Abstract
The purpose of this review is to discuss the management of the low cardiac output syndrome (LCOS) following surgery for congenital heart disease. The LCOS is a well-recognized, frequent post-operative complication with an accepted collection of hemodynamic and physiologic aberrations. Approximately 25% of children experience a decrease in cardiac index of less than 2 L/min/m2 within 6-18 hours after cardiac surgery. Post-operative strategies that may be used to manage patients as risk for or in a state of low cardiac output include the use of hemodynamic monitoring, enabling a timely and accurate assessment of cardiovascular function and tissue oxygenation; optimization of ventricular loading conditions; the judicious use of inotropic agents; an appreciation of and the utilization of positive pressure ventilation for circulatory support; and, in some circumstances, mechanical circulatory support. All interventions and strategies should culminate in improving the relationship between oxygen supply and demand, ensuring adequate tissue oxygenation.
Keywords: Acute kidney injury, heart failure, hemodynamic monitoring, low cardiac output, mechanical circulatory devices, Pediatrics, vasoactive therapies.
INTRODUCTION
The LCOS refers to the reduction in cardiac output that may occur following cardiopulmonary bypass (CPB) for correction of congenital heart disease. It is a well-recognized, post-operative phenomenon that may be seen following pediatric heart surgery. Although no stringent diagnostic criteria exist, an accepted collection of hemodynamic and physiologic aberrations occur which alert the cardiac intensivist to its presence. A variety of therapeutic strategies can be applied to support cardiac function and cardiac output and include optimization of ventricular preload; inotropic and afterload reducing agents; positive pressure ventilation; and in extreme circumstances mechanical circulatory support. Early recognition of and intervention for the LCOS is paramount due to its adverse impact on perioperative morbidity and mortality.
INCIDENCE
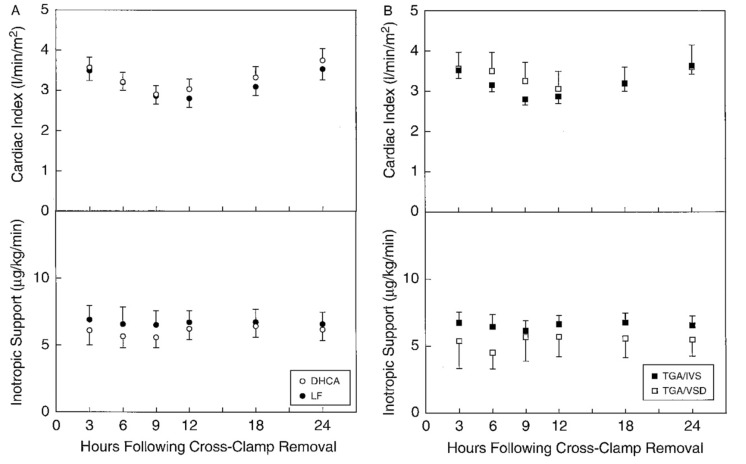
The LCOS was first described by Parr and colleagues in 1975 [1]. They used dye dilution to measure cardiac index (CI) and discovered that 25% of children after cardiac surgery have a CI of less than 2.0 L/min/m2. Some 20 years later, Wernovsky and colleagues demonstrated a similar incidence of LCOS in patients after the arterial switch operation [2]. They determined that measurable drops in CI to less than 2L/min/m2 occur in 25% of patients in the post-operative period with most nadirs occurring between 6-18 hours following admission to the intensive care unit (Fig. 1).
Fig. (1).
Scatterplots showing serial measurements of cardiac index (top) as determined by thermodilution techniques and inotropic support (bottom) in 122 patients after the arterial switch operation for transposition of the great arteries. Cardiac index fell during the first postoperative night, returning to baseline values by 24 hours after surgery. TGA/IVA, transposition of the great arteries with intact ventricular septum; TGA/VSD, TGA with a ventricular septal defect; DHCA, deep hypothermic circulatory arrest; Wernovsky G et al. Circulation 1995; 92: 2226-35. [used with permission].
PATHOPHYSIOLOGY AND CAUSES
In order to understand the contributing factors that culminate in the LCOS, one must grasp the pathophysiologic aberrations that may impair cardiac function following cardiac surgery. The etiology of the LCOS is generally multifactorial. It may result from either left ventricular (LV), right ventricular (RV) or systemic ventricular (single ventricle anatomy) dysfunction and may include systolic and or diastolic dysfunction. Ventricular dysfunction may be exacerbated by inadequate preload or alterations in RV and or LV afterload. The underlying physiology resulting from the congenital defect may include a volume and or pressure load, which may dramatically alter ventricular function, impacting postoperative management. A residual volume load may be caused by an intra-cardiac shunt at the atrial or ventricular level or a patent ductus arteriosus. A residual pressure load may result from stenotic valves or conduits, or from alterations in systemic vascular resistance (SVR) or pulmonary vascular resistance (PVR). The RV is especially sensitive to changes in afterload, as it has significantly less contractile reserve than the systemic or LV. Increases in RV afterload may result from conduit stenosis, pulmonary artery narrowing, heightened pulmonary vascular reactivity, pulmonary venous obstruction, left atrial hypertension, mitral stenosis, or poor LV function.
In addition to post-operative alterations in loading conditions, the intraoperative effects of cardiac surgery and CPB contribute to post-operative myocardial injury and dysfunction. The exposure of the patient’s blood elements to the foreign surface of the bypass circuit induces a systemic inflammatory response, leading to capillary leak, edema formation, and myocardial diastolic and systolic dysfunction [3-6]. The adverse affects of the CPB induced systemic inflammatory response on myocardial injury is compounded by the regional inflammatory phenomenon resulting from myocardial reperfusion injury following cardioplegic arrest. Further, re-establishment of pulmonary perfusion leads to pulmonary endothelial injury and impaired nitric oxide production, contributing to heightened pulmonary vascular reactivity. Pulmonary reperfusion injury also damages the endothelial alveolar epithelial barrier, leading to pulmonary edema and impaired oxygenation. The systemic and regional inflammatory response that occurs with cardiopulmonary bypass and reperfusion injury increases total body oxygen demand while impairing oxygenation, cardiac output and systemic oxygen delivery [3-6].
MONITORING AND DIAGNOSTICS
The key to mitigating the LCOS in the post-operative period is early recognition and timely intervention. Many physiologic, hemodynamic, and serologic variables can be assessed and re-assessed in order to follow a trend more valuable than any single point measurement or evaluation. Both invasive and non-invasive monitoring strategies are available and are discussed elsewhere in this issue. It is important to appreciate that estimations of cardiac function, cardiac output and tissue oxygenation, based on the interpretation of standard clinical parameters, such as the physical examination, and hemodynamic parameters such as the central venous pressure, heart rate and blood pressure are often discordant from measured values [7, 8]. The use of adjunctive monitoring modalities is invaluable in making a timely and accurate assessment of cardiovascular function and the adequacy of tissue oxygenation.
THERAPEUTIC OPTIONS FOR THE LCOS
A myriad of strategies exists for managing LCOS, including optimization of ventricular preload; vasoactive medications; mechanical ventilation, and mechanical circulatory support. The optimal timing for therapeutic interventions for the LCOS is prior to the onslaught of end-organ ischemic injury and the development of organ failure. Serologic markers of anaerobic metabolism such as serum lactate levels while generally indicative of inadequate tissue oxygenation are relatively late signs of cellular hypoxia, further emphasizing the importance of monitoring modalities such as venous and NIRS oximetry, which are discussed under monitoring. As mentioned above, the LCOS is an umbrella-term encompassing any set of conditions leading to an imbalance of oxygen supply and demand. A deliberate evaluation of the cause(s) of compromised cardiac output must take place and consideration must be given to the impact of those lesions on ventricular loading conditions and function, as well as on heart rate and the conduction system. Strategies to optimize cardiac output and minimize oxygen demand will be reviewed.
OPTIMIZING PRELOAD
A determination of where the ventricles reside on their pressure stroke volume curves is essential for determining the optimal ventricular filling pressure (central venous or right atrial and left atrial pressures). It is important to note that a given atrial pressure does not correlate with ventricular volume or stroke volume due to alterations in ventricular compliance. Further, in the setting of cardiopulmonary disease there is no correlation between right atrial and left atrial pressures, making it even more challenging to determine the optimal filling pressure for the LV. Administering volume and objectively assessing the response provides some indication of where the ventricles reside on their pressure stroke volume curve. A prompt decrease in heart rate, or increase in venous oxygen saturations or invasive blood pressure immediately following volume administration indicates that preload reserve is present, and that the ventricles are operating on the ascending portion of their pressure stroke volume curves. The lack of a response suggests that the ventricles are residing on the flat portion of their function curves. In this case, preload reserve is exhausted and inotropic and or afterload reducing agents are indicated to improve stroke volume and cardiac output. Additional volume expansion will only increase ventricular filling pressures, increasing myocardial oxygen demand and the formation of pulmonary edema. Decreasing systemic venous return decreases the extent of congestion without compromising stroke volume or cardiac output. Such an assessment of preload reserve is of particular importance in patients where ventricular compliance is impaired such as following cardioplegic arrest and myocardial reperfusion injury and following the repair of certain lesions such as tetralogy of Fallot and following the cavopulmonary anastomoses (bidirectional Glenn or Fontan). The use of positive pressure ventilation further confounds the interpretation of atrial pressures by decreasing the effective compliance of each ventricle by decreasing their respective diastolic transmural pressures (inside – surrounding pressure), which is discussed in cardiopulmonary interactions. In addition to establishing where the ventricles reside on their function curves, it is also important to realize that the adequate and optimal ventricular filling pressure may vary over time, as conditions such as respiration and ventricular function vary.
MANIPULATING SYSTOLIC FUNCTION
Once adequate preload has been established, inotropic support may be indicated. Many options exist with various side effect profiles and dose-dependent hemodynamic effects. Although not a classic inotrope, the calcium ion is essential to myofibril contraction. The neonatal myocardium is especially sensitive to changes in serum calcium levels due to a poorly developed sarcoplasmic reticulum with limited calcium stores. Intravenous calcium administration causes both increased contractility as well as increased smooth muscle tone in the peripheral vasculature. Thus, ensuring adequate levels of ionized calcium is essential in the management of patients following cardiac surgery, particularly in the neonate.
Catecholamines are the mainstay of inotropic support. Augmentation of myocardial contractility is discussed in detail elsewhere in this issue. In brief, dopamine and dobutamine provide modest inotropic support with greater inotropy provided by epinephrine and norepinephrine. Dobutamine and epinephrine in low doses (<0.05-0.1 mcg/kg/min) are particularly attractive agents for severe systolic impairment as they also reduce systemic ventricular afterload. Catecholamines also have a few drawbacks including that they all increase myocardial oxygen demand as well as heart rate and therefore the propensity for developing tachyarrhythmias.
MANIPULATING AFTERLOAD
Another strategy to improve cardiac output is to reduce ventricular afterload. The benefits of afterload reduction increase as systolic function wanes. Phosphodiesterase (PDE) type III inhibitors are an attractive agent for this purpose as they provide modest inotropic support while reducing PVR and SVR. In addition, they are less chronotropic, less arrhythmogenic, and have less of an impact on myocardial oxygen demand than catecholamines. PDE type III inhibitors work by preventing the break down of cyclic adenosine monophosphate (cAMP). The accumulation of cAMP in vascular smooth muscle cells leads to vasodilation while in the cardiomyocyte it leads to improved contractility. In addition, PDE type III inhibitors do not rely on adrenergic receptors and are therefore immune to adrenergic receptor downregulation, which begins to occur within hours of exposure to endogenous and exogenous catecholamines. The use of milrinone to prevent or treat LCOS following pediatric cardiac surgery has been well studied [9-2]. In a double-blind, placebo controlled trial of infants following pediatric cardiac surgery Hoffman and colleagues showed a 64% relative risk reduction of LCOS in the first 36 hours following cardiac surgery in infants randomized to high dose milrinone (0.75mcg/kg/min) compared to placebo, low or moderate doses [11].
Other agents useful for reducing ventricular afterload include the nitric oxide donors nitroprusside and nitroglycerin, and calcium channel blockers. Nitric oxide works via cGMP causing vascular smooth muscle cell relaxation. Nitroprusside is a potent, readily titratable agent that vasodilates venous capacitance and arterial resistance vessels in a dose-dependent manner. Nitroglycerin is low doses primary vasodilates venous capacitance vessels while in higher does it also vasodilates arterial resistance vessels. Nicardipine belongs to a class of dihydropyridine calcium channel blockers, which predominantly act on the systemic arterial resistance vessels.
VASOPRESSOR THERAPY
Vasomotor paresis is characterized by a pathologic decrease in vascular tone, which increases venous capacitance and decreases SVR. Several agents may be used to restore adequate vascular function. Vasopressin, an endogenous hormone produced in the hypothalamus, has recently been applied to children with refractory hypotension due to vasoplegia after cardiac surgery [13-15]. Vasopressin acts on V1 receptors in the peripheral vasculature to cause intense vasoconstriction via activation of protein kinase C, ultimately leading to an influx of intracellular calcium. The rationale for using vasopressin as a therapy to treat refractory hypotension originated from the research produced by Landry and colleagues, which demonstrated that vasopressin levels were lower in adults with refractory vasodilatory septic shock [16]. It is important to note that augmenting SVR, while improving the mean arterial pressure, may cause a reduction in stroke volume and cardiac output, particularly in patients with impaired systolic function. Some children after CPB also exhibit lower than expected levels of vasopressin, providing a rationale for its use [17]. In 2007, Lechner and colleagues evaluated neonates with catecholamine-resistant shock in the following CPB and showed a reduction in catecholamine requirement and an increase in mean arterial pressure with the use of vasopressin [18]. Other studies have also demonstrated a decrease need for fluid and catecholamine requirements and improved blood pressure after complex heart surgery in neonates with the use of vasopressin [14, 15]. Another agent that may be used to treat vasodilatory shock is norepinephrine, which possesses significant α-adrenergic activity as well as providing inotropic support.
The use of glucocorticoids for catecholamine-resistant shock in the neonatal and pediatric population is another strategy that may be of benefit, including following cardiac surgery [19-21]. Glucocorticoids may work through a number of mechanisms, including an increase in the expression of adrenoreceptors. The use of glucocorticoids however is not without its challenges. In children with post-operative LCOS, the response to hydrocortisone does not appear to be related to the baseline cortisol level [22]. In addition, the administration of high dose glucocorticoids may be associated with increased morbidity [23-25]. Post-operative hydrocortisone administration has been identified as an independent risk factor for the development of a catheter-associated bloodstream infection in infants after heart surgery [23]. Most recently, cumulative steroid exposure (7 vs. 4 days, p<0.001) has been shown to be independently associated with occurrence of infection in postoperative cardiac patients [24]. Multi-center data analysis has not demonstrated a mortality benefit with the use of perioperative steroids but rather an increase in postoperative morbidity [25].
THE ROLE OF POSITIVE PRESSURE VENTILATION IN THE LCOS
The impact of positive pressure ventilation (PPV) on cardiovascular function is reviewed in detail elsewhere in this issue. In brief, PPV is an invaluable tool in the armamentarium to treat the LCOS. PPV increases intrathoracic pressure and in doing so decreases systemic ventricular afterload, which is of particular benefit to patients with impaired systemic ventricular systolic dysfunction or in patients experiencing exaggerated negative pressure breathing, as is seen in pulmonary edema or airway disease. Another benefit of PPV results from the mechanical unloading of the respiratory muscles when cardiac output is limited. When oxygen demand of the respiratory muscles is elevated, as in unobstructed hyperventilation or when respiratory mechanics are impaired, perfusion of the respiratory pump must increase to meet the increase in metabolic demand. By mechanically unloading the respiratory pump, respiratory muscle perfusion requirements decrease, and a limited cardiac output may be redistributed to other vital organs, including the brain and myocardium [26, 27].
MECHANICAL CIRCULATORY SUPPORT
When residual mechanical lesions have been ruled out and inadequate cardiac output persists despite maximal medical therapy, extracorporeal life support (ECLS) should be considered. Patients placed on venoarterial ECLS can either be cannulated via a central approach (right atrium and aorta) or via the right neck (right carotid artery and right internal jugular vein). Progressive LCOS is the cause for 36% of post-operative ECLS cannulations, making it one of the most common indications for ECLS in the post-operative period [28-32]. Survival of pediatric patients who receive ECLS after cardiac surgery is approximately 41-49% [28-32]. Timing of ECLS initiation is a key determinant of outcome. Ongoing end organ hypoperfusion and subsequent organ injury such as the development of acute kidney injury contribute to morbidity and mortality associated with ECLS [32].
CONCLUSION
LCOS is an expected, frequent physiologic challenge following pediatric cardiac surgery that requires exquisitely diligent bedside monitoring and thoughtful intervention. Identification of reversible conditions such as residual surgical lesions or transient impairment of ventricular function must be diagnosed and addressed. The initiation of therapeutic strategies such as inotropes, steroids, inodilators, afterload reducing agents, and mechanical ventilation may all have a role in augmenting cardiac output, decreasing oxygen demand, and improving the relationship between oxygen supply and demand. When medical interventions fail, transition to extracorporeal support should be pursued to support end organ function, allowing for myocardial recovery.
AUTHOR CONTRIBUTIONS
Authors Heather Chandler and Roxanne Kirsch contributed equally to the research, preperation, revisions and completion of the Management of the low cardiac output syndrome following surgery for congenital heart disease Manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENT
Declared none.
REFERENCES
- 1.Parr G.V., Blackstone E.H., Kirklin J.W. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975;51(5):867–874. doi: 10.1161/01.CIR.51.5.867. [DOI] [PubMed] [Google Scholar]
- 2.Wernovsky G., Wypij D., Jonas R.A., Mayer J.E., Jr, Hanley F.L., Hickey P.R., Walsh A.Z., Chang A.C., Castañeda A.R., Newburger J.W., Wessel D.L. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–2235. doi: 10.1161/01.CIR.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 3.Asimakopoulos G., Taylor K.M. Effects of cardiopulmonary bypass on leukocyte and endothelial adhesion molecules. Ann. Thorac. Surg. 1998;66(6):2135–2144. doi: 10.1016/S0003-4975(98)00727-9. [DOI] [PubMed] [Google Scholar]
- 4.Nagashima M, Imai Y, Seo K, et al. Effect of hemofiltrated whole blood pump priming on hemodynamics and respiratory function after the arterial switch operation in neonates. ann Thorac Surg. 2000;70:1901–1906. doi: 10.1016/s0003-4975(00)02024-5. [DOI] [PubMed] [Google Scholar]
- 5.Davies M.J., Nguyen K., Gaynor J.W., Elliott M.J. Modified ultrafiltration improves left ventricular systolic function in infants after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1998;115(2):361–369. doi: 10.1016/S0022-5223(98)70280-6. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu T., Imai Y., Kurosawa H., Takanashi Y., Aoki M., Shinoka T., Nakazawa M. Effects of dilutional and modified ultrafiltration in plasma endothelin-1 and pulmonary vascular resistance after the Fontan procedure. Ann. Thorac. Surg. 2002;73(3):861–865. doi: 10.1016/S0003-4975(01)03564-0. [DOI] [PubMed] [Google Scholar]
- 7.Connors A.F., Jr, McCaffree D.R., Gray B.A. Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N. Engl. J. Med. 1983;308(5):263–267. doi: 10.1056/NEJM198302033080508. [DOI] [PubMed] [Google Scholar]
- 8.Lobos A-T., Lee S., Menon K. Capillary refill time and cardiac output in children undergoing cardiac catheterization. Pediatr. Crit. Care Med. 2012;13(2):136–140. doi: 10.1097/PCC.0b013e318220afdc. [DOI] [PubMed] [Google Scholar]
- 9.Bailey J.M., Miller B.E., Lu W., Tosone S.R., Kanter K.R., Tam V.K. The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology. 1999;90(4):1012–1018. doi: 10.1097/00000542-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Chang A.C., Atz A.M., Wernovsky G., Burke R.P., Wessel D.L. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit. Care Med. 1995;23(11):1907–1914. doi: 10.1097/00003246-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman T.M., Wernovsky G., Atz A.M., Kulik T.J., Nelson D.P., Chang A.C., Bailey J.M., Akbary A., Kocsis J.F., Kaczmarek R., Spray T.L., Wessel D.L. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107(7):996–1002. doi: 10.1161/01.CIR.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 12.Wright E.M., Skoyles J., Sherry K.M. Milrinone in the treatment of low output states following cardiac surgery. Eur. J. Anaesthesiol. Suppl. 1992;5(5) Suppl.:21–26. [PubMed] [Google Scholar]
- 13.Alten J.A., Borasino S., Toms R., Law M.A., Moellinger A., Dabal R.J. Early initiation of arginine vasopressin infusion in neonates after complex cardiac surgery. Pediatr. Crit. Care Med. 2012;13(3):300–304. doi: 10.1097/PCC.0b013e31822f1753. [DOI] [PubMed] [Google Scholar]
- 14.Burton G.L., Kaufman J., Goot B.H., da Cruz E.M. The use of Arginine Vasopressin in neonates following the Norwood procedure. Cardiol. Young. 2011;21(5):536–544. doi: 10.1017/S1047951111000370. [DOI] [PubMed] [Google Scholar]
- 15.Mastropietro C.W., Davalos M.C., Seshadri S., Walters H.L., III, Delius R.E. Clinical response to arginine vasopressin therapy after paediatric cardiac surgery. Cardiol. Young. 2013;23(3):387–393. doi: 10.1017/S1047951112000996. [DOI] [PubMed] [Google Scholar]
- 16.Landry D.W., Levin H.R., Gallant E.M., Ashton R.C., Jr, Seo S., D’Alessandro D., Oz M.C., Oliver J.A. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125. doi: 10.1161/01.CIR.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 17.Mastropietro C.W., Rossi N.F., Clark J.A., Chen H., Walters H., III, Delius R., Lieh-Lai M., Sarnaik A.P. Relative deficiency of arginine vasopressin in children after cardiopulmonary bypass. Crit. Care Med. 2010;38(10):2052–2058. doi: 10.1097/CCM.0b013e3181eed91d. [DOI] [PubMed] [Google Scholar]
- 18.Lechner E., Hofer A., Mair R., Moosbauer W., Sames-Dolzer E., Tulzer G. Arginine-vasopressin in neonates with vasodilatory shock after cardiopulmonary bypass. Eur. J. Pediatr. 2007;166(12):1221–1227. doi: 10.1007/s00431-006-0400-0. [DOI] [PubMed] [Google Scholar]
- 19.Menon K. Use of hydrocortisone for refractory shock in children. Crit. Care Med. 2013;41(10):e294–e295. doi: 10.1097/CCM.0b013e31828cf478. [DOI] [PubMed] [Google Scholar]
- 20.Shore S., Nelson D.P., Pearl J.M., Manning P.B., Wong H., Shanley T.P., Keyser T., Schwartz S.M. Usefulness of corticosteroid therapy in decreasing epinephrine requirements in critically ill infants with congenital heart disease. Am. J. Cardiol. 2001;88(5):591–594. doi: 10.1016/S0002-9149(01)01751-9. [DOI] [PubMed] [Google Scholar]
- 21.Suominen P.K., Dickerson H.A., Moffett B.S., Ranta S.O., Mott A.R., Price J.F., Heinle J.S., McKenzie E.D., Fraser C.D., Jr, Chang A.C. Hemodynamic effects of rescue protocol hydrocortisone in neonates with low cardiac output syndrome after cardiac surgery. Pediatr. Crit. Care Med. 2005;6(6):655–659. doi: 10.1097/01.PCC.0000185487.69215.29. [DOI] [PubMed] [Google Scholar]
- 22.Verweij E.J., Hogenbirk K., Roest A.A., van Brempt R., Hazekamp M.G., de Jonge E. Serum cortisol concentration with exploratory cut-off values do not predict the effects of hydrocortisone administration in children with low cardiac output after cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2012;15(4):685–689. doi: 10.1093/icvts/ivs292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello J.M., Graham D.A., Morrow D.F., Potter-Bynoe G., Sandora T.J., Laussen P.C. Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr. Crit. Care Med. 2009;10(4):453–459. doi: 10.1097/PCC.0b013e318198b19a. [DOI] [PubMed] [Google Scholar]
- 24.Mastropietro C.W., Barrett R., Davalos M.C., Zidan M., Valentine K.M., Delius R.E., Walters H.L., III Cumulative corticosteroid exposure and infection risk after complex pediatric cardiac surgery. Ann. Thorac. Surg. 2013;95(6):2133–2139. doi: 10.1016/j.athoracsur.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali S.K., Hall M., Li J.S., Peterson E.D., Jaggers J., Lodge A.J., Marino B.S., Goodman D.M., Shah S.S. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122(21):2123–2130. doi: 10.1161/CIRCULATIONAHA.110.948737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viires N., Sillye G., Aubier M., Rassidakis A., Roussos C. Regional blood flow distribution in dog during induced hypotension and low cardiac output. Spontaneous breathing versus artificial ventilation. J. Clin. Invest. 1983;72(3):935–947. doi: 10.1172/JCI111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain S.N., Roussos C. Distribution of respiratory muscle and organ blood flow during endotoxic shock in dogs. J. Appl. Physiol. 1985;59(6):1802–1808. doi: 10.1152/jappl.1985.59.6.1802. [DOI] [PubMed] [Google Scholar]
- 28.Lequier L., Joffe A.R., Robertson C.M., Dinu I.A., Wongswadiwat Y., Anton N.R., Ross D.B., Rebeyka I.M., Western Canadian Complex Pediatric Therapies Program Follow-up Group Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J. Thorac. Cardiovasc. Surg. 2008;136(4):976–983.e3. doi: 10.1016/j.jtcvs.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Joffe A.R., Lequier L., Robertson C.M. Pediatric outcomes after extracorporeal membrane oxygenation for cardiac disease and for cardiac arrest: a review. ASAIO J. 2012;58(4):297–310. doi: 10.1097/MAT.0b013e31825a21ff. [DOI] [PubMed] [Google Scholar]
- 30.Kumar T.K., Zurakowski D., Dalton H., Talwar S., Allard-Picou A., Duebener L.F., Sinha P., Moulick A. Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome. J. Thorac. Cardiovasc. Surg. 2010;140(2):330–336.e2. doi: 10.1016/j.jtcvs.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Wolf M.J., Kanter K.R., Kirshbom P.M., Kogon B.E., Wagoner S.F. Extracorporeal cardiopulmonary resuscitation for pediatric cardiac patients. Ann. Thorac. Surg. 2012;94(3):874–879. doi: 10.1016/j.athoracsur.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 32.Chai P.J., Jacobs J.P., Dalton H.J., Costello J.M., Cooper D.S., Kirsch R., Rosenthal T., Graziano J.N., Quintessenza J.A. Extracorporeal cardiopulmonary resuscitation for post-operative cardiac arrest: indications, techniques, controversies, and early results--what is known (and unknown). Cardiol. Young. 2011;21(Suppl. 2):109–117. doi: 10.1017/S1047951111001685. [DOI] [PubMed] [Google Scholar]