Abstract
Effective cellular function requires both compartmentalization of tasks in space and time, and coordination of those efforts. The endoplasmic reticulum’s (ER) expansive and ramifying structure makes it ideally suited to serve as a regulatory platform for organelle–organelle communication through membrane contacts. These contact sites consist of two membranes juxtaposed at a distance less than 30 nm that mediate the exchange of lipids and ions without the need for membrane fission or fusion, a process distinct from classical vesicular transport. Membrane contact sites are positioned by organelle-specific membrane–membrane tethering proteins and contain a growing number of additional proteins that organize information transfer to shape membrane identity. Here we briefly review the role of ER-containing membrane junctions in two important cellular functions: calcium signalling and phosphoinositide processing.
Keywords: calcium signalling, endoplasmic reticulum, membrane-membrane junctions, organelle contact sites, phosphoinositide regulation
This mini-review considers the role of endoplasmic reticulum–membrane contact sites in the regulation of cellular calcium and phosphoinositide levels, with an emphasis on mammalian cells.
Eukaryotic cells have evolved compartmentalization that orchestrates and segregates a myriad of important cellular processes. One organizing tool is the partitioning of the cytoplasm into membrane-enclosed organelles each with a distinct milieu and function. Although each organelle performs important independent tasks, they are not simply autonomous platforms of regulation. The success of complicated cellular reactions such as receptor signalling or apoptosis requires coordinated action by multiple organelles. One way that organelles communicate to organize specific cellular tasks is via membrane–membrane junctions or contact sites [1]. These sites of communication between organelles can be stable or dynamic, are mediated via specific proteins (Figure 1), are closely apposed (~10–30 nm), and facilitate the transfer of ions (e.g. calcium, Figure 1), proteins or lipids (e.g. phosphoinositides, Figure 2).
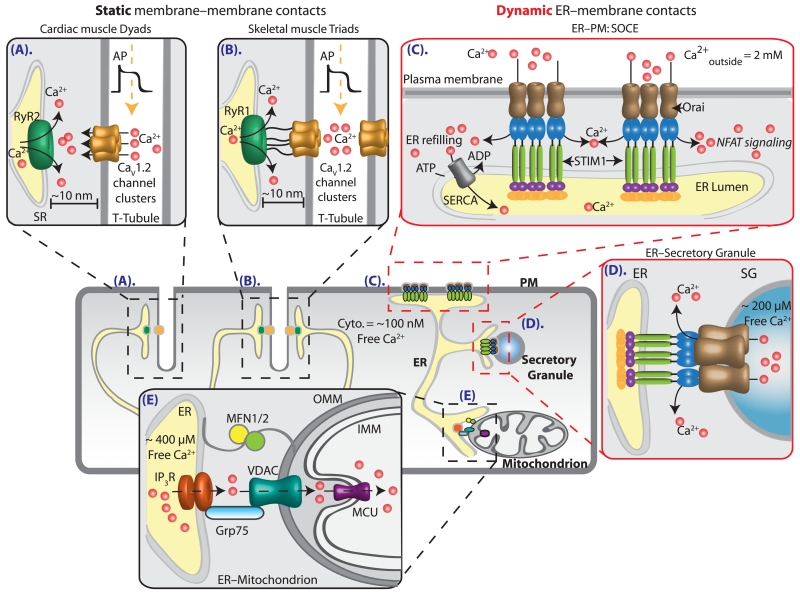
Figure 1. Static and dynamic ER–membrane junctions regulate cellular calcium concentrations.
(A) Cardiac muscle dyad. A static single T-tubule paired with a terminal cisterna of the SR. Action potentials propagating along cardiac myocyte T-tubules open clustered voltage-gated calcium channels (CaV1.2) to facilitate calcium-induced calcium release from the SR and elicit muscle contraction. (B) Skeletal muscle triad. Two static terminal cisternae of the SR paired with a T-tubule. Physical coupling between CaV1.2 channels and RyR1 gates the release of calcium from SR stores to produce muscle contraction. (C) SOCE. Diagram represents the dynamic reorganization of STIM1 dimers in ER membranes and Orai1 channels in the PM following depletion of ER calcium. Orai1–STIM1 binding initiates calcium influx to the cytoplasm, the refilling of ER stores via the SERCA pump and stimulation of transcription factors via nuclear factor of activated T-cells (NFAT). (D) Dynamic SG–ER membrane junction. G protein-coupled receptor activation promotes interaction between ER STIM1 and SG Orai channels, releasing SG calcium to the cytoplasm. (E) ER–mitochondrion junction. Interactions between IP3R and VDAC facilitate the flux of calcium ions across ER–mitochondrial junctions.
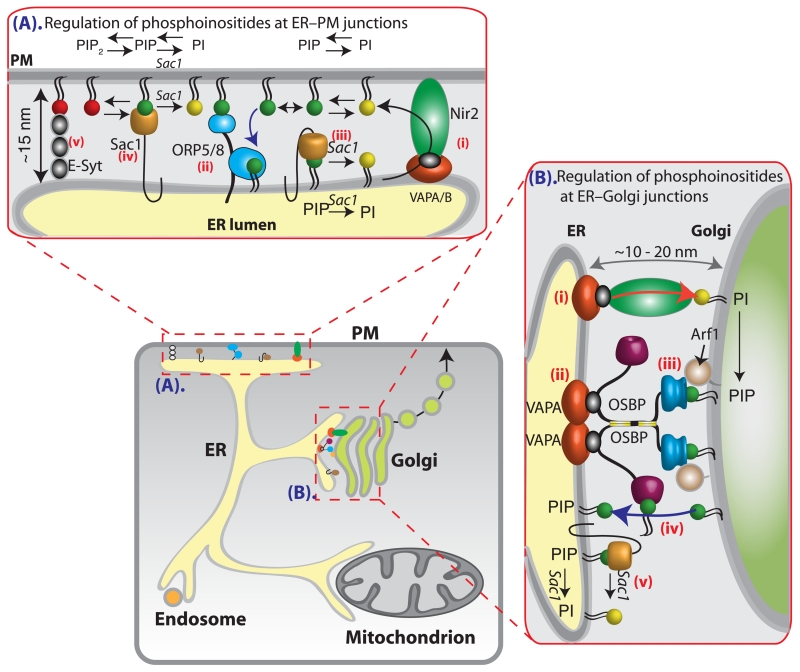
Figure 2. Phosphoinositide regulation at membrane–membrane junctions.
(A) ER–PM junctions. (i) Following Gq protein-coupled receptor activation, Nir2 translocates and binds to VAPA/B on ER membranes to transfer PI to the PM. (ii) ORP5/8 complexes tether ER–PM junctions via interactions between their PH domain and PM PI(4)P to transfer PI(4)P to the ER membrane. (iii) ER PI(4)P is dephosphorylated to PI by the ER-resident lipid phosphatase Sac1 acting in cis. (iv) PM PI(4)P is dephosphorylated to PI via Sac1 acting in trans. (v) E-Syt proteins tether ER–PM junctions via a hair-pin insertion into the ER membrane and interactions with PM PI(4,5)P2 via their C2 domains. (B) ER–Golgi junctions (ER–Golgi). (i) Nir2 or Nir3 bind to VAPA/B on ER membranes and transfer PI to Golgi membranes. (ii) OSBP tethers ER–Golgi junctions through interactions with VAPA/B on ER membranes and (iii) PI(4)P and Arf1 on Golgi membranes. (iv) The tethering of ER–Golgi membranes by OSBP allows its oxysterol-binding domain (ORD) to transfer PI(4)P to ER membranes and sterol to Golgi membranes. (v) PI(4)P on ER membranes is dephosphorylated by Sac1 to PI. The distance between ER and Golgi membrane junctions is approximately 10–20 nm.
Although diverse membrane–membrane contact sites are present throughout mammalian cells, those involving the endoplasmic reticulum (ER) are the most abundant and best studied. This is perhaps not surprising considering the ER’s extensive network of ramifying tubules that project throughout the cell and its roles in protein trafficking and calcium storage. Such properties allow the ER to form functional platforms of communication, both static and dynamic, with the plasma membrane (PM) [2–4], Golgi [5,6], mitochondria [7,8], secretory granules (SGs) [9] and endo/lysosomes [10–12]. Two important functions of ER–membrane junctions are (1) the transfer of calcium ions to generate and coordinate cytoplasmic calcium signals and (2) the transfer of lipids. This review briefly highlights the major principles and molecular identity of the proteins involved in both processes; for more extensive reviews on the regulation of calcium or transfer of additional lipids at ER–membrane contact sites see [13,14] and [15,16] respectively.
Calcium signalling at endoplasmic reticulum–organelle contact sites
Local or global elevations in cytoplasmic calcium concentrations are the trigger for a host of cellular events including memory, fertilization, contraction, secretion, migration and transcription. Each cell’s cytoplasmic calcium concentration is tightly regulated to facilitate this array of tasks. Increases in cytoplasmic calcium typically arise from two major calcium sources: the ER [via inositol 1,4,5-trisphosphate (IP3) receptors or ryanodine receptors (RyR)] and the extracellular space (via voltage-dependent calcium channels and store-operated channels). Calcium influx is opposed by pumps and transporters on the ER [sarcoplasmic reticulum (SR)/ER Ca2+ ATPases, SERCA pump], PM (plasma membrane Ca2+ ATPases, PMCA, NCX) and mitochondria (mitochondrial calcium uniporter, MCU). Many cell membranes maintain a steep calcium gradient at rest, facilitating rapid (millisecond) and large (micromolar) increases in local or global cytoplasmic calcium that initiate cellular signalling events. Given the importance of the ER and PM in shaping cellular calcium dynamics, understanding their communication at ER–PM contacts is key to a clear view of cellular function.
Excitation-contraction coupling
A compelling and historically significant example of ER–PM contact sites is the regulation of cytoplasmic calcium during excitation–contraction (EC) coupling in striated muscle cells. In both cardiac (Figure 1A) and skeletal muscle (Figure 1B) deep PM (sarcolemma) invaginations called transverse-tubules (T-tubules) penetrate into the myocyte and are functionally coupled to the SR. These SR–sarcolemma contacts form dyads (1 T-tubule:1 SR terminal cisterna; Figure 1A) in cardiac muscle [17] and triads (1 T-tubule:2 SR terminal cisternae; Figure 1B) in skeletal muscle [18,19]. In both cases the functional coupling arises from the close apposition between dihydropyridine-sensitive voltage-gated calcium channels (DHPR) on the sarcolemma and RyR on the SR membrane (Figures 1A and 1B). The DHPR channels are activated by action potential propagation depolarizing the sarcolemma in both muscle types. However, the mode of SR coupling is muscle-specific: for skeletal muscle there is direct physical coupling between DHPR channels and RyR1 [19], whereas cardiac muscle relies on cooperative gating of clustered CaV1.2 calcium channels [20,21] to increase calcium in the cleft between membranes and thereby activate RyR2 via calcium-induced calcium release [22]. In both cases, the release of SR calcium rapidly elevates cytoplasmic calcium to initiate muscle contraction. These stereotyped interactions between the SR and PM in myocytes are long lasting and essential for regulated EC-coupling.
Store-operated calcium entry
Another example of ER–PM cooperation is store-operated calcium entry (SOCE), wherein Ca2+ decrease in the ER lumen triggers Ca2+ influx through the PM. SOCE is a major calcium signalling pathway in excitable and non-excitable cells often initiated through activation of Gq protein-coupled receptors. The resulting hydrolysis of PM phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] produces diffusible IP3 which binds to IP3 receptors on the ER membrane to stimulate release of ER calcium into the cytoplasm. It is this loss of calcium from the ER lumen that triggers SOCE and thus influx of calcium into the cytoplasm from the extracellular space.
The key determinants orchestrating this calcium-signalling cascade (for a comprehensive review see [23]) are stromal interaction molecules (STIM1 and STIM2 in mammals) in the ER membrane and Orai channels (Orai1, Orai2, Orai3 in mammals) in the PM (Figure 1C). How do these two proteins interact to facilitate the influx of calcium into the cytoplasm? At rest, STIM1 molecules (typically dimers) and Orai channels (reportedly hexamers) can freely diffuse along the ER and PM respectively. Depletion of ER calcium causes loss of calcium from the luminal EF hand domains on STIM1 dimers and a conformational change in their structure that promotes oligomerization and subsequent translocation to regions of the ER closely apposed to the PM. The clustering of multiple STIM1 dimers at ER–PM junctions then traps diffusing Orai hexamers. Higher-order oligomers form after activation. This intimate molecular choreography results in the influx of calcium into the ER–PM junction (Figure 1C) that aids refilling of ER stores and controls gene expression, proliferation, cell migration and cell excitability. SOCE is terminated by refilling of ER calcium via the SERCA pump (Figure 1C).
Endoplasmic reticulum–endo/lysosome
Dynamic membrane–membrane junctions that mediate calcium-signalling cascades are not restricted to ER–PM junctions. Recently, junctions between the ER and acidic organelles have been identified as platforms for calcium regulation at these non-canonical calcium stores. For example, Gq-coupled receptor-stimulated release of calcium from SG (free calcium concentration ~200 μM) is mediated by STIM1 molecules in ER membranes and Orai1 channels in SG membranes (Figure 1D; [9]). In addition, ER cisternae make junctions with phagosomes via STIM1 and junctate, opening phagosomal Ca2+ channels to generate localized Ca2+ elevations that promote phagocytosis [24,25]. Finally, recent work suggests that junctions between the ER and lysosomes can help shape receptor signalling mediated by IP3 [26,27] or nicotinic acid adenine dinucleotide phosphate (NAADP; [11,28]). NAADP activates two-pore channels in lysosomal membranes, increasing calcium at sites close to the ER. These calcium microdomains are proposed to initiate calcium-induced calcium release via IP3R or RyR on ER membranes to increase cytosolic calcium throughout the cell. Thus, ER–membrane interactions underlie calcium release from a diverse pool of acidic granules.
Endoplasmic reticulum–mitochondrion
Membrane junctions between the ER and mitochondria, often referred to as mitochondrial-associated membranes are essential for the transfer of Ca2+ from the ER lumen to the mitochondrial matrix (Figure 1E). The main molecular components involved are the ER–mitochondrial membrane tethering proteins mitofusin 1 and 2 [29], IP3 receptor channels in the ER membrane and a non-specific ‘voltage-dependent anion channel’ (VDAC1) in the outer mitochondrial membrane (OMM) (Figure 1E; [30]). The IP3R and VDAC1 are functionally coupled at the ER–mitochondrial membrane interface via a chaperone protein, glucose-regulated protein GRP75 (Figure 1E). Upon release of calcium from the ER by IP3R, the calcium is transported rapidly across the OMM by VDAC1 resulting in elevated calcium concentrations within the mitochondrial intermembrane space that are sufficient to trigger uptake by the low-affinity MCU. Consequently, when intermembrane calcium is elevated to micromolar concentrations, calcium is transferred down the steep electrical gradient into the mitochondrial matrix (Figure 1E). Although the fluidity of ER–mitochondrial contacts remains to be fully established, the physiological functions of calcium transferred from the ER into the mitochondrial matrix appear to include buffering cytoplasmic calcium, facilitating calcium-dependent respiration and regulating cell death (apoptosis).
Regulation of phosphoinositides at membrane–membrane junctions
Phosphoinositides are a family of eight minority phospholipids found on the cytoplasmic leaflet of all endomembranes [31,32]. They serve as negatively charged molecular beacons recruiting cytosolic proteins to lipid membranes or binding to the cytosolic domain(s) of membrane proteins. The parent lipid, phosphatidylinositol (PI), is generated and synthesized on ER membranes via PI synthase enzymes conjugating ER-derived CDP-diacylglycerol and cytoplasmic myo-inositol. Following the synthesis of PI, specific lipid kinases and phosphatases add or remove phosphate groups at the 3′, 4′ or 5′ position on the inositol ring to generate seven additional phosphoinositides (PI(3)P, PI(4)P, PI(5)P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, PI(3,4,5)P3). The localization and activity of phosphoinositide metabolizing enzymes generates a heterogeneous distribution of phosphoinositide species throughout cells, providing each membrane an identifying phosphoinositide signature despite membrane traffic between organelles (e.g. ER: PI; Golgi: PI(4)P; PM: PI(4,5)P2 etc.). Recently, non-vesicular lipid transfer at ER–membrane junctions has been implicated as a mechanism through which cells maintain lipid compositions despite constant membrane recycling [33]. The functional importance of phosphoinositides is underscored by the large number of diseases and developmental malformations that result from mutations in phosphoinositide metabolizing enzymes [32,34–36] and by the variety of cellular activities they regulate [31,32,37–40].
Phosphoinositide regulation at ER–PM junctions
The signature phosphoinositide of the PM is PI(4,5)P2. Here it serves as an essential substrate for many biological activities, including endocytosis, exocytosis and ion channel function [32,37]. In mammalian cells, PI(4,5)P2 is generated in two steps: PI is phosphorylated by a PI 4-kinase to generate PI(4)P, which is subsequently phosphorylated by PI(4)P 5-kinase to generate PI(4,5)P2. Both the PM and the Golgi contain pools of PI(4)P that supply PM PI(4,5)P2 and PI(4,5)P2 itself helps organize contact sites between the ER and the PM via the extended synaptotagmin (E-Syt) proteins [32,37].
Transfer of PI at ER–PM junctions
Following Gq-receptor stimulation, PM PI(4,5)P2 is hydrolysed and proteins containing phosphoinositide transfer domains (phosphoinositide transfer proteins, PITP), called Nir2 and Nir3, are recruited to ER–PM junctions [Figure 2A(i)] [33,41,42]. This recruitment is triggered through the combined actions of phosphatidic acid and diacylglycerol [41], and mediated through an interaction with the FFAT motif on the ER vesicle-associated membrane protein (VAMP)-associated proteins A and B (VAP-A and VAP-B) [43] [Figure 2A(i)]. The stimulated recruitment of Nir2 and Nir3 at ER–PM junctions delivers PI to the PM to replenish PM phosphoinositides, and concurrently delivers phosphatidic acid to the ER for future PI synthesis [41].
Regulation of PI(4)P at ER–PM junctions
The majority of PM PI(4)P seems to be generated by the activity of the lipid kinase PI4KIIIα and its associated factors [44,45]. This pool of PM PI(4)P is important for the maintenance of PM PI(4,5)P2 [45,46]. Recently, two integral ER membrane proteins, oxysterol-binding protein (OSBP)-related protein 5 (ORP5) and ORP8 have been identified as key regulators of PM PI(4)P [45]. Through the action of a hydrophobic tail sequence that anchors it in the ER membrane and a pleckstrin homology (PH) domain that interacts with PM PI(4)P, ORP5/8 tethers ER–PM junctions [Figure 2A(ii)] and facilitates the exchange of phosphatidylserine (PS) for PI(4)P at ER–PM junctions [2,47]. Evidence for this includes: (i) the purified OSBP-related domain (ORD) of ORP8 binds PI(4)P and PS, (ii) overexpression of ORP5 reduces resting PM PI(4)P whereas increasing PM PS signals, (iii) rapamycin-induced recruitment of ORP5 lacking its PH domain (ΔPH-ORP5) or ΔPH-ORP8 to the PM reduces PI(4)P and (iv) rapamycin-induced recruitment of ΔPH-ORP5 to the PM increases PM PS following treatment with a PI4KIII inhibitor [2,47]. It remains to be seen if endogenous ORP5/8 is sufficient to induce ER–PM junctions in mammalian cells or if it stabilizes pre-existing ER–PM contacts. In addition to the ORP proteins, in yeast an ER PI(4)P phosphatase, Sac1, is found in ER–PM junctions and has been proposed to regulate PM PI(4)P. There is evidence for Sac1 dephosphorylating PI(4)P in cis on ER membrane (Figure 2A(iii); [48]) and in trans on PM (Figure 2A(iv); [49,50]). Although Sac1 has been shown to decrease PM PI(4)P in mammalian cells [51], its presence, localization and role in regulating phosphoinositides at ER–PM junctions remain to be fully determined. This is the subject of our ongoing work.
Phosphoinositide regulation at endoplasmic reticulum–Golgi junctions
The predominant phosphoinositide found on Golgi membranes is PI(4)P. Here it binds to specific effector proteins to orchestrate constitutive membrane trafficking and maintain the structural integrity of the Golgi complex.
Transfer of PI at ER–Golgi junctions
As at ER–PM junctions, PI transfer proteins (Nir) are thought to transfer PI down its concentration gradient from the ER to Golgi. This transfer is facilitated through an interaction of Nir2 with VAP-A and -B on ER membranes [6] [Figure 2B(i)], and mediated via the N-terminal PI-transfer domain of Nir2. Upon being transferred to Golgi membranes, PI is subsequently phosphorylated by Golgi-localized PI4K enzymes to produce PI(4)P [52].
Regulation of PI(4)P at ER–Golgi junctions
The accumulation of PI(4)P on the Golgi membrane, coupled with the presence of VAP-A and -B on ER membranes allows the cytosolic protein OSBP to be recruited to and subsequently to tether ER–Golgi junctions. The molecular components facilitating such membrane tethering are VAP proteins on the ER membrane and interactions between PI(4)P and the PH domain of OSBP on Golgi membranes [Figure 2B(ii)–(iv)]. Specifically, the FFAT motif of OSBP binds a highly conserved positive patch on the VAP protein surface, in a 2:2 stoichiometry, to anchor OSBP at the ER–cytosol interface (Figure 2B(ii); [53]). This positions OSBP and allows its N-terminal PH domain to detect two determinants of the trans-Golgi: Golgi PI(4)P and the small G protein Arf1-GTP [Figure 2B(iii)], thereby promoting ER–Golgi tethering. Consequently, the lipid transfer domain (ORD) of OSBP is positioned to facilitate sterol transfer to Golgi membranes, for the synthesis of sphingomyelin [54] and glycosphingolipids [55] and the transfer of PI(4)P to ER membranes [Figure 2B(iv)] for dephosphorylation by the ER lipid phosphatase Sac1 (Figure 2B(v)); [5,56]).
Regulation of phosphoinositides at other ER–membrane junctions
ER–endosome and ER–lysosome membrane contact sites have also been reported. They may regulate phosphoinositides. Recent evidence has implicated ER–endosome membrane junctions as regulators of microtubule-dependent endosomal transport. These junctions, mediated via VAP-A and protrudin on ER membranes binding to Rab7-GTP and PI(3)P on endosomal membranes [57], promote endosome translocation and neurite outgrowth. Given that several OSBP and ORP proteins may also localize to endosome membranes [58–61], it is possible that mechanisms at ER–PM or ER–Golgi junctions could be conserved at ER–endosome junctions to regulate endosomal PI(3)P or PI(4)P, for instance via Sac1 on ER membranes or recruitment of Sac2 via Rab5-GTP [62]. Finally, although ER–mitochondrial contacts are clearly important for calcium handling and mitochondrial division, they are not known to participate in phosphoinositide regulation.
Conclusions and perspectives
Current research on organelle–organelle junctions is advancing at a rapid pace despite technological limitations to monitor membrane–lipid composition in real time and to identify the full repertoire of participating proteins. As protein/lipid imaging and screening technologies advance we will better understand the role of membrane–membrane junctions in regulating membrane identity and shaping cellular responses. For example, improvements in imaging single molecules [63] and in fluorescent labelling of endogenous lipids and proteins [64–66] will enable us to monitor native membrane–membrane junctions in living cells. Future experiments must address not just the nanoscopic structural organization and function of membrane–membrane junctions, but also their role in coordinating macroscopic cellular events. We have summarized the role of membrane–membrane junctions in the exchange and regulation of calcium and phospholipids and the implications for cellular function, but many questions remain. Are other molecules exchanged at membrane–membrane junctions? In addition to acting as a portal for metabolite exchange, do these junctions serve other regulatory tasks? How does membrane–membrane communication interface with vesicular transport? Do various tethering molecules work synergistically or independently? Is there cooperative action between different membrane–membrane junctions? For example, certain cellular events, such as activation of PM Gq protein-coupled receptors would be expected to initiate a cellular cascade that impacts at least three ER–membrane junctions (ER–PM, ER–Golgi and ER–mitochondrial junctions). A more comprehensive view of membrane–membrane junctions will consider their combined effects across the cell.
Membrane–membrane junctions serve important functions in regulating membrane proteins, lipids and ion composition. We anticipate that future studies will uncover additional proteins involved in information transfer at membrane–membrane junctions and elucidate their impact on cellular pathways and organismal function and health.
Acknowledgments
Funding
This work was supported by the NIH National Institute of Neurological Disorders and Stroke [grant number R37NS008174]; and the Wayne E. Crill Endowed Professorship.
Abbreviations
- DHPR
dihydropyridine-sensitive voltage-gated calcium channel
- EC
excitation–contraction
- ER
endoplasmic reticulum
- E-Syt
extended synaptotagmin
- IP3
inositol 1,4,5-trisphosphate
- MCU
mitochondrial calcium uniporter
- NAADP
nicotinic acid adenine dinucleotide phosphate
- OMM
outer mitochondrial membrane
- ORD
oxysterol-binding protein-related domain
- ORP5
oxysterol-binding protein-related protein 5
- OSBP
oxysterol-binding protein
- PH
pleckstrin homology
- PI
phosphatidylinositol
- PI(4)P
phosphatidylinositol 4-phosphate
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PM
plasma membrane
- PS
phosphatidylserine
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum/endoplasmic reticulum ATPase
- SG
secretory granule
- SOCE
store-operated calcium entry
- SR
sarcoplasmic reticulum
- STIM
stromal interaction molecule
- VAMP
vesicle-associated membrane protein
- VAP
vesicle-associated membrane protein-associated protein
- VDAC
voltage-dependent anion channel
References
- 1.Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J. Cell Biol. 2014;205:759–769. doi: 10.1083/jcb.201401126. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P2-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu. Rev. Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lackner LL, Ping H, Graef M, Murley A, Nunnari J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E458–E467. doi: 10.1073/pnas.1215232110. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson EJ, Duman JG, Moody MW, Chen L, Hille B. Orai-STIM-mediated Ca2+ release from secretory granules revealed by a targeted Ca2+ and pH probe. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E3539–E3548. doi: 10.1073/pnas.1218247109. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER contact sites define the position and timing of endosome fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penny CJ, Kilpatrick BS, Eden ER, Patel S. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium. 2015;58:387–396. doi: 10.1016/j.ceca.2015.03.006. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam AK, Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Helle SC, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B. Organization and function of membrane contact sites. Biochim. Biophys. Acta. 2013;1833:2526–2541. doi: 10.1016/j.bbamcr.2013.01.028. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 2013;25:434–442. doi: 10.1016/j.ceb.2013.02.020. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr. Opin. Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore DH, Ruska H. Electron microscope study of mammalian cardiac muscle cells. J. Biophys. Biochem. Cytol. 1957;3:261–268. doi: 10.1083/jcb.3.2.261. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Dixon RE, Moreno CM, Yuan C, Opitz-Araya X, Binder MD, Navedo MF, Santana LF. Graded Ca2+ /calmodulin-dependent coupling of voltage-gated CaV1.2 channels. eLife. 2015;4:e05608. doi: 10.7554/eLife.05608. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon RE, Yuan C, Cheng EP, Navedo MF, Santana LF. Ca2+ signaling amplification by oligomerization of L-type CaV1.2 channels. Proc. Natl. Acad. Sci. U.S.A. 2012;109:1749–1754. doi: 10.1073/pnas.1116731109. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guido D, Demaurex N, Nunes P. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell Sci. 2015;128:4074–4082. doi: 10.1242/jcs.172510. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Nunes P, Cornut D, Bochet V, Hasler U, Oh-Hora M, Waldburger JM, Demaurex N. STIM1 juxtaposes ER to phagosomes, generating Ca2+ hotspots that boost phagocytosis. Curr. Biol. 2012;22:1990–1997. doi: 10.1016/j.cub.2012.08.049. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Lopez Sanjurjo CI, Tovey SC, Taylor CW. Rapid recycling of Ca2+ between IP3-sensitive stores and lysosomes. PLoS One. 2014;9:e111275. doi: 10.1371/journal.pone.0111275. CrossRefPubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Sanjurjo CI, Tovey SC, Prole DL, Taylor CW. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013;126:289–300. doi: 10.1242/jcs.116103. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgoyne T, Patel S, Eden ER. Calcium signaling at ER membrane contact sites. Biochim. Biophys. Acta. 2015;1853:2012–2017. doi: 10.1016/j.bbamcr.2015.01.022. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 29.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta. 2014;1843:2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 31.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S. The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO Rep. 2013;14:891–899. doi: 10.1038/embor.2013.113. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staiano L, De Leo MG, Persico M, De Matteis MA. Mendelian disorders of PI metabolizing enzymes. Biochim. Biophys. Acta. 2015;1851:867–881. doi: 10.1016/j.bbalip.2014.12.001. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Bankaitis VA. Phosphoinositide phosphatases in cell biology and disease. Prog. Lipid Res. 2010;49:201–217. doi: 10.1016/j.plipres.2009.12.001. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 2009;24:8–16. doi: 10.1152/physiol.00035.2008. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta. 2015;1851:844–856. doi: 10.1016/j.bbalip.2014.09.010. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posor Y, Eichhorn-Grunig M, Haucke V. Phosphoinositides in endocytosis. Biochim. Biophys. Acta. 2015;1851:794–804. doi: 10.1016/j.bbalip.2014.09.014. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B. Phosphoinositides: lipid regulators of membrane proteins. J. Physiol. 2010;588:3179–3185. doi: 10.1113/jphysiol.2010.192153. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Angelo G, Vicinanza M, Di Campli A, De Matteis MA. The multiple roles of PtdIns(4)P – not just the precursor of PtdIns(4,5)P2. J. Cell Sci. 2008;121:1955–1963. doi: 10.1242/jcs.023630. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 41.Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-phosphatidic acid exchange by Nir2 at ER-PM contact sites maintains phosphoinositide signaling competence. Dev. Cell. 2015;33:549–561. doi: 10.1016/j.devcel.2015.04.028. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CL, Hsieh TS, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao JC, Liou J. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Amarilio R, Ramachandran S, Sabanay H, Lev S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J. Biol. Chem. 2005;280:5934–5944. doi: 10.1074/jbc.M409566200. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Chung J, Nakatsu F, Baskin JM, De Camilli P. Plasticity of PI4KIIIα interactions at the plasma membrane. EMBO Rep. 2015;16:312–320. doi: 10.15252/embr.201439151. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakatsu F, Baskin JM, Chung J, Tanner LB, Shui G, Lee SY, Pirruccello M, Hao M, Ingolia NT, Wenk MR, De Camilli P. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 2012;199:1003–1016. doi: 10.1083/jcb.201206095. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dickson EJ, Jensen JB, Hille B. Golgi and plasma membrane pools of PI(4)P contribute to plasma membrane PI(4,5)P2 and maintenance of KCNQ2/3 ion channel current. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E2281–E2290. doi: 10.1073/pnas.1407133111. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 48.Cai Y, Deng Y, Horenkamp F, Reinisch KM, Burd CG. Sac1-Vps74 structure reveals a mechanism to terminate phosphoinositide signaling in the Golgi apparatus. J. Cell Biol. 2014;206:485–491. doi: 10.1083/jcb.201404041. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 50.Manford A, Xia T, Saxena AK, Stefan C, Hu F, Emr SD, Mao Y. Crystal structure of the yeast Sac1: implications for its phosphoinositide phosphatase function. EMBO J. 2010;29:1489–1498. doi: 10.1038/emboj.2010.57. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammond GR, Machner MP, Balla T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 2014;205:113–126. doi: 10.1083/jcb.201312072. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIβ at the Golgi complex. Nat. Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser SE, Brickner JH, Reilein AR, Fenn TD, Walter P, Brunger AT. Structural basis of FFAT motif-mediated ER targeting. Structure. 2005;13:1035–1045. doi: 10.1016/j.str.2005.04.010. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 54.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 55.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 56.Blagoveshchenskaya A, Cheong FY, Rohde HM, Glover G, Knodler A, Nicolson T, Boehmelt G, Mayinger P. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J. Cell Biol. 2008;180:803–812. doi: 10.1083/jcb.200708109. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 58.Vihervaara T, Uronen RL, Wohlfahrt G, Bjorkhem I, Ikonen E, Olkkonen VM. Sterol binding by OSBP-related protein 1L regulates late endosome motility and function. Cell. Mol. Life Sci. 2011;68:537–551. doi: 10.1007/s00018-010-0470-z. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobuna H, Inoue T, Shibata M, Gengyo-Ando K, Yamamoto A, Mitani S, Arai H. Multivesicular body formation requires OSBP-related proteins and cholesterol. PLoS Genet. 2010;6:e1001055. doi: 10.1371/journal.pgen.1001055. CrossRef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Li S, Mayranpaa MI, Zhong W, Back N, Yan D, Olkkonen VM. OSBP-related protein 11 (ORP11) dimerizes with ORP9 and localizes at the Golgi-late endosome interface. Exp. Cell Res. 2010;316:3304–3316. doi: 10.1016/j.yexcr.2010.06.008. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 61.Du X, Brown AJ, Yang H. Novel mechanisms of intracellular cholesterol transport: oxysterol-binding proteins and membrane contact sites. Curr. Opin. Cell Biol. 2015;35:37–42. doi: 10.1016/j.ceb.2015.04.002. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 62.Nakatsu F, Messa M, Nandez R, Czapla H, Zou Y, Strittmatter SM, De Camilli P. Sac2/INPP5F is an inositol 4-phosphatase that functions in the endocytic pathway. J. Cell Biol. 2015;209:85–95. doi: 10.1083/jcb.201409064. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen F, Tillberg PW, Boyden ES. Optical imaging. Expansion microscopy. Science. 2015;347:543–548. doi: 10.1126/science.1260088. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dean KM, Palmer AE. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 2014;10:512–523. doi: 10.1038/nchembio.1556. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]