Peplies J., Glöckner F. O. (2012). SINA: accurate high- throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28 1823–1829. 10.1093/bioinformatics/bts252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. S., Aggarwal R. K., Matsumoto G. I., Shivaji S. (2000). Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley, Antarctica. Int. J. Syst. Evol. Microbiol. 50 1553–1561. 10.1099/00207713-50-4-1553 [DOI] [PubMed] [Google Scholar]
- Salter S. J., Cox M. J., Turek E. M., Calus S. T., Cookson W. O., Moffatt M. F., et al. (2014). Reagent and laboratory contamination can critically impact sequence-based microbiome analysis. BMC Biol. 12:87 10.1186/s12915-014-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson M. O. (1985). Dissimilatory nitrate reduction to nitrate, nitrous oxide, and ammonium by Pseudomonas putrefaciens. Appl. Environ. Microbiol. 50 812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. (2014). Prokka: rapid prokaryotic genome annotation. Bioinformatics 30 2068–2069. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- Shade A., Hogan C. S., Klimowicz A. K., Linske M., McManus P. S., Handelsman J. (2012). Culturing captures members of the soil rare biosphere. Environ. Microbiol. 14 2247–2252. 10.1111/j.1462-2920.2012.02817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. C., Spivack A. J., Fisk M. R., Haveman S. A., Staudigel H. (2000). Tracer-based estimates of drilling-induced microbial contamination of deep sea crust. Geomicrobiol. J. 17 207–219. 10.1080/01490450050121170 [DOI] [Google Scholar]
- Staley J. T., Konopka A. (1985). Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 39 321–346. 10.1146/annurev.mi.39.100185.001541 [DOI] [PubMed] [Google Scholar]
- Straub K. L., Buchholz-Cleven B. E. E. (1998). Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl. Environ. Microbiol. 64 4846–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebo B. M., Johnson H. A., McCarthy J. K., Templeton A. S. (2005). Geomicrobiology of manganese (II) oxidation. TRENDS Microbiol. 13 421–428. 10.1016/j.tim.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Templeton A. S., Staudigel H., Tebo B. M. (2005). Diverse Mn (II)-oxidizing bacteria isolated from submarine basalts at Loihi Seamount. Geomicrobiol. J. 22 127–139. 10.1080/01490450590945951 [DOI] [Google Scholar]
- Teske A. P. (2006). Microbial communities of deep marine subsurface sediments: molecular and cultivation surveys. Geomicrobiol. J. 23 357–368. 10.1080/01490450600875613 [DOI] [Google Scholar]
- Toffin L., Bidault A., Pignet P., Tindall B. J., Slobodkin A., Kato C., et al. (2004). Shewanella profunda sp. nov., isolated from deep marine sediment of the Nankai Trough. Int. J. Syst. Evol. Bacteriol. 54 1943–1949. 10.1099/ijs.0.03007-0 [DOI] [PubMed] [Google Scholar]
- Tsubokura A., Yoneda H., Mizuta H. (1999). Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin- producing bacterium. Int. J. Syst. Evol. Bacteriol. 49 277–282. 10.1099/00207713-49-1-277 [DOI] [PubMed] [Google Scholar]
- Vetriani C., Chew Y. S., Miller S. M., Yagi J., Coombs J., Lutz R. A., et al. (2005). Mercury adaptation among bacteria from a deep-sea hydrothermal vent. Appl. Environ. Microbiol. 71 220–226. 10.1128/AEM.71.1.220-226.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier S., Pagni M. (2002). The elusive roles of bacterial glutathione S-transferases: new lessons from genomes. Appl. Microbiol. Biotechnol. 58 138–146. 10.1007/s00253-001-0836-0 [DOI] [PubMed] [Google Scholar]
- Wankel S. D., Buchwald C., Ziebis W., Wenk C. B., Lehmann M. F. (2015). Nitrogen cycling in the deep sedimentary biosphere: nitrate isotopes in porewaters underlying the oliogtrophic North Atlantic. Biogeosciences 12 7483–7502. 10.5194/bg-12-7483-2015 [DOI] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M. A., Clamp M., Barton G. J. (2009). Jalview version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics 25 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S., Amy C. R., Stephen S. R. (2011). Techniques for cultivating methanogens. Methods Enzymol. 494 1–22. 10.1016/B978-0-12-385112-3.00001-9 [DOI] [PubMed] [Google Scholar]
- Wrighton K. C., Thrash J. C., Melnyk R. A., Bigi J. P., Byrne-Bailey K. G., Remis J. P., et al. (2011). Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 77 7633–7639. 10.1128/AEM.05365-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-W., Tang Y.-H., Tringe S. G., Simmons B. A., Singer S. W. (2014). MaxBin: an automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome 2:26 10.1186/2049-2618-2-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.