Abstract
Morel-Lavallée lesions are hemolymphatic, nonanatomic fluid collections that result from a separation of the subcutaneous tissue from the underlying fascia. Ultrasound and MRI characteristics of such lesions have been previously described and can be helpful in establishing a diagnosis and guiding clinical management. We present a case of a Morel-Lavallée lesion of the elbow, with ultrasound and MRI correlation, which has not been reported in the radiology literature heretofore.
Introduction
Morel-Lavallée lesions (MLL), first described in 1853, are closed degloving injuries that result in an abrupt separation of the subcutaneous tissue from the underlying fascia (1). The vessels and lymphatics that penetrate through this fascial layer are disrupted, and as a result, hemolymphatic fluid and debris accumulate in this potential space. These traumatic injuries are well known to orthopedic surgeons, and their imaging features have been described in radiology literature over the past two decades (2, 3, 4, 5, 6). MLLs are most commonly seen in the trochanteric region of the hip and around the proximal thigh, but they have also been reported in the knee, trunk, lower lumbar region, and calf. We present a case of a MLL of the elbow in an adult, with MRI and ultrasound correlation in the acute phase, which to our knowledge has not been previously reported in radiology literature.
Case report
A 57-year-old male presented to the Emergency Department with right elbow swelling and discomfort. The patient reported falling on the elbow and skidding on the grass with the elbow flexed forward while playing football, four days before presentation. While in the Emergency Department, the patient was seen by Orthopedics, had elbow radiographs performed (Fig. 1), and had approximately 5 cc of fluid (described as “venous blood”) aspirated from the area of swelling.
Figure 1.

An AP radiograph of the elbow shows soft-tissue swelling at the medial aspect of the elbow (white arrow), in the region of the medial epicondyle, with loss of the normal fat planes.
The patient presented for a followup appointment at the Orthopedic Clinic two days later complaining of pain and increased swelling at the medial aspect of the elbow. The pain woke him from sleep and was only minimally relieved with pain medication. Additionally, there were two palpable cords in the region of elbow swelling. The patient was clinically diagnosed with traumatic bursitis, and a compressive bandage was applied. Over the next week, the patient’s swelling worsened, and he had an ultrasound examination of the area of pain and palpable mass (Fig. 2).
Figure 2.
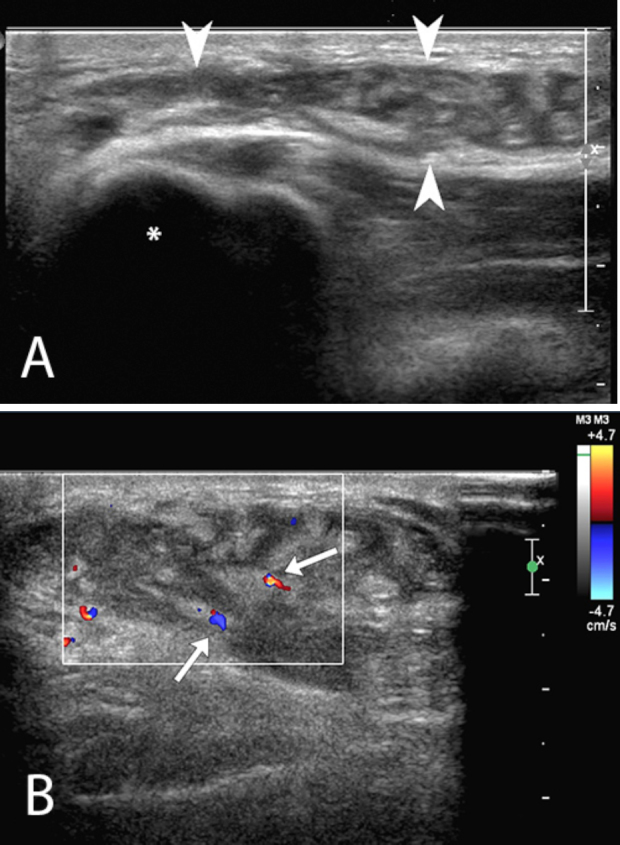
A. Targeted ultrasound evaluation of the soft palpable mass located over medial epicondyle (asterisk) with a high-frequency linear transducer (12 to 5-MHz transducer) demonstrates a complex collection (arrowheads) located between the subcutaneous fat and fascia. The lesion contains highly echogenic lobules of fat, interspersed with areas of lower echogenicity. B. Color Doppler imaging of the Morel-Lavallée lesion demonstrates minimal flow in two small vessels (arrows) traversing the collection, and absence of flow in the lesion otherwise.
On ultrasound, the mass showed lobules of increased echogenicity, interspersed with areas of slightly complex lower echogenicity. Unenhanced MRI, performed the following week, demonstrated an irregularly shaped, 4.2 × 3.7 × 1.4-cm (longitudinal × AP × transverse) lentiform unilocular T2 hyperintense collection that contained numerous fat globules overlying the deep fascia of the elbow and draping over the medial epicondyle (Figure 3, Figure 4).
Figure 3.

Axial T1W (TR/TE 800/10) (A) and T2W fat saturated (TR/TE 3617/64) (B) sequences. A Morel-Lavallée lesion overlies the fascia (black arrows) in the region of the medial epicondyle (asterisks). The lesion is predominately T1 hypointense, and T2 hyperintense. There are scattered T1 hyperintense foci (white arrow), with loss of signal on the fat-saturated sequences, consistent with fat lobules.
Figure 4.
Coronal T1W(TR/TE 633/19) (A) and PD fat saturated (TR/TE 2833/32) (B) sequences. Morel-Lavallée lesion is seen superficial to the fascia (black arrow), overlying the medial epicondyle. A tubular structure (white arrow) surrounded by fat is also seen coursing through the collection, representing sheared-off subcutaneous venous branch.
The imaging findings were consistent with an acute MLL. The patient continued to follow up with Orthopedics over the course of the next few months, opting for conservative management, including physical therapy and pain medication. The collection resolved approximately 6 months after the traumatic event.
Discussion
MLLs are the result of closed degloving injuries that cause the skin and subcutaneous tissues to separate from the underlying fascial layer. Patients often present with a history of trauma (with a blunt tangential force shearing the hypodermis from the fascia), as seen in motor vehicle accidents associated with pelvic or acetabular fractures, or often after a skid or a fall (1, 7, 8), as with our patient. On physical exam, there is a fluctuant, tender mass in the area of injury. It is important to note, however, that the traumatic event may not be specifically recalled by the patient (1, 9) and that diagnosis of MLL may be missed or mistaken for a tumor.
Imaging of MLL often begins with radiographs; however, findings are nonspecific, as the most common radiographic finding is soft-tissue swelling involving the affected area. Ultrasound and MRI have proven useful in evaluating for MLL.
Neal et al (6) performed a retrospective review of sonographic findings of 21 Morel-Lavallée lesions. The most common imaging findings were predominately hypoechoic or anechoic lesions. The lesions were also described as heterogeneous, particularly in the acute stage. This finding reflects the various components of fat, blood, lymph, and other debris within an acute lesion. This study also showed that as the lesion evolves over time, it will become more homogeneous and well-defined.
The MRI appearance of MLL also depends on the acuity of the lesion. In the acute phase, blood products and debris of varying signal intensity are seen within a T2-hyperintense cavity. Mellado et al (4) described three different patterns of longstanding MLL on MRI. First, as the lesion evolves from an acute state, deoxyhemoglobin is converted into methemoglobin, resulting in intermediate to slightly increased T1 signal. As the red blood cells undergo lysis, the MLL takes on a more homogeneous T1 hypointense and T2 hyperintense appearance, similar to that of a seroma. A third pattern of longstanding MLL includes variable signal intensity on T1 and heterogeneous hyperintensity on T2 with patchy internal enhancement. The lesion often appears well-circumscribed and ovoid. MLL can also develop a fibrous pseudocapsule, which would have the appearance of a T1- and T2-hypointense peripheral rim on MRI.
MLLs can present a diagnostic challenge, especially when they are in an atypical location. They are most commonly seen in the trochanteric regions and around the proximal thigh, but have also been reported at the knee (5, 7), trunk and lower lumbar region (10, 11), and the calf (12). Postoperative MLLs also have been reported in the abdominal wall after liposuction and abdominoplasty (13, 14).
Main differential considerations for MLL include bursitis, hematoma, and seroma. Borrero et al (5) described four cases of MLLs of the knee, and discussed the similarities and differentiating features of MLLs and prepatellar bursitis. MLLs were often larger than the fluid collections seen in prepatellar bursitis, and often in a slightly different location. However, size criteria and location are not diagnostic of either entity. The best differentiating feature might be following the natural history of the lesion and its response to steroid injection. For instance, chronic MLL may develop a pseudocapsule that prevents reabsorption of fluid, whereas most cases of prepatellar bursitis will decrease in size, provided that the causative stress is removed. Bursitis is also more likely to respond to a steroid injection.
The majority of MLLs are treated conservatively with compressive therapy. If these lesions do not respond to conservative management, percutaneous aspiration with or without sclerotherapy or surgical debridement are considered the next line of treatment. More chronic MLLs, which often develop a fibrous pseudocapsule, commonly require surgical intervention. Other indications for surgery include superimposed infection, compression of adjacent structures, or association with an open fracture (1, 7, 8, 9, 15). A recent review of clinical management of MLL in the setting of trauma by Nickerson et al (16) stated that aspiration of more than 50 mL of fluid from MLL was much more common among lesions that recurred (83%) than among those that resolved (33%), advocating for early operative intervention for larger-volume lesions. Two out of 87 lesions in this study were located at the elbow; however, the imaging correlation was not described.
The ultrasound and MRI appearances in our case, in conjunction with the patient’s mechanism of injury and clinical course, are diagnostic of an acute MLL. When the immobile medial epicondyle and relatively mobile overlying dermis are subjected to a sheering tangential force, it creates a typical injury mechanism that has been previously described for other MLLs. Differential diagnostic considerations in our case included bursitis and hematoma, as there is an overlap in imaging appearance between these entities. The presence of floating fat particles within the collection on both ultrasound and MRI would indicate a MLL, and would not be typical for either bursitis or a hematoma. In addition to the floating fat particles, we observed two subcutaneous venous branches traversing the lesion on MRI, which corresponded to the palpable cords on physical exam. The medial location of the lesion distinguishes it from a post-traumatic olecranon bursitis. Also, as discussed by Borrero et al (5), bursitis should resolve once the causative stress is removed. In our case, there was persistent swelling and tenderness over the course of 6 months, despite conservative therapy. Similarly, the recalcitrant course of this lesion argues against a hematoma. The lesion was aspirated and re-accumulated shortly thereafter, whereas the natural time course for hematomas in a patient without coagulopathy would be to reabsorb gradually.
In conclusion, this is, to our knowledge, the first reported case of an acute Morel-Lavallée lesion of the elbow with ultrasound and MRI correlation. The ultrasound and MRI demonstrated typical findings of an acute MLL, and in conjunction with the mechanism of injury and clinical course of the lesion, helped establish the diagnosis and direct patient management.
Footnotes
Published: August 31, 2014
References
- 1.Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma. 1997;42:1046–1051. doi: 10.1097/00005373-199706000-00010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Parra JA, Fernandez MA, Encinas B, Rico M. Morel-Lavallée effusions in the thigh. Skeletal Radiol. 1997;26:239–241. doi: 10.1007/s002560050228. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee K, Perrin SM, Hughes PM. Morel-Lavallée lesion in an adolescent with ultrasound and MRI correlation. Skeletal Radiol. 2007;36:43–45. doi: 10.1007/s00256-006-0122-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Mellado JM, Perez del Palomar L, Diaz L. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol. 2004;182:1289–1294. doi: 10.2214/ajr.182.5.1821289. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Borrero CG, Maxwell N, Kavanagh E. MRI findings of prepatellar Morel-Lavallée effusions. Skeletal Radiol. 2008;37(5):451–455. doi: 10.1007/s00256-008-0450-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Neal C, Jacobson JA, Brandon C. Sonography of Morel-Lavallée lesions. J Ultrasound Med. 2008;27:1077–1081. doi: 10.7863/jum.2008.27.7.1077. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallée lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35:1162–1167. doi: 10.1177/0363546507299448. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Dawre S, Lamba S, Gupta S. The Morel-Lavallée lesion: a review and a proposed algorithmic approach. Eur J Plast Surg. 2012;35:489–494. [Google Scholar]
- 9.Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg. 1992;89:853–855. doi: 10.1097/00006534-199205000-00013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Sawkar AA, Swischuk LE, Jadhav SP. Morel-lavallée seroma: a review of two cases in the lumbar region in the adolescent. Emerg Radiol. 2011;18:495–498. doi: 10.1007/s10140-011-0975-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Moran DE, Napier NA, Kavanagh EC. Lumbar morel-lavallée effusion. Spine J. 2012;12:1165–1166. doi: 10.1016/j.spinee.2012.11.019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Moriarty JM, Borrero CG, Kavanagh EC. A rare cause of calf swelling: the morel-lavallée lesion. Ir J Med Sci. 2011;180:265–268. doi: 10.1007/s11845-009-0386-5. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zecha PJ, Missotten FE. Pseudocyst formation after abdominoplasty—extravasations of Morel-Lavallée. Br J Plast Surg. 1999;52:500–502. doi: 10.1054/bjps.1999.3154. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Scaranelo AM, Davanco RA. Pseudocyst formation after abdominal liposuction-extravasations of Morel-Lavallée on MR images. Br J Plastic Surgery. 2005;58:849–851. doi: 10.1016/j.bjps.2004.12.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Tseng S, Tornetta P., III Percutaneous management of Morel-Lavallée lesions. J Bone Joint Surg Am. 2006;88:92–96. doi: 10.2106/JBJS.E.00021. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Nickerson TP, Zielinski MD, Jenkins DH, Schiller HJ. The Mayo Clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J Trauma Acute Care Surg. 2014 Feb;76(2):493–497. doi: 10.1097/TA.0000000000000111. [PubMed] [DOI] [PubMed] [Google Scholar]



