Abstract
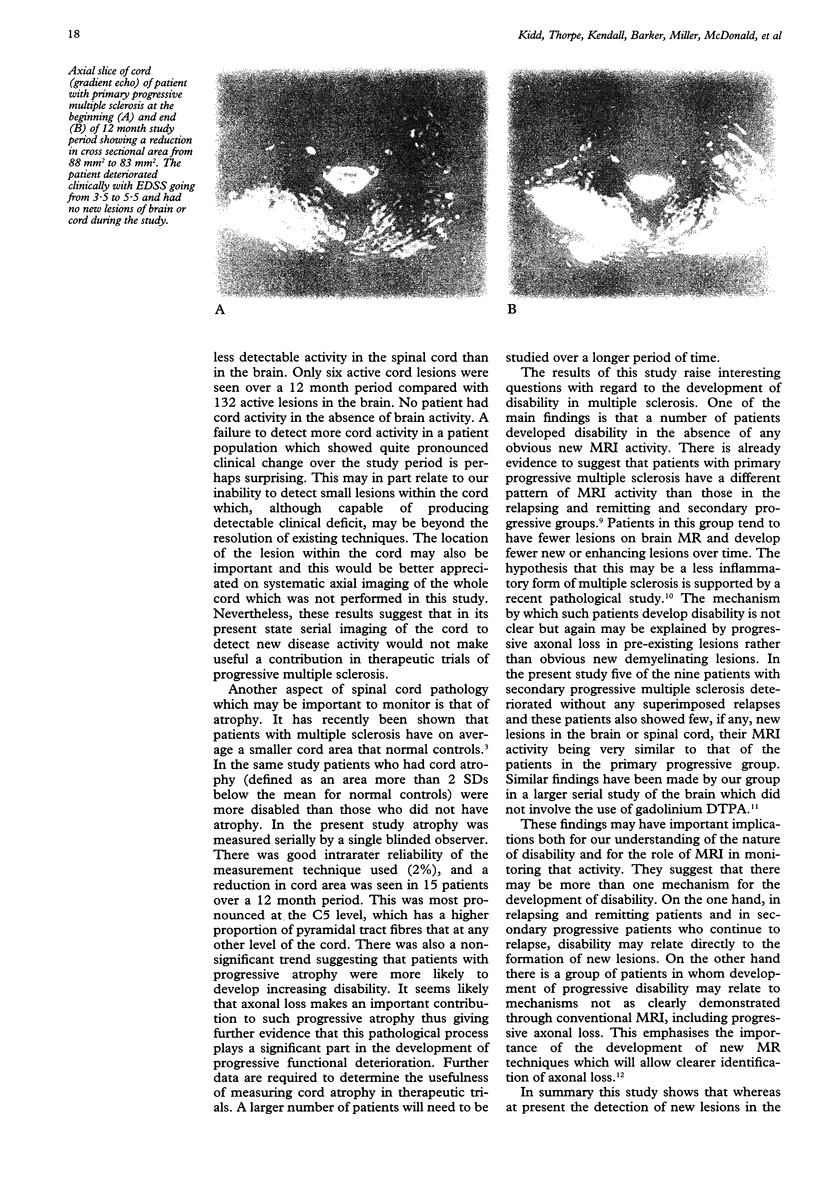
OBJECTIVE--To assess the usefulness of serial cord MRI in patients with progressive multiple sclerosis. METHODS--Monthly MRI of the brain and spinal cord with and without gadolinium enhancement was carried out in 19 patients with progressive multiple sclerosis (10 primary progressive, nine secondary progressive) over the course of one year. RESULTS--During this period there were 132 active lesions in the brain and only six in the cord. One hundred and twelve (85%) active brain lesions occurred in the secondary progressive group; three new cord lesions occurred in each group. In the secondary progressive group MRI activity was high in patients who had superimposed relapses, whereas in those who progressed without relapse and in the primary progressive group it was low. Cross sectional areas of the cord decreased at the C5 level in both groups, implying progressive atrophy of fibre tracts. There was no relation between either brain or cord MRI activity and change in disability over the study period. CONCLUSIONS--Although the detection of new lesions by frequent cord imaging using current technology has little role in the monitoring of disease activity in progressive multiple sclerosis, the serial measurement of cord cross sectional area may be important. There is also evidence to suggest that the mechanism underlying irreversible disability in patients with progressive multiple sclerosis may be different in patients who continue to relapse than in those who do not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kidd D., Thorpe J. W., Thompson A. J., Kendall B. E., Moseley I. F., MacManus D. G., McDonald W. I., Miller D. H. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993 Dec;43(12):2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Miller D. H., Barnes D. The pathological evolution of multiple sclerosis. Neuropathol Appl Neurobiol. 1992 Aug;18(4):319–334. doi: 10.1111/j.1365-2990.1992.tb00794.x. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Miller D. H., Thompson A. J. Are magnetic resonance findings predictive of clinical outcome in therapeutic trials in multiple sclerosis? The dilemma of interferon-beta. Ann Neurol. 1994 Jul;36(1):14–18. doi: 10.1002/ana.410360106. [DOI] [PubMed] [Google Scholar]
- Paty D. W., Li D. K. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993 Apr;43(4):662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Revesz T., Kidd D., Thompson A. J., Barnard R. O., McDonald W. I. A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain. 1994 Aug;117(Pt 4):759–765. doi: 10.1093/brain/117.4.759. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., Wicks D., MacManus D. G., Kendall B. E., Kingsley D. P., McDonald W. I. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991 Jan;29(1):53–62. doi: 10.1002/ana.410290111. [DOI] [PubMed] [Google Scholar]
- Thorpe J. W., Kidd D., Kendall B. E., Tofts P. S., Barker G. J., Thompson A. J., MacManus D. G., McDonald W. I., Miller D. H. Spinal cord MRI using multi-array coils and fast spin echo. I. Technical aspects and findings in healthy adults. Neurology. 1993 Dec;43(12):2625–2631. doi: 10.1212/wnl.43.12.2625. [DOI] [PubMed] [Google Scholar]




