Abstract
The field of pediatric mechanical circulatory support has undergone a significant evolution with the advent of devices designed for children and the implementation of new strategies for deployment. With the ongoing shortage of organs the demand for new devices specifically designed for children will only increase. This review discusses the evolution of mechanical circulatory support, available devices, and the implementation of new strategies for their deployment.
Keywords: Cardiac, heart failure, mechanical circulatory support, pediatric, transplantation, remodeling.
INTRODUCTION
The last decade has witnessed a significant maturation in the field of mechanical circulatory support (MCS) for adults. As a result of continuing improvements in device technology as well as advances in clinical management, outcomes of MCS continue to improve, resulting in a rapid increase in the number of patients who receive MCS [1]. By contrast, the field of MCS for children has significantly lagged behind its adult counterpart [2]. This is primarily because of limitations in MCS devices available for children, particularly in infants and small children. This frustrating reality, however, has begun to change. With widespread acceptance and application of the Berlin Heart EXCOR®, which is available in a wide range of sizes, even the smallest infants can now benefit from long-term ventricular assist device (VAD) support. This represents a substantially better option when compared to extracorporeal membrane oxygenation (ECMO), which formerly was the only MCS option for small children. In addition, the relatively smaller size of the newer “adult” continuous-flow VADs has allowed for their deployment in larger children and adolescents [3]. In this review, we discuss the current state of pediatric MCS.
DRAMATIC CHANGES IN HEART FAILURE MANAGEMENT
In recent years, durable VADs have become part of the standard care of adults with end-stage heart failure. The newest devices are small (relatively), implantable continuous-flow VADs, which have only a power cord traversing the skin. In North America, these devices include the HeartMate II (Thoratec, Corp., Pleasanton, CA; Fig. (1) and the HeartWare HVAD (HeartWare Inc., Framingham, Massachusetts; Fig. (2). Because of the favorable low morbidity profile of these devices as compared to prior pulsatile pumps, the indications for device placement have changed, making early device implantation an acceptable approach in preference to escalating medical management [4]. Indeed, VADs are now used not only for bridging patients to cardiac transplantation, but also as destination therapy, i.e. VAD implantation is the final therapy, in those patients not deemed to be transplant candidates. In fact, the number of patients with the destination therapy indication has grown rapidly and now accounts for at least 40% of implants [1]. Currently, destination therapy is offered only to those who are not transplant eligible. However with the evolution of the VAD, the heart failure community is nearing the point where VADs are becoming a primary alternative to cardiac transplantation [5], with the concept of delaying transplantation indefinitely, even in patients who would otherwise be good transplant candidates.
Fig. (1).
Heart Mate II.
Fig. (2).
Heart Ware HVAD.
There has been an increasing interest in pediatric MCS for several reasons with the primary factor being the number of children with end-stage heart failure far exceeding the number of potential donors. The number of pediatric heart transplants worldwide has remained relatively stable at 300 to 400 per year and the annual number of heart failure-related hospitalizations in the United States has also remained stable [6, 7]. However, the hospital length of stay has increased from a mean of 13.8 days in 1997 to a mean of 19.4 days in 2006, supporting the notion that the wait time for transplantation has increased substantially [8].
Although ECMO has been used for bridging patients to transplantation, the outcome of patients bridged with ECMO is generally poor, with a significant number of patients dying before receiving a transplant. This is particularly true in recent years as the time to transplant increases. Even for those ECMO-bridged candidates fortunate enough to receive a transplant, post-transplant outcomes are substantially worse compared to patients not bridged with ECMO [9]. Using data from the Extracorporeal Life Support Organization Registry, Almond et al. have shown that a third of pediatric heart transplant recipients bridged with ECMO die during the same admission [10]. Clearly, there is a need for an alternative MCS strategy for bridging children to heart transplantation.
TIMING OF MECHANICAL CIRCULATORY SUPPORT
There is no simple answer as to when MCS support is indicated in children. As with all complex therapy, a risk benefit assessment must be made. Since this calculation in children varies widely depending on unique patient specific-factors (age, size, anatomic features, re-do status, etc.), institutional experience, and types of devices available, the decision has to be made on a case-by-case basis. In general, MCS support is indicated when the severity of heart failure exceeds the efficacy of maximal medical therapy. However, the definition of “maximal medical therapy” is imprecise and variable, as is the definition of failure of that therapy. Furthermore, the spectrum and frequency of device-associated complications is difficult to quantify for children, but is certainly more formidable than for their adult counterparts. In fact in the adult population there has been a clear trend toward earlier institution of VAD support, prior to the patient incurring end organ injury. This is in large part the result of a significant reduction in device-associated morbidity and overall improved outcomes [4]. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) scale has been widely accepted for use in adult MCS candidates and stratifies patients according to the severity of illness (profile 1 representing the most ill patients and profile 7 the least ill). It is clear that patients with less severe heart failure are now being implanted [11, 12], with dramatic improvements in the outcomes of long-term VAD implantation [1]. It must be emphasized, however, that this aggressive approach is reasonable because of the low morbidity profiles of the devices available for adult patients.
By contrast, physicians caring for children with heart failure are much less enthusiastic about implantation earlier on in the course of illness and prior to the patient becoming critically ill. This is particularly relevant for small children who can only be supported with pulsatile, paracorporeal VADs, such as the Berlin Heart EXCOR (Fig. 3) (Berlin Heart Inc., The Woodlands, TX) that have significantly higher risk profiles [13] than the implantable, continuous-flow devices used in adults. The experience with the Berlin Heart EXCOR (n=42 as of December 2014) and its morbidity profiles at Texas Children’s Hospital (Fig. 4) were recently reviewed. The incidence of pump thrombosis requiring pump exchange was approximately 0.6 events per patient-month. This is nearly 100-fold higher than the incidences for HeartMate II and HeartWare HVAD, which are about 0.04 to 0.08 events per patient-year, and occurs even in the presence of a much more intense anticoagulation protocol for the EXCOR device. Currently, most centers consider application of the EXCOR only in critically ill children with cardiogenic shock or evidence of impending end-organ injury (i.e. INTERMACS profile 1 or 2), or those who have “crashed” onto emergent ECMO support. Although waiting too long to implant the EXCOR sharply increases the risk of mortality, initiating EXCOR support too early can also increase the risk of morbidities. The dilemma for clinicians is that the window of opportunity for implantation between “too early” and “too late” for this particular device may be quite slim [14]. If the patient is deemed large enough to accommodate an adult-sized implantable device, VAD support is offered sooner, typically at INTERMACS profile 2 or 3 because the risk profiles for these devices justify an earlier deployment. The worldwide experience of HVAD use in small children is still limited. The smallest patient in the Texas Children’s Hospital series is a 5-year-old male with body weight of 13 kg and body surface area of 0.6 m2.
Fig. (3).
Berlin Heart EXCOR.
Fig. (4).
Temporary distribution of thrombotic and hemorrhagic complications during Berlin Heart EXCOR ventricular assist device support at Texas Children’s Hospital.
The use of implantable “adult” VADs in small children mandates several important considerations. One of the most critical of these is the angle of the inflow cannula relative to the interventricular septum [15]. If the HeartWare HVAD is placed in the standard manner, the so-called ‘intrapericardial technique’ recommended by the manufacturer, the inflow cannula may come to lie nearly perpendicular to the interventricular septum (Fig. 5). Such an unfavorable angle may be tolerable in a severely dilated adult heart, but in children may result in obstruction of the inflow cannula. However, even in the adult population, it is known that conditions with a smaller left ventricular cavity (restrictive or hypertrophic cardiomyopathy) may be much less forgiving of minor imperfections in the position of the inflow cannula [16]. The standard technique in adult patients may therefore not be the best option for small children who in effect have ‘device-patient size mismatch.’ Our approach to this problem is to place the HVAD pump in the infradiaphragmatic space, which allows the inflow axis to align nearly parallel to the interventricular septum (Fig. 5). Previous experience with the HeartMate II has shown that a horizontal orientation of the inflow cannula (and hence perpendicular relationship to the septum) predisposes to pump thrombosis [17]. This lesson learned from the experience with the HeartMate II may not be directly applicable to the HVAD, but certainly great attention must be paid to the orientation of the inflow cannula when implanting this device in small children.
Fig. (5).
The different angles of the inflow cannula of the HeartWare HVAD.
In addition to size related considerations, anatomic factors may require deliberation when planning VAD implantation in children, particularly in those with congenital heart disease, as the anatomic variations may pose technical challenges to cannulation (e.g., abnormal size and location of the aorta, unusual cardiac situs, or shape of the ventricle). Previous surgical palliation may also complicate the application of MCS, particularly in children with univentricular hearts where pulmonary blood flow is provided by systemic to pulmonary shunts or cavopulmonary anastomosis (or anastomoses).
SELECTION OF DEVICE
There are two major factors to consider when selecting a modality of MCS. First, the anticipated duration of support should be determined, which is primarily based on the etiology of the heart failure (acute [e.g. viral myocarditis] vs. chronic [e.g. cardiomyopathy or end-pathy or end-stage congenital heart disease]). A decision has to be made as to whether the patient needs temporary support (mostly for acute etiologies) or long-term support (for chronic etiologies). Temporary MCS is also the support of choice when the etiology is unknown or transplant candidacy is uncertain. Stabilization with temporary MCS will allow for further evaluation in order to make a decision regarding further support, an approach termed “bridge-to-decision”. If long-term support is deemed necessary as a bridge-to-transplant or to allow for possible cardiac recovery, then temporary MCS is transitioned to a long-term VAD as a “bridge-to-bridge”.
Second, a determination must be made as to the extent of support that will be required, which includes left ventricular (LVAD), right ventricular (RVAD), or biventricular (BiVAD) support. Since acute decompensated heart failure may result in significant pulmonary dysfunction, some children with severe heart failure may require not only cardiac but also pulmonary support in the form of ECMO. Extracorporeal cardiopulmonary resuscitation (ECPR) [18] is another example of when ECMO may be indicated for MCS. If cardiac support is all that is necessary in a rapidly declining patient, VAD support alone is preferred, which represents something of a paradigm shift away from the somewhat more familiar first line use of ECMO. The rationale for the recommendation of temporary VAD support rather than ECMO for pure cardiac support will be discussed in the next section.
WHY NOT ECMO FOR CIRCULATORY SUPPORT?
There are two major disadvantages of ECMO for pure circulatory support. The first of these relates to the inclusion of the oxygenator in the circulation, which stimulates a pro-inflammatory response, rendering the management of a patient on MCS far more challenging than it need be. The second major disadvantage of ECMO for pure circulatory support relates to the inability to adequately decompress the pulmonary atrium, which in turn will worsen pulmonary dysfunction (Fig. 6). Though an atrial septostomy or placement of a percutaneous trans-atrial catheter may be helpful in ameliorating this circumstance, left atrial hypertension remains an important drawback of ECMO. That said, there are certain circumstances in which ECMO may be preferred including the presence of significant pulmonary hypertension with right ventricular dysfunction, hemodynamic instability due to septic shock, or severe pulmonary edema resulting from ventricular dysfunction [19, 20]. Aside from these exceptional situations, short-term VAD support, for periods less than two weeks, is a reasonable option for pure cardiac support, in preference to the “traditional” use of ECMO for temporary support.
Fig. (6).
The difference between ECMO and VAD in terms of decompression of the left heart.
CENTRAL CANNULATION FOR SHORT-TERM VAD SUPPORT
Although an LV apical cannulation is an option [21], our preferred approach is to place an inflow cannula in the left atrium, either via the Waterston’s groove or the left atrial appendage. We prefer this approach in order to avoid an incision in the potentially salvageable and recoverable left ventricle. In our experience, left atrial cannulation provides stable inflow and adequate decompression of the left heart. In the Texas Children’s Hospital experience of more than 40 patients, left ventricular thrombus formation, one theoretical drawback of left atrial cannulation, has not been seen. The necessity of sternotomy for central cannulation might be considered a disadvantage of the short-term VAD support strategy compared to ECMO, but one advantage of this approach is the opportunity to place larger cannulae, which allow for optimal drainage of the left heart. This is particularly true when there is increased cardiac return (e.g. systemic-pulmonary collaterals) that is often the case in the pediatric population. Adequate decompression of the systemic ventricle is of critical importance for myocardial recovery and subsequent VAD explantation. Furthermore, the use of a central cannulation strategy avoids the potential for major vascular complications, which is ever-present if peripheral (ECMO) cannulation is employed in small children.
PERIPHERAL CANNULATION FOR SHORT-TERM VAD SUPPORT
Although the most typical approach for short-term VAD support is central cannulation via sternotomy, peripheral cannulation can be an alternative option in selected cases if the patient is of sufficient size. An inflow venous cannula is inserted via the femoral vein and advanced into the left atrium by crossing the atrial septum [22], while the return is through a femoral arterial cannula. One advantage of this technique is the ability to rapidly establish VAD support without a sternotomy, which is particularly useful when hemodynamic instability is present and there is a history of a sternotomy. The TandemHeart (CardiacAssist, Inc., Pittsburgh, PA) is a currently available short-term VAD system that relies on peripheral cannulation. This system provides a 21 Fr transseptal venous cannula (62 or 72 cm in length). Due to the size of this venous cannula, its application in the pediatric population is limited to larger adolescents (at least 40 kilograms). Aside from the size limitation, a potential problem with a transseptal venous cannula is dislodgement, which would result in severe hypoxemia if the cannula were to retract into the right atrium. This is much more of a concern in children with acute heart failure wherein the size of the left atrium is smaller than in adult patients.
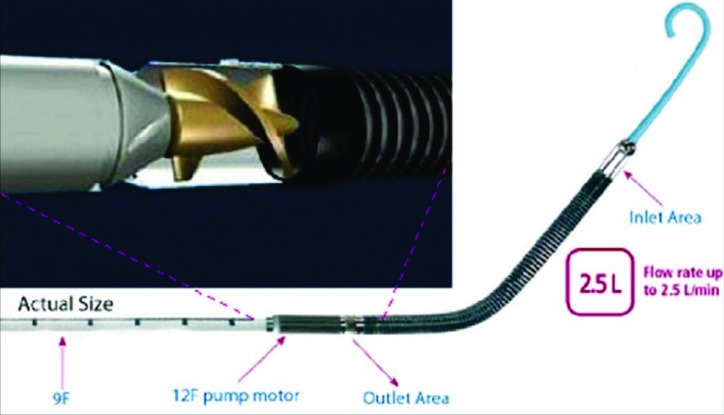
A catheter-based VAD such as the Impella (Abiomed, Danvers, MA) can also be placed percutaneously via the femoral artery or a graft sewn on the right axillary artery and comes in several different sizes. The smallest device in the Impella family is called 2.5® (Fig. 7), which can flow up to 2.5 L/minute. The size of the catheter is 9 Fr and its largest part (a pump motor) is 12 Fr. Successful use of this device has been reported in the pediatric population [23, 24]. One potential challenge to the use of this device in small children is the length (rather than diameter) of the left ventricular cavity. Since the distance from the outlet area (which must sit distal to the aortic valve) to the tip of the catheter is relatively long, the device may not be appropriate for smaller hearts. If the heart is too small, contact of the pigtail tip of the catheter with the myocardium may induce arrhythmias. Because experience with the Impella in children is very limited (only 7 children in the U.S. as of January 2015), it is unclear how small is too small for this device. The smallest known patient, a 6-year-old female (weight 22kg, BSA of 0.85 m2) with acute viral myocarditis (Fig. 8), was well supported with the Impella 2.5 without complications until her cardiac function recovered at which time she developed ventricular ectopy, presumably due to mechanical stimulation of the LV from the catheter. A true pediatric version of the Impella with a shorter catheter length is now under development [25].
Fig. (7).
Impella 2.5.
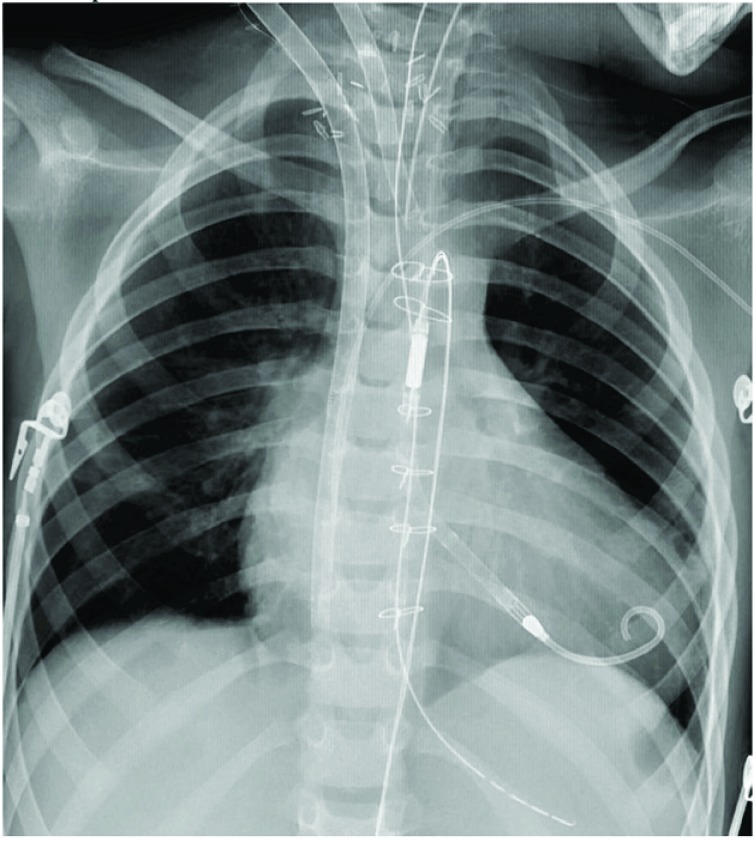
Fig. (8).
A chest-°© Xray with in a child with ECMO (via neck cannulation) and Impella 2.5 support for left heart decompression.
SHORT-TERM VAD SUPPORT FOR INITIAL STA-BILIZATION
Short-term VAD support may also play a role in the management of acute decompensated chronic heart failure. In this setting, cardiac recovery with temporary VAD support is unlikely due to the chronic nature of the disease, and a transition to a more durable form of MCS support is anticipated. However, some patients may present in such dire condition that logistical challenges preclude placement of a durable device. In the past, such a patient might have been “medically managed” unsuccessfully or may have even deteriorated to ECPR (ECMO). A more aggressive and in our opinion more reasonable approach is to stabilize the patient’s hemodynamics with short-term VAD support, reverse existing end-organ dysfunction, and proceed electively to implantation of a durable VAD.
Another example of the application of a short-term device is in small children for whom Berlin Heart implantation is ultimately planned. In such children, the cannulas may be placed for the EXCOR device but attached to a temporary continuous flow device such as the Pedimag (Thoratec Corporation, Pleasanton, California), which allows for a lesser degree of anti-coagulation early after surgery when the risk of bleeding is highest. This temporary/long-term hybrid approach has become the standard at Duke Children’s Hospital, in an attempt to lower the risk profile for these smallest children.
LONG-TERM VENTRICULAR ASSIST DEVICE
When the etiology of heart failure is chronic in nature and the patient is relatively stable, a long-term VAD should be considered as a first line device. The selection of device is mainly determined by the size of the patient. In North America, the Berlin EXCOR is the device of choice in children with a body surface area of 0.7 m2 or smaller. As discussed, implantable, continuous-flow devices are preferred in larger children. In the past, the HeartMate II (Thoratec Corp.; Pleasanton, CA) was used in patients with a body surface area of 1.3 m2 or larger [26], but more recently it has been supplanted by the HeartWare HVAD.
Most children with severe heart failure can be supported with an LVAD alone, despite the fact that some degree of right heart dysfunction is virtually always present. With a reduction in the left atrial pressure following LVAD implantation, right ventricular afterload decreases. In most circumstances, a LVAD alone will suffice if right ventricular failure is ‘secondary’ to pulmonary venous hypertension and LV failure. If significant tricuspid regurgitation is present, tricuspid valve repair, typically with a ring annuloplasty, may be helpful. Conversely, the addition of a RVAD may be necessary if the right ventricular myocardium is inherently compromised. Also, when pulmonary vascular resistance is severely elevated, as seen in restrictive cardiomyopathy [27], BiVAD support may be necessary. Since previous studies have consistently shown that support with a BiVAD is associated with worse outcomes, it is prudent to apply BiVAD support selectively.
MANAGEMENT OF THE RIGHT VENTRICLE IN PATIENTS WITH A LVAD
In patients with isolated LVAD support, the focus of postoperative care is directed at optimizing right ventricular output, since this will be the limiting factor in determining systemic output. The basic principles of maintaining adequate right heart output include: minimizing pulmonary vascular resistance with the use of pulmonary vasodilators such as inhaled nitric oxide acutely and sildenafil chronically; maintaining adequate preload to the right heart, with care taken not to over distend the right heart and interfere with LV function through diastolic ventricular interdependence; and provision of RV inotropy. Although echocardiography is a useful tool to assess right ventricular function, one must not solely rely on echocardiographic findings. The fundamental question is whether an LVAD is filling adequately or not. If the filling of the LVAD is adequate, right heart function is by definition adequate, so long as the right atrial pressure is not inordinately high. In our experience, it is not uncommon to see adequate filling of the LVAD despite the demonstration of severely depressed RV function with echocardiography. With an extracorporeal, pulsatile pump, such as the Berlin Heart EXCOR, the filling status of a pump can be assessed by direct inspection of the chamber, providing a good indication of right heart output.
REACTIVATION OF THE PATIENT FOLLOWING VAD PLACEMENT
Generally speaking, the average wait time for heart transplantation is shorter in children with VAD support than in their adult counterparts [8]. A potential contributing factor is the difference in listing criteria between children and adults defined by the United Network for Organ Sharing (UNOS). Adult patients are given 1A status (the highest priority on the basis of medical urgency) for 30 days following VAD implantation. After the 30-day ‘grace’ period, the patient’s status is downgraded to 1B, unless the patient is experiencing VAD-related complications. However children with ongoing VAD support remain status 1A until transplantation, regardless of the type of VAD they are supported with. This explains, in part, why wait times for children are relatively shorter than for adults. In the Berlin Heart IDE trial [13], the mean duration of VAD support was approximately 1 month (28 days in children with BSA of 0.7 m2 or less and 43 days in larger children). Since the Berlin Heart device is associated with a high incidence of pump-related complications, such as stroke (29%), major bleeding (42- 50%) or infection (50-65%) [13], it is critically important to reactivate the patient as soon as possible after VAD placement in order to minimize the risks of such complications. The timing of reactivation of children with continuous flow “adult” devices, however, may be approached differently because the risk of device related complications is substantially lower. In this setting a “less morbid VAD” may provide the opportunity for physical rehabilitation during VAD support, rendering the patient a much better candidate for eventual heart transplantation. Because most children who undergo VAD placement are profoundly debilitated, months of rehabilitation may be required to optimize their pre-transplant condition. Given the lower risk of device-related complications with a durable continuous flow VAD, it may be reasonable to maintain the patient inactive for a period of time following VAD placement. At Texas Children’s Hospital the current protocol for patients receiving a continuous flow device is to remain on inactive status for the first 3 months following implantation. This period allows for rehabilitation and the possibility, albeit rare, to detect evidence of myocardial recovery.
While a paracorporeal, pulsatile-flow VAD such as the Berlin heart EXCOR is associated with a higher risk of device-related complications, pulsatile VADs may have several potential advantages over continuous-flow VADs, the most significant of which may be a higher potential for myocardial recovery [28]. This concept derives from the anecdotal experience that myocardial recovery appeared to be more frequent in the prior era when pulsatile VADs were the predominant mode of support used in adult patients. Although there is a paucity of data, pediatric myocardium may have a greater propensity for recovery compared with adults due to a greater abundance of cardiac progenitor cells [29]. Several publications on pediatric VAD support include a small proportion of patients (ranging from 5 to 73%) that were weaned from a VAD following myocardial recovery [30-38]. In a report from the German Heart Institute [30] Hezter et al. observed myocardial recovery in 15% of their pediatric patients (9 out of 62). Reports from Zimmerman et al. [32] and Ihnat et al. [33] are exceptional in that the incidences of recovery were substantially higher compared to other reports at 72% (8 out of 11 patients) and 62% (8 out of 13 patients), respectively. In a recent report from the United Kingdom [39] 10 of 53 patients with the EXCOR (19%) showed signs of myocardial recovery and underwent VAD explantation with a median support time of 36 (7 to 120) days. Three of these patients (30%) had a relapse of heart failure, requiring subsequent VAD replacement and eventual heart transplantation however the remaining patients demonstrated that myocardial recovery with VAD support is a real possibility in children. A better understanding of reverse remodeling specific to the pediatric myocardium will be crucial to improving clinical strategies directed at using a VAD as a bridge-to-recovery.
OUTCOMES AFTER LONG-TERM VAD SUPPORT
In the past the success of pediatric VAD support was measured primarily by survival to transplant or weaning, and avoidance of major complications during support (e.g. stroke, bleeding, or infection). As the field of pediatric MCS matures, attention must be directed towards the long-term impact of MCS on outcomes following transplantation. An important factor that impacts outcomes is the degree of human leukocyte antigen (HLA) sensitization during VAD support. Previous data from adults have suggested VAD support is associated with a higher risk of HLA sensitization [40, 41]. Although children may have a different immunological response to human tissue exposure compared to adults, it is likely that exposure to a VAD is also associated with HLA sensitization. Currently available pediatric single-center studies [42-44] have reported the incidence of HLA sensitization in children with VAD (or ECMO) support to be 18 to 35%. A recent study using the Organ Procurement and Transplantation Network database confirms these findings [45]. Specifically, the use of VADs as a bridge to transplant in children with dilated cardiomyopathy is associated with a 3-fold increase in HLA sensitization, a 2-fold increase in the risk of a positive cross-match at transplant, and a higher incidence of rejection after transplant. Another study from the multi-institutional Pediatric Heart Transplant Study Group has shown a higher rate of waitlist mortality and lower rate of post transplant survival [46]. A better understanding of the basic mechanisms of HLA sensitization in children undergoing VAD support will be required to develop strategies to minimize HLA sensitization in this patient population.
Equally important is the long-term effect of MCS on the quality of life in children following transplantation. To date, only two studies have addressed this issue [47, 48]. Using a validated generic measure, the Pediatric Quality of Life Inventory, which assesses the quality of life in pediatric heart transplant recipients, has shown that the quality of life of children bridged to transplant with a MCS is not inferior to those who were not bridged with a device. It will be of paramount importance to provide stable long-term VAD support without complications in order to achieve a truly successful long-term outcome.
DESTINATION THERAPY IN CHILDREN
In the adult world, long-term VAD support is increasingly being used for destination therapy, accounting for 40% of all implants [1]. Destination therapy is currently not a widely accepted strategy in the pediatric population, but may gain a more prominent role in the management of heart failure with ongoing advances in the development of devices. An example of conditions that could potentially benefit from the use of VADs as a destination therapy is Duchenne muscular dystrophy, which is an X-linked recessive disorder that is characterized by progressive skeletal muscle weakness and cardiomyopathy [49]. Successful long-term VAD support as a destination therapy in Duchenne muscular dystrophy in adolescents and young adults has recently been reported [50, 51]. It remains to be seen if this type of therapy gains wider acceptance from the pediatric medical community, though certainly there has been proof-of-concept in highly selected patients at centers capable of sophisticated, multimodal support.
Another population that may be considered for destination therapy is children who have developed cardiomyopathy as a consequence of chemotherapy. Some of these patients present with profound heart failure early after completion of treatment, at the time when an oncologic cure may not be certain. A smaller subset with well-controlled metastatic disease may also merit consideration. In circumstances wherein transplantation may be relatively contraindicated, durable VAD implantation may serve as a bridge-to-decision for transplant in some, or destination therapy in others.
SUMMARY
The number of children with severe heart failure is increasing, whereas the number of organ donors has not kept pace. As a result, the evolution of pediatric MCS has become increasingly more important. The appropriate selection and timing of device deployment is key to optimizing outcomes in patients with end stage heart failure.
ACKNOWLEDGEMENT
Declared none.
AUTHOR CONTRIBUTIONS
Authors Iki Adachi and Robert Jaquiss contributed equally to the manuscript’s research, preparation, editing, revisions and completion.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kirklin J.K., Naftel D.C., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., Miller M.A., Baldwin J.T., Young J.B. Sixth INTERMACS annual report: a 10,000-patient database. J. Heart Lung Transplant. 2014;33(6):555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Adachi I., Fraser C.D., Jr Mechanical circulatory support for infants and small children. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2011;14(1):38–44. doi: 10.1053/j.pcsu.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Miera O., Potapov E.V., Redlin M., Stepanenko A., Berger F., Hetzer R., Hübler M. First experiences with the HeartWare ventricular assist system in children. Ann. Thorac. Surg. 2011;91(4):1256–1260. doi: 10.1016/j.athoracsur.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin J.K., Naftel D.C., Kormos R.L., Stevenson L.W., Pagani F.D., Miller M.A., Baldwin J.T., Young J.B. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J. Heart Lung Transplant. 2013;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Birks E.J. The comparative use of ventricular assist devices: differences between Europe and the United States. Tex. Heart Inst. J. 2010;37(5):565–567. [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk R., Edwards L.B., Aurora P., Taylor D.O., Christie J., Dobbels F., Kucheryavaya A.Y., Rahmel A.O., Hertz M.I. Registry of the International Society for Heart and Lung Transplantation: eleventh official pediatric heart transplantation report--2008. J. Heart Lung Transplant. 2008;27(9):970–977. doi: 10.1016/j.healun.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Rossano J.W., Kim J.J., Decker J.A., Price J.F., Zafar F., Graves D.E., Morales D.L., Heinle J.S., Bozkurt B., Towbin J.A., Denfield S.W., Dreyer W.J., Jefferies J.L. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J. Card. Fail. 2012;18(6):459–470. doi: 10.1016/j.cardfail.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Colvin-Adams M., Smithy J.M., Heubner B.M., Skeans M.A., Edwards L.B., Waller C., Schnitzler M.A., Snyder J.J., Israni A.K., Kasiske B.L. OPTN/SRTR 2012 annual data report: heart. Am. J. Transplant. 2014;14(Suppl. 1):113–138. doi: 10.1111/ajt.12583. [DOI] [PubMed] [Google Scholar]
- 9.Davies R.R., Russo M.J., Hong K.N., O’Byrne M.L., Cork D.P., Moskowitz A.J., Gelijns A.C., Mital S., Mosca R.S., Chen J.M. The use of mechanical circulatory support as a bridge to transplantation in pediatric patients: an analysis of the United Network for Organ Sharing database. J. Thorac. Cardiovasc. Surg. 2008;135(2):421–427, 427.e1. doi: 10.1016/j.jtcvs.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Almond C.S., Singh T.P., Gauvreau K., Piercey G.E., Fynn-Thompson F., Rycus P.T., Bartlett R.H., Thiagarajan R.R. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the United States: analysis of data from the Organ Procurement and Transplant Network and Extracorporeal Life Support Organization Registry. Circulation. 2011;123(25):2975–2984. doi: 10.1161/CIRCULATIONAHA.110.991505. [DOI] [PubMed] [Google Scholar]
- 11.Barbone A., Pini D., Rega F., Ornaghi D., Vitali E., Meyns B. Circulatory support in elderly chronic heart failure patients using the CircuLite® Synergy® system. Eur. J. Cardiothorac. Surg. 2013;44(2):207–212. doi: 10.1093/ejcts/ezt041. [DOI] [PubMed] [Google Scholar]
- 12.Meyns B., Klotz S., Simon A., Droogne W., Rega F., Griffith B., Dowling R., Zucker M.J., Burkhoff D. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J. Am. Coll. Cardiol. 2009;54(1):79–86. doi: 10.1016/j.jacc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Fraser C.D., Jr, Jaquiss R.D., Rosenthal D.N., Humpl T., Canter C.E., Blackstone E.H., Naftel D.C., Ichord R.N., Bomgaars L., Tweddell J.S., Massicotte M.P., Turrentine M.W., Cohen G.A., Devaney E.J., Pearce F.B., Carberry K.E., Kroslowitz R., Almond C.S., Berlin Heart Study Investigators Prospective trial of a pediatric ventricular assist device. N. Engl. J. Med. 2012;367(6):532–541. doi: 10.1056/NEJMoa1014164. [DOI] [PubMed] [Google Scholar]
- 14.Adachi I., Fraser C.D., Jr Berlin Heart EXCOR Food and Drug Administration Investigational Device Exemption Trial. Semin. Thorac. Cardiovasc. Surg. 2013;25(2):100–106. doi: 10.1053/j.semtcvs.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Adachi I., Guzmán-Pruneda F.A., Jeewa A., Fraser C.D., Jr, Dean McKenzie E. A modified implantation technique of the HeartWare ventricular assist device for pediatric patients. J. Heart Lung Transplant. 2015;34(1):134–136. doi: 10.1016/j.healun.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Kirklin J.K., Naftel D.C., Kormos R.L., Pagani F.D., Myers S.L., Stevenson L.W., Acker M.A., Goldstein D.L., Silvestry S.C., Milano C.A., Baldwin J.T., Pinney S., Eduardo Rame J., Miller M.A. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J. Heart Lung Transplant. 2014;33(1):12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Taghavi S., Ward C., Jayarajan S.N., Gaughan J., Wilson L.M., Mangi A.A. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann. Thorac. Surg. 2013;96(4):1259–1265. doi: 10.1016/j.athoracsur.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 18.Fagnoul D., Combes A., De Backer D. Extracorporeal cardiopulmonary resuscitation. Curr. Opin. Crit. Care. 2014;20(3):259–265. doi: 10.1097/MCC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 19.Horton S., d’Udekem Y., Shann F., Butt W., Bennett M., Best D., Brizard C. Extracorporeal membrane oxygenation via sternotomy for circulatory shock. J. Thorac. Cardiovasc. Surg. 2010;139(2):e12–e13. doi: 10.1016/j.jtcvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 20.MacLaren G., Butt W., Best D., Donath S. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr. Crit. Care Med. 2011;12(2):133–136. doi: 10.1097/PCC.0b013e3181e2a4a1. [DOI] [PubMed] [Google Scholar]
- 21.Takayama H., Chen J.M., Jorde U.P., Naka Y. Implantation technique of the CentriMag biventricular assist device allowing ambulatory rehabilitation. Interact. Cardiovasc. Thorac. Surg. 2011;12(2):110–111. doi: 10.1510/icvts.2010.252908. [DOI] [PubMed] [Google Scholar]
- 22.Pavie A., Léger P., Nzomvuama A., Szefner J., Regan M., Vaissier E., Gandjbakhch I. Left centrifugal pump cardiac assist with transseptal percutaneous left atrial cannula. Artif. Organs. 1998;22(6):502–507. doi: 10.1046/j.1525-1594.1998.06145.x. [DOI] [PubMed] [Google Scholar]
- 23.Dimas V.V., Murthy R., Guleserian K.J. Utilization of the Impella 2.5 micro-axial pump in children for acute circulatory support. Catheter. Cardiovasc. Interv. 2014;83(2):261–262. doi: 10.1002/ccd.25042. [DOI] [PubMed] [Google Scholar]
- 24.Murthy R., Brenes J., Dimas V.V., Guleserian K.J. Ringed polytetrafluoroethylene (Gore-Tex) tunneled “chimney” graft for pediatric use of Impella 2.5 axial flow pump. J. Thorac. Cardiovasc. Surg. 2014;147(4):1421–1422. doi: 10.1016/j.jtcvs.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Webb M.K., Lutts E.R., Price D.T., et al. A Porcine Survival Model for the Novel Impella Pediatric Prototype. ASAIO. 2014;P91:A123. [Google Scholar]
- 26.Stiller B., Adachi I., Fraser C.D., Jr Pediatric ventricular assist devices. Pediatr. Crit. Care Med. 2013;14(5) Suppl. 1:S20–S26. doi: 10.1097/PCC.0b013e318292df5f. [DOI] [PubMed] [Google Scholar]
- 27.Kimberling M.T., Balzer D.T., Hirsch R., Mendeloff E., Huddleston C.B., Canter C.E. Cardiac transplantation for pediatric restrictive cardiomyopathy: presentation, evaluation, and short-term outcome. J. Heart Lung Transplant. 2002;21(4):455–459. doi: 10.1016/S1053-2498(01)00400-4. [DOI] [PubMed] [Google Scholar]
- 28.Kato T.S., Chokshi A., Singh P., Khawaja T., Cheema F., Akashi H., Shahzad K., Iwata S., Homma S., Takayama H., Naka Y., Jorde U., Farr M., Mancini D.M., Schulze P.C. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail. 2011;4(5):546–553. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra R., Vijayan K., Colletti E.J., Harrington D.A., Matthiesen T.S., Simpson D., Goh S.K., Walker B.L., Almeida-Porada G., Wang D., Backer C.L., Dudley S.C., Jr, Wold L.E., Kaushal S. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123(4):364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hetzer R., Potapov E.V., Stiller B., Weng Y., Hübler M., Lemmer J., Alexi-Meskishvili V., Redlin M., Merkle F., Kaufmann F., Hennig E. Improvement in survival after mechanical circulatory support with pneumatic pulsatile ventricular assist devices in pediatric patients. Ann. Thorac. Surg. 2006;82(3):917–924. doi: 10.1016/j.athoracsur.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 31.Blume E.D., Naftel D.C., Bastardi H.J., Duncan B.W., Kirklin J.K., Webber S.A., Pediatric Heart Transplant Study Investigators Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113(19):2313–2319. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman H., Covington D., Smith R., Ihnat C., Barber B., Copeland J. Recovery of dilated cardiomyopathies in infants and children using left ventricular assist devices. ASAIO J. 2010;56(4):364–368. doi: 10.1097/MAT.0b013e3181e1d228. [DOI] [PubMed] [Google Scholar]
- 33.Ihnat C.L., Zimmerman H., Copeland J.G., Meaney F.J., Sobonya R.E., Larsen B.T., Blair B., Lax D., Barber B.J. Left ventricular assist device support as a bridge to recovery in young children. Congenit. Heart Dis. 2011;6(3):234–240. doi: 10.1111/j.1747-0803.2011.00494.x. [DOI] [PubMed] [Google Scholar]
- 34.Morales D.L., Almond C.S., Jaquiss R.D., Rosenthal D.N., Naftel D.C., Massicotte M.P., Humpl T., Turrentine M.W., Tweddell J.S., Cohen G.A., Kroslowitz R., Devaney E.J., Canter C.E., Fynn-Thompson F., Reinhartz O., Imamura M., Ghanayem N.S., Buchholz H., Furness S., Mazor R., Gandhi S.K., Fraser C.D., Jr Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J. Heart Lung Transplant. 2011;30(1):1–8. doi: 10.1016/j.healun.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 35.Reinhartz O., Keith F.M., El-Banayosy A., McBride L.R., Robbins R.C., Copeland J.G., Farrar D.J. Multicenter experience with the thoratec ventricular assist device in children and adolescents. J. Heart Lung Transplant. 2001;20(4):439–448. doi: 10.1016/S1053-2498(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 36.Imamura M., Dossey A.M., Prodhan P., Schmitz M., Frazier E., Dyamenahalli U., Bhutta A., Morrow W.R., Jaquiss R.D. Bridge to cardiac transplant in children: Berlin Heart versus extracorporeal membrane oxygenation. Ann. Thorac. Surg. 2009;87(6):1894–1901. doi: 10.1016/j.athoracsur.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y., Weng Y-G., Xiao Y-B., Huebler M., Franz N., Potapov E., Hetzer R. Outcomes of ventricular assist device support in young patients with small body surface area. Eur. J. Cardiothorac. Surg. 2011;39(5):699–704. doi: 10.1016/j.ejcts.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Valeske K., Thul J., Müller M., et al. Mechanical circulatory support in pediatric patients – a review of 15 years. Thorac. Cardiovasc. Surg. 2012;•••:60–PP46. [Google Scholar]
- 39.Irving C.A., Crossland D.S., Haynes S., Griselli M., Hasan A., Kirk R. Evolving experience with explantation from Berlin Heart EXCOR ventricular assist device support in children. J. Heart Lung Transplant. 2014;33(2):211–213. doi: 10.1016/j.healun.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Massad M.G., Cook D.J., Schmitt S.K., Smedira N.G., McCarthy J.F., Vargo R.L., McCarthy P.M. Factors influencing HLA sensitization in implantable LVAD recipients. Ann. Thorac. Surg. 1997;64(4):1120–1125. doi: 10.1016/S0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 41.John R., Lietz K., Schuster M., Naka Y., Rao V., Mancini D.M., Rose E.A., Smith C.R., Oz M.C., Edwards N.M., Itescu S. Immunologic sensitization in recipients of left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2003;125(3):578–591. doi: 10.1067/mtc.2003.30. [DOI] [PubMed] [Google Scholar]
- 42.Yang J., Schall C., Smith D., Kreuser L., Zamberlan M., King K., Gajarski R. HLA sensitization in pediatric pre-transplant cardiac patients supported by mechanical assist devices: the utility of Luminex. J. Heart Lung Transplant. 2009;28(2):123–129. doi: 10.1016/j.healun.2008.11.908. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor M.J., Menteer J., Chrisant M.R., Monos D., Lind C., Levine S., Gaynor J.W., Hanna B.D., Paridon S.M., Ravishankar C., Kaufman B.D. Ventricular assist device-associated anti-human leukocyte antigen antibody sensitization in pediatric patients bridged to heart transplantation. J. Heart Lung Transplant. 2010;29(1):109–116. doi: 10.1016/j.healun.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Hong B.J., Delaney M., Guynes A., Warner P., McMullan D.M., Kemna M.S., Boucek R.J., Law Y.M. Human leukocyte antigen sensitization in pediatric patients exposed to mechanical circulatory support. ASAIO J. 2014;60(3):317–321. doi: 10.1097/MAT.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 45.Almond C.S., Daly K.P., Singh T.P., et al. Effect of VAD use on HLA sensitization and risk of rejection post-heart transplant in US children with dilated cardiomyopathy. J. Heart Lung Transplant. 2013;32:S108. doi: 10.1016/j.healun.2013.01.1038. [DOI] [Google Scholar]
- 46.Mahle W.T., Tresler M.A., Edens R.E., Rusconi P., George J.F., Naftel D.C., Shaddy R.E., Pediatric Heart Transplant Study Group Allosensitization and outcomes in pediatric heart transplantation. J. Heart Lung Transplant. 2011;30(11):1221–1227. doi: 10.1016/j.healun.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Wray J., Lunnon-Wood T., Smith L., Orrells C., Iguchi A., Burch M., Brown K. Perceived quality of life of children after successful bridging to heart transplantation. J. Heart Lung Transplant. 2012;31(4):381–386. doi: 10.1016/j.healun.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Ezon D.S., Khan M.S., Adachi I., Jeewa A., Morris S.A., Nagy C.Z., Morales D.L., Heinle J.S. Pediatric ventricular assist device use as a bridge to transplantation does not affect long-term quality of life. J. Thorac. Cardiovasc. Surg. 2014;147(4):1334–1343. doi: 10.1016/j.jtcvs.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Manzur A.Y., Kinali M., Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch. Dis. Child. 2008;93(11):986–990. doi: 10.1136/adc.2007.118141. [DOI] [PubMed] [Google Scholar]
- 50.Amodeo A, Adorisio R. Left ventricular assist device in Duchenne cardiomyopathy: can we change the natural history of cardiac disease? Int J Cardiol. 2012;161(3):e43. doi: 10.1016/j.ijcard.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Ryan TD, Jefferies JL, Sawnani H. Implantation of the HeartMate II and HeartWare left ventricular assist devices in patients with Duchenne muscular dystrophy: lessons learned from the first applications. ASAIO. 2014;60(2):246–248. doi: 10.1097/MAT.0000000000000050. [DOI] [PubMed] [Google Scholar]