Abstract
Despite the growing number of patients affected, the understanding of diastolic dysfunction and heart failure with preserved ejection fraction (HFpEF) is still poor. Clinical trials, largely based on successful treatments for systolic heart failure, have been disappointing, suggesting that HFpEF has a different pathology to that of systolic dysfunction. In this review, general concepts, epidemiology, diagnosis, and treatment of diastolic dysfunction are summarized, with an emphasis on new experiments suggesting that oxidative stress plays a crucial role in the pathogenesis of at least some forms of the disease. This observation has lead to potential new diagnostics and therapeutics for diastolic dysfunction and heart failure caused by diastolic dysfunction.
Keywords: Diastolic dysfunction, Heart failure, Myofilament Ca2+ sensitivity, Oxidative stress, Ventricular relaxation
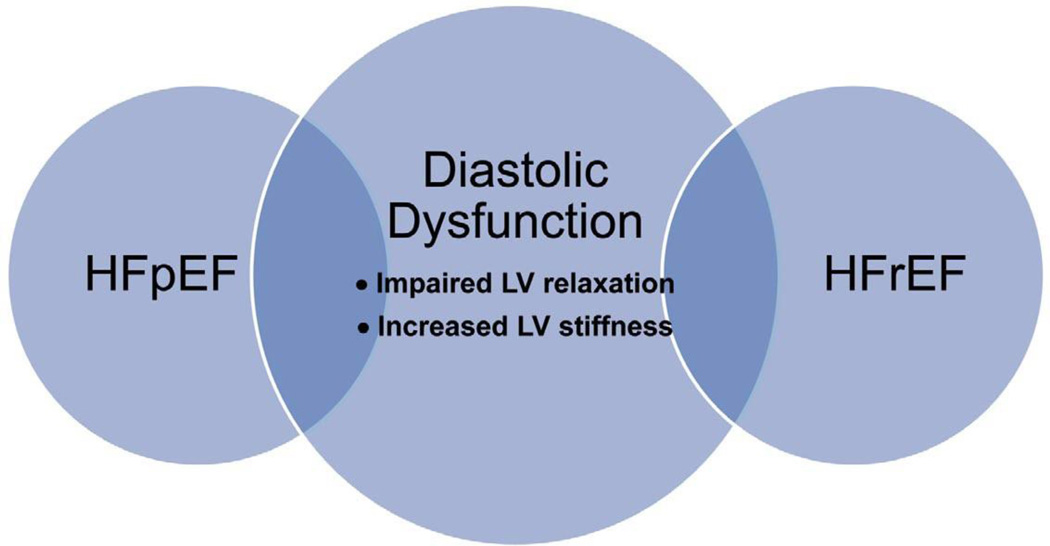
Since the first report of the syndrome of heart failure (HF) with a preserved ejection fraction (HFpEF) nearly 30 years ago,1 the diagnosis, pathophysiology, and most effective therapies for diastolic dysfunction and HFpEF caused by diastolic dysfunction (ie, diastolic HF) have remained controversial. Some of the confusion exists because diastolic dysfunction can be present in asymptomatic patients, patients with preserved EF, and patients with reduced EF (Figure 1).2 Moreover, not all cases of HFpEF or HF with reduced EF (HFrEF) are associated with diastolic dysfunction.3 Therefore, the relationship of diastolic dysfunction to the clinical syndrome of HF is somewhat ill-defined.
Figure 1.
Relationship of diastolic dysfunction to HFpEF and HFrEF. Diastolic heart failure is a subset of HFpEF, diastolic dysfunction can exist in HFrEF, and many patients with diastolic dysfunction are asymptomatic. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Epidemiology
HF is a major and growing public health problem in the USA, affecting approximately 5.1 million patients, and over 23 million patients worldwide.4 In Japan, approximately 1–2 million patients have chronic HF and nearly 170,000 patients die annually because of heart disease.5 More than 650,000 new patients are diagnosed with HF in the USA each year, and approximately half of them show diastolic dysfunction.6,7 Aging is an independent factor in HF incidence. The absolute mortality rate is high, and the prevalence of asymptomatic left ventricular (LV) dysfunction is increasing annually.6,8,9 Major risk factors for diastolic dysfunction include age, hypertension, diabetes mellitus, and LV hypertrophy.3,7,10 Diastolic dysfunction is common in diabetic patients and is associated with increased LV mass, wall thickness, and arterial stiffness.7 Of note, 34% of patients with diabetes have diastolic dysfunction.6
Although these risk factors are similar to those for HFrEF, growing evidence indicates that the mechanism of diastolic dysfunction is quite different from that in systolic dysfunction. Many effective treatments for HFrEF have shown disappointing results when applied to HFpEF patients.11 There are also clear clinical differences between HFpEF and HFrEF. Patients with HFpEF are older and more likely to be female.6 In HFpEF, the LV end-diastolic volume is not increased relative to the stroke volume, and there is concentric remodeling. In contrast, HFrEF has eccentric remodeling with LV dilation.12 The major risk factors for diastolic dysfunction are shared between HFpEF and HFrEF.6
Relationship of Diastolic Dysfunction to Diastolic HF
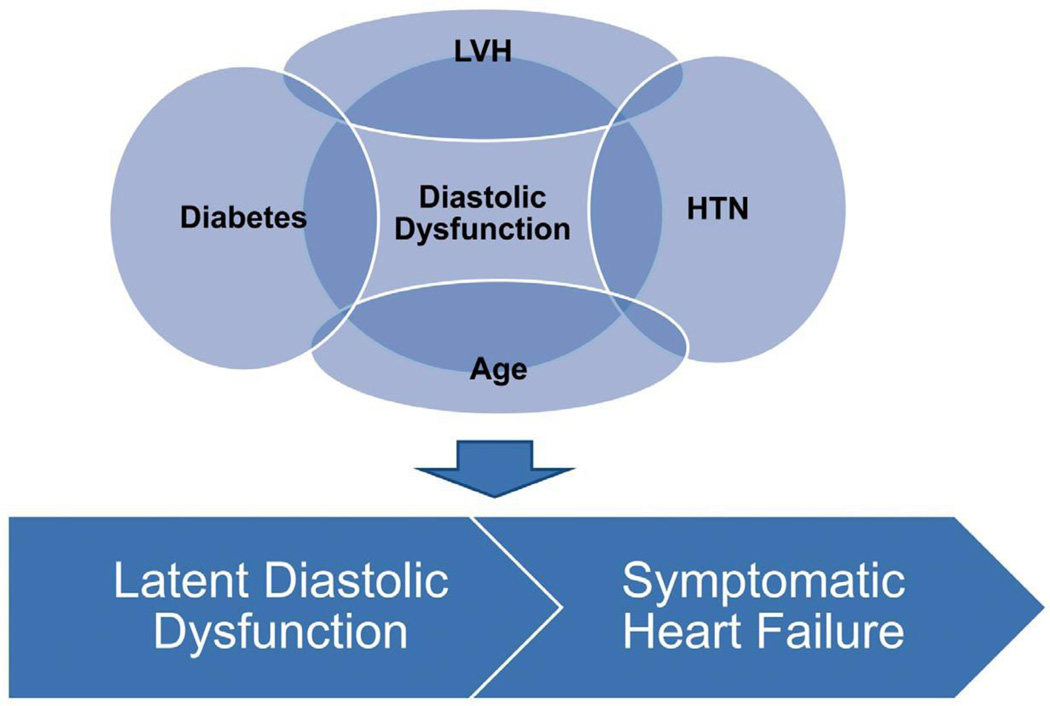
Epidemiological evidence suggests there is a latent phase in which diastolic dysfunction is present and progresses in severity before the symptoms of HF arise.3 Asymptomatic mild LV diastolic dysfunction is found in 21%, and moderate or severe diastolic dysfunction is present in 7% of the population.3 Both moderate and severe diastolic dysfunction is associated with an increased risk of symptomatic HF and mortality.3 This asymptomatic phase represents a potential time to intervene to prevent symptomatic HF. Suggesting the success of possible interventions, a mortality benefit has been observed in those whose diastolic dysfunction improved compared with those whose diastolic dysfunction remained the same or worsened.13 In early diastolic dysfunction, elevated LV stiffness is associated with diastolic filling abnormalities and normal exercise tolerance. Asymptomatic diastolic dysfunction may be present for significant periods before it develops into a symptomatic clinical event. When the disease progresses, pulmonary pressures increase abnormally during exercise, producing reduced exercise tolerance. When filling pressures increase further, clinical signs of HF appear.10 In a significant number of cases of diastolic HF, patients have atrial fibrillation at the time of diagnosis, suggesting an association and a possible common pathogenesis.14 With atrial fibrillation, diastolic dysfunction may rapidly lead to overt diastolic HF (Figure 2).15
Figure 2.
Major risk factors for diastolic dysfunction, which can lead to asymptomatic or symptomatic diastolic dysfunction. HTN, hypertension; LVH, left ventricular hypertrophy.
Mechanisms of Diastolic Dysfunction
Many mechanisms have been proposed. Recently, cardiac oxidative stress has been associated with diastolic dysfunction.16 Increased levels of cardiac reactive oxygen species (ROS) may explain some of the changes in Ca2+ handling proteins and the increased Ca2+ sensitivity of myofilaments in diastolic dysfunction.11,17 Some of the proposed mechanisms that represent therapeutic targets are reviewed next.
Alterations in Intracellular Ca2+ Transients
Increased diastolic Ca2+, delayed Ca2+ extrusion from the cytoplasm, or increased myofilament Ca2+ sensitivity could theoretically cause diastolic dysfunction. A prolonged Ca2+ transient results in elevation of intracellular Ca2+ during diastole, leading to abnormalities in both active relaxation and passive stiffness.18 Ca2+ homeostasis is regulated by a number of Ca2+ handling proteins, including the sarcoplasmic reticulum (SR) Ca2+ release channel (the ryanodine receptor, RyR), the SR Ca2+ pump (ie, the SR Ca2+-ATPase-SERCA2a), the sarcolemmal L-type Ca2+ channel, and the sodium-calcium exchanger (NCX). Increased diastolic intracellular Ca2+ may be a result of 3 possible mechanisms: (1) decreased SR Ca2+ pump activity, (2) SR Ca2+ leakage, or (3) abnormalities in the ionic channels responsible for calcium transport.19,20 For example, the NCX couples Ca2+ extrusion to the transmembrane Na+ gradient.9 In the failing heart, a small number of Na+ channels fail to inactivate, creating a late Na+ current (INa),10–13 which increases Na+ entry into the cell, reducing Ca2+ extrusion by the NCX.14 Oxidative stress may contribute to diastolic dysfunction by RyR S-nitrosylation, resulting in diastolic SR Ca2+ leaks and relaxation stiffness of cardiomyocytes.21 In addition, ROS-activated, cardiac-specifi Ca/calmodulin kinase (CaMK) II expression can regulate relaxation through SERCA2A.22 Redox-mediated SERCA2A sulfonation on the cysteine residue may also play a role.23
Many of these changes are not unique to diastolic dysfunction, however, and are seen in systolic dysfunction. Nevertheless, it is possible that these changes may contribute to both diastolic and systolic dysfunction or explain the presence of diastolic dysfunction during systolic dysfunction.
Titin Isoform Shifts
Another sarcomere macromolecule, titin, has been recognized as a determinant of diastolic relaxation.19 Titin is expressed in 2 isoforms: a smaller, stiffer N2B and a larger, more compliant N2A. In HFpEF, there is a higher proportion of the N2B isoform.24 Moreover, titin is modulated by phosphorylation.25,26 The cGMP-protein kinase (PK) G-dependent pathway has been suggested to play an important role in regulating diastolic tone and ventricular fi through titin phosphorylation and troponin I phosphorylation.26
Fibrosis
Changes in fibrillar collagen may be responsible for the development of diastolic dysfunction and diastolic HF. Hypertension and aging are associated with diastolic dysfunction and are accompanied by fibrosis. In turn, this fibrosis is associated with increased oxidative stress and profi cytokines. Reed et al reported that senescence-accelerated mice have diastolic dysfunction in the absence of alterations in systolic function.27 This change in diastolic dysfunction was associated with increased interstitial and perivascular collagen 1A1, collagen 3A, and fibronectin.27 Cardiac fibrosis was accompanied by increased levels of transforming growth factor-β and connective tissue growth factor.
Alterations in collagen degradation have also been associated with diastolic dysfunction. Changes in matrix metalloproteases (MMPs), which degrade collagen, and tissue inhibitors of MMPs (TIMPs) result in LV remodeling.15 A knockout of MMP-9 results in increased myocardial collagen with increased LV stiffness.28 Increased MMP-1 activity results in excessive collagen deposition and diastolic dysfunction.28 Elevations of MMP-2 and MMP-9 or a decrease of TIMP-1 occur in patients with asymptomatic diastolic dysfunction, as well as in diastolic HF.29 In addition, the magnitude of collagen turnover correlates directly with the severity of diastolic dysfunction.29 In systemic sclerosis, TIMP-1 levels are associated with diastolic dysfunction and LV matrix remodeling.30 In premenopausal, obese women with asymptomatic diastolic dysfunction, plasma MMP-2/-9 and TIMP profiles are altered.31
Posttranslational Modification of Cardiac Myosin Binding Protein C (cMyBP-C)
cMyBP-C regulates cross-bridge kinetics. Many of the mutations in cMyBP-C are known to induce diastolic dysfunction, and cMyBP-C knockout mice show higher myofilament Ca2+ sensitivity and lower diastolic sarcomere length with impaired relaxation without Ca2+ handling proteins changes or fibrosis.32 Phosphorylation of cMyBP-C by protein kinase A accelerates cross-bridge turnover rates, and dephosphorylation of cMyBP-C slows the dissociation of actin and myosin.33 Recently, Jeong EM et al reported that oxidative S-glutathionylation of cMyBP-C correlated with relaxation impairment. The mechanism for this diastolic dysfunction is increased myofilament Ca2+ sensitivity.34
Diagnosis of Diastolic Dysfunction
The presence and severity of diastolic dysfunction is commonly evaluated by echocardiography using color Doppler and tissue Doppler imaging (Table 1). Alternative modalities include strain analysis from cardiac magnetic resonance imaging (CMR) and speckle tracking echocardiography (STE). The diagnosis of diastolic HF, a subset of HFpEF, requires 3 conditions to be simultaneously satisfi (1) the presence of signs and symptoms of HF; (2) the presence of normal or only slightly reduced LVEF (EF >50%) and (3) the presence of increased diastolic pressure or impaired filling as indicated by delayed isovolumic relaxation or elevated stiffness.
Table 1.
Grades of Diastolic Dysfunction as Categorized by Echocardiography
| Normal | Grade I Abnormal relaxation |
Grade II Pseudonormal |
Grade III Restrictive (reversible) |
Grade IV Restrictive (fixed) |
|
|---|---|---|---|---|---|
| NYHA | I–II | II–III | III–IV | IV | |
| Mitral inflow (PW) | 0.75<E/A<1.5 150<DT<240 ms IVRT 70–90 ms |
E/A≤0.75 DT >240 ms IVRT >90 ms |
0.75<E/A<1.5 150<DT<200 ms IVRT <90 ms |
E/A >1.5 DT <150 ms IVRT <70 ms |
E/A >1.5 DT <15 ms IVRT <70 ms |
| Mitral inflow on valsalva |
ΔE/A >0.5 | ΔE/A ≤0.5 | ΔE/A ≥0.5 | ΔE/A ≥0.5 | ΔE/A <0.5 |
| Mitral anular motion (TDI) |
E/e’ <10 e’ >8 |
E/e’ <10 e’ <8 |
E/e’ ≥10 e’ <8 |
E/e’ ≥10 e’ <8 |
E/e’ ≥10 e’ <8 |
| Vp (Color M-mode) | Vp >55 | Vp >45 | Vp <45 | Vp <45 | Vp <45 |
| Pulmonary venous flow (PW-Doppler) |
S≥D ARdur-Adur <0 ms |
S>D ARdur-Adur <0 ms |
S<D or ARdur-Adur ≥30 ms |
S<D or ARdur-Adur ≥30 ms |
S<D or ARdur-Adur ≥30 ms |
| LV relaxation (tau) | Normal | Impaired | Impaired | Impaired | Impaired |
| LV compliance | Normal | Normal to ↓ | ↓↓ | ↓↓↓ | ↓↓↓↓ |
| LA pressure | Normal | Normal | ↑↑ | ↑↑↑ | ↑↑↑↑ |
| LV blood filling | Normal | ↓ | ↓↓ | ↓↓↓ | ↓↓↓ |
| LV volume index | <34 ml/m2 | <34 ml/m2 | >34 ml/m2 | >34 ml/m2 | >34 ml/m2 |
A, late diastolic mitral velocity; Adur, duration of A wave; ARdur, peak pulmonary venous atrial reversal flow velocity duration; D, a diastolic wave in pulmonary vein flow; E, early diastolic mitral velocity; e’, peak early diastolic mitral annulus velocity; IVRT, isovolumic relaxation time; PW-Doppler, pulse-wave Doppler; S, a larger systolic wave in pulmonary vein flow; TDI, tissue Doppler imaging; Vp, color M-mode Doppler blood velocity. (Modified from Maharaj R15)
Two-dimensional echocardiography with Doppler flow measurements is commonly used to assess diastolic dysfunction.35 Exercise may be required to clearly demonstrate diastolic functional changes.36 During diastole, blood flows through the mitral valve when the LV relaxes, causing an early diastolic mitral velocity (E), and then additional blood is pumped through the valve when the left atrium contracts during late diastole (A). The E/A ratio can be altered in diastolic dysfunction. Tissue Doppler imaging is an echocardiographic technique that measures the velocity of the mitral annulus. This velocity has been shown to be a sensitive marker of early myocardial dysfunction. With abnormal active relaxation, mitral annulus velocity during early diastole (e’) is decreased while mitral annulus velocity during late diastole (a’) is increased, resulting in a lowered e’/a’ ratio. In animal models, tissue Doppler imaging has been validated as a reliable tool for the evaluation of diastolic dysfunction.15,35,37 LV inflow propagation velocity (VP) by color M-mode Doppler is another relatively preload-insensitive index of LV relaxation.38 It has been shown to correlate well with the time constant of isovolumic relaxation (τ), both in animals and humans.35
Recently, STE has emerged as a promising technique for the evaluation of myocardial wall motion by strain analysis. By tracking the displacement of speckles during the cardiac cycle, STE allows semiautomated delineation of myocardial deformation.
CMR imaging is a newer technique for measuring diastolic dysfunction.39 Myocardial tagging allows the labeling of specific myocardial regions. Following these regions during diastole enables them to be analyzed in a manner similar to STE. In addition, the rapid diastolic untwisting motion followed by CMR tagging is directly related to isovolumic relaxation and can be used as an index of the rate and completeness of relaxation.39
Biomarkers may contribute to the diagnosis. B-type natriuretic peptide (BNP) and TnI have been used as HF biomarkers and exhibit strong association with hospitalization.40 Nevertheless, they are nonspecifi and not well correlated with diastolic dysfunction. Recently, it has been reported that cMyBP-C could be a new biomarker releases from damaged myofilaments.41 Additionally, elevated S-glutathionylated cMyBP-C level can be detected in the blood of patients with diastolic dysfunction.42 Hypertension and diabetes lead to cardiac oxidation and S-glutathionylation of cMyBP-C, a cardiac contractile protein, which leads to impaired relaxation, and modified cMyBP-C in the blood may represent a circulating biomarker for diastolic dysfunction.17
Novel Therapeutic Strategies
To date, there are no specific treatments for diastolic dysfunction to selectively enhance myocardial relaxation. Moreover, no drug has been developed to improve long-term outcomes for diastolic HF.9 Nevertheless, recent trials and new hypotheses about the mechanism of diastolic dysfunction suggest possible directions for specific therapies.
Current Treatment for HFpEF
Recent clinical trials using drugs of advantage in systolic dysfunction have failed to demonstrate improvement in long-term outcome for diastolic HF, further emphasizing differences in the underlying pathophysiology of diastolic dysfunction. Several trials of these drugs for HFpEF are summarized in Table 2. Despite abundant evidence of the efficacy of reninangiotensin system inhibition in systolic dysfunction, the PEP-CHF trial using perindopril showed no overall difference in mortality and or need for HF hospitalization.43 In the Hong Kong Diastolic Heart Failure study, only diuretics in combination with irbesartan or ramipril marginally improved diastolic function and lowered NT-proBNP over 1 year.44 Angiotensin II receptor blockers show a similar lack of efficacy. The CHARM-preserved trial, which randomized 3,023 patients between candesartan and placebo, showed no beneficial effect in cardiovascular death at 3-year follow-up.45 In the I-PRESERVE trial, which randomized 4,128 patients, irbesartan showed no reduction in all-cause mortality or hospitalization for a cardiovascular cause at 49.5-month follow-up.46 In OPTIMIZE-HF, carvedilol, a β-blocker, did not affect primary or long-term outcomes for HFpEF.47 In the SENIORS trial, nebivolol showed limited beneficial effect in the elderly HFpEF group (age >70).48 The CORONA trial used a statin and showed only LV remodeling improvement without changes in the primary outcomes.49 Aldosterone antagonists are known to prevent the development of cardiac hypertrophy and fibrosis.50 Aldo-DHF, using spironolactone, revealed little improvement in LV relaxation and no change in the primary outcome in HFpEF patients.51 In the TOPCAT trial, there was no reduction in mortality, aborted cardiac arrest or hospitalization for HFpEF patients using spironolactone.52 Furthermore, the inotropic agent digoxin showed no significant advantage in HFpEF.53
Table 2.
Randomized Preclinical or Clinical Trials for HFpEF
| Pre- or clinical trial |
Drug type | Drug | n | Years* | Comments | Outcome** Mortality/ Hospitalization |
Reference |
|---|---|---|---|---|---|---|---|
| PEP-CHF | ACEI | Perindopril | 850 | 1 | Improved HF symptom, exercise capacity |
No/partial at 1 st year |
Cleland et al43 |
| Hong Kong DHF |
ACEI+diuretics | Ramipril+ Irbesartan |
150 | 1 | Improved HF symptoms and LV function with diuretics combination, but no effect with irbesartan or ramipril alone |
No/No | Yip et al44 |
| CHARM- preserved |
ARB | Candesartan | 3,023 | 1–3 | Moderate effect in preventing admissions for CHF among HFpEF patients |
No/partial | Persson et al45 |
| I-PRESERVE | Irbesartan | 4,128 | <1 | No improvement | No/No | Massie et al46 |
|
| OPTIMIZE-HF | β-blocker | Carvediol | 24,689 | 1 | No beneficial effect on mortality | No/No | Hernandez et al47 |
| SENIOR | Nebivolol | 2,128 | <2 | Beneficial on primary outcome in seniors >70 years, HFpEF Well- tolerated, vasodilation |
Yes/Yes | Flather et al48 |
|
| CORONA | HMG-CoA inhibitor |
Rosuvastatin | 2,514 | 0.5 | Beneficial on LV remodeling, hyper- trophy & fibrosis |
No/No | Kjekshus et al49 |
| Aldo-DHF | Aldosterone antagonist |
Spironolactone | 209 | 1 | Beneficial on LV stiffness, but no better exercise capacity |
No/No | Edelmann et al51 |
| TOPCAT | Spironolactone | 3,445 | 3.3 | No benefit in HFpEF | No/No | Pitt et al52 | |
| DIG | Inotropic vasodilator |
Digoxin | 3,397 | 2 | Beneficial to reduce LV blood over- load, pulmonary congestion |
No/No | DIG53 |
| RALI-DHF† | Late INa inhibitor | Ranolazine | 20 | Acute | 24 h infusion, 14-day oral treatment; improved hemodynamics No relaxation improvement, no NT-proBNP changes |
No/No | Maier et al58 |
| RELAX | PDE-5 inhibitor | Sidenafil | 206 | 6 | Increase NO production to improve relaxation, but no better exercise capacity, 24-week treatment |
No/No | Redfield et al55 |
Study period (years);
primary outcome of mortality and hospitalization;
preclinical trial with acute treatment.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; DHF, diastolic heart failure; DIG, Digitalis Investigation Group trial; HFpEF, heart failure with preserved ejection fraction; HMG-CoA, hydroxymethylglutaryl-coenzyme A; INa, sodium current; PDE, phosphodiesterase.
There is accumulating evidence indicating diastolic dysfunction is associated with oxidative stress and the nitric oxide (NO) pathway. Oxidative stress is often associated with reduced NO and cGMP levels, leading to vasoconstriction and cardiac stiffness.54 Therefore, it might stand to reason that increasing NO-cGMP signaling by phosphodiesterase (PDE)-5 inhibition would improve diastolic function. Nevertheless, the RELAX trial, which used sildenafil to treat NYHA class II/III HFpEF patients showed no significant difference in clinical outcomes.55 This suggests that diastolic dysfunction is independent of downstream cGMP-dependent signaling, but the result does not clearly rule out the oxidative stress hypothesis.
Ranolazine
Ranolazine, an anti-anginal drug with multiple putative mechanisms of action, has shown some promise as a treatment for diastolic dysfunction. In an animal model of hypertension-induced diastolic dysfunction, ranolazine worked directly on myofilaments to correct the defect in relaxation.56 Ranolazine is also known to decrease the late Na+ current, which may lower internal Na+ and Ca2+ levels in diastole.57 In the randomized clinical trial, RALI-DHF, acute infusion of ranolazine in HFpEF patients resulted in modest improvements in hemodynamics, but no improvement in LV relaxation.58 It is possible that ranolazine may have therapeutic efficacy in diastolic dysfunction, even if the mechanism is unclear.
Tetrahydrobiopterin (BH4)
NO synthase (NOS) usually produces NO, which relaxes the heart.59 When the NOS cofactor, BH4, becomes oxidized and depleted, NOS begins to produce superoxide, an oxidant, rather than NO. This situation is called NOS uncoupling. In hypertension-induced diastolic dysfunction, cardiac NOS is uncoupled, BH4 is reduced, and NO is decreased. Cardiac oxidation generated diastolic dysfunction independent of changes in the vasculature. Supplementation with oral BH4 prevented or reversed the cardiac changes, including the diastolic dysfunction.
The cellular level of BH4 also regulates SERCA2A activity.60 HMG-CoA reductase inhibitors (statins) or resveratrol increase BH4 availability and improve LV relaxation in diabetes61 and in a hyperlipidemia animal model.62 Therefore, increasing BH4 may be a promising therapeutic target for diastolic dysfunction. Currently, oral BH4 is used to treat atypical phenylketonuria and shows a favorable safety profile.34,63
Mitochondria-Targeted Antioxidants
Oxidative stress has been implicated in the pathophysiology of cardiac remodeling and diastolic dysfunction.16 Mitochondria are a major source of cardiac oxidative stress, especially in diabetes, and diabetes is a risk factor for diastolic dysfunction. In preliminary data, we have shown that diabetes is associated with cardiac mitochondrial oxidative stress and diastolic dysfunction.64 Injecting animals with a mitochondria-targeted antioxidant, mitoTEMPO, prevented diabetic-associated diastolic dysfunction.65 Other mitochondria-targeted antioxidants that have shown beneficial effects in muscle include MitoQ1066 and the mitochondria-selective peptide, SS-31.67 Any of these may represent a novel therapeutic strategy for diastolic dysfunction.
Summary
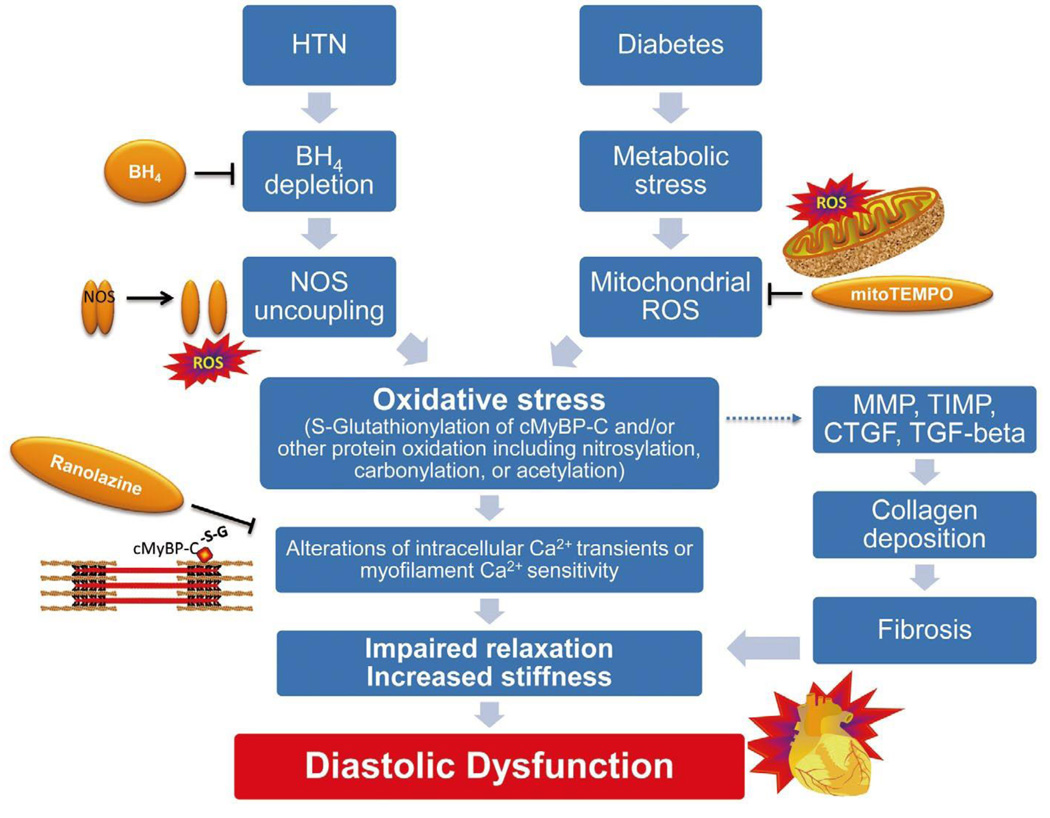
The fact that many drugs beneficial in HFrEF are not efficacious in HFpEF, and that systolic and diastolic dysfunction can exist in isolation or together, suggests that diastolic dysfunction has a fundamentally different pathology to that of systolic dysfunction. Among the more promising avenues of ongoing research is the concept that cardiac oxidation can lead to diastolic dysfunction (Figure 3). This hypothesis explains why many of the risk factors for diastolic dysfunction are associated with increased oxidative stress and why cardiac oxidation has been associated with diastolic dysfunction. Also, it explains why select antioxidant therapies have shown potential efficacy in preventing or reversing diastolic dysfunction. The oxidant theory can also explain why the cardiac or circulating level of S-glutathionylated cMyBP-C is associated with diastolic dysfunction.
Figure 3.
Selected possible mechanisms of diastolic dysfunction. Hypertension (HTN) and diabetes lead to oxidative modification of proteins including cMyBP-C and a decreased myofilament relaxation rate. Targeted antioxidants appear to prevent or treat oxidant stress-induced diastolic dysfunction in animal models, and circulating modified cMyBP-C may serve as a biomarker of disease. Independent of myocyte biology, increased extracellular matrix may cause abnormal LV relaxation. BH4, tetrahydrobiopterin; cMyBP-C, cardiac myosin binding protein C; CTGF, connective tissue growth factor; MMP, matrix metalloprotease; NOS, nitric oxide synthase; ROS, reactive oxygen species; TIMP, tissue inhibitors of MMP; TGF, transforming growth factor.
Although it seems likely that more than one hypothesis will be necessary to explain all cases of diastolic dysfunction, new insights into the pathogenesis of the disease should lead to novel diagnostics and therapies. The latency between dysfunction and symptoms represents an ideal time for using these diagnostics and therapies.
Acknowledgments
This study was supported by National Institutes of Health grants R01 HL104025, R01 HL106592 (S.C.D.), and Veterans Affairs MERIT grant BX000859, and R41 HL112355 to S.C.D.
Footnotes
Disclosures
S.C.D. is the inventor on patent applications: (1) 11/895,883 Methods and Compositions for Treating Diastolic Dysfunction, (2) 13/503,812 Methods of Diagnosing Diastolic Dysfunction, (3) 13/397,622 Methods for Treating Diastolic Dysfunction and Related Conditions, (4) 13/658,943 Method of Improving Diastolic Dysfunction, (5) 13/841,843 Myosin Binding Protein-C for Use in Methods Relating to Diastolic Heart Failure, and (6) 61/728,302 Mitochondrial Antioxidants and Diabetes.
References
- 1.Dougherty AH, Naccarelli GV, Gray EL, Hicks CH, Goldstein RA. Congestive heart failure with normal systolic function. Am J Cardiol. 1984;54:778–782. doi: 10.1016/s0002-9149(84)80207-6. [DOI] [PubMed] [Google Scholar]
- 2.LeWinter MM, Meyer M. Mechanisms of diastolic dysfunction in heart failure with a preserved ejection fraction: If it’s not one thing it’s another. Circ Heart Fail. 2013;6:1112–1115. doi: 10.1161/CIRCHEARTFAILURE.113.000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redfi MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart Disease and Stroke Statistics 2010 Update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health, Labour and Welfare. Summary of vital statistics. [accessed January 18, 2015];2005 http://www.mhlw.go.jp/english/database/db-hw/populate/index.html. [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfi MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 7.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, et al. Prevention of heart failure: A scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 8.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: A follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res. 2000;45:813–825. doi: 10.1016/s0008-6363(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 11.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 12.Little WC, Zile MR. HFpEF: Cardiovascular abnormalities not just comorbidities. Circ Heart Fail. 2012;5:669–671. doi: 10.1161/CIRCHEARTFAILURE.112.972265. [DOI] [PubMed] [Google Scholar]
- 13.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: A mechanistic appraisal. Circulation. 2012;126:2353–2362. doi: 10.1161/CIRCULATIONAHA.112.113233. [DOI] [PubMed] [Google Scholar]
- 15.Maharaj R. Diastolic dysfunction and heart failure with a preserved ejection fraction: Relevance in critical illness and anaesthesia. J Saudi Heart Assoc. 2012;24:99–121. doi: 10.1016/j.jsha.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maack C, Bohm M. Targeting mitochondrial oxidative stress in heart failure: Throttling the afterburner. J Am Coll Cardiol. 2011;58:83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Jeong EM, Dudley SC., Jr New diagnostic and therapeutic possibilities for diastolic heart failure. RI Med J. 2014;97:35–37. [PMC free article] [PubMed] [Google Scholar]
- 18.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: Causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 19.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 20.Shannon TR, Lew WY. Diastolic release of calcium from the sarcoplasmic reticulum: A potential target for treating triggered arrhythmias and heart failure. J Am Coll Cardiol. 2009;53:2006–2008. doi: 10.1016/j.jacc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin F, Siwik DA, Lancel S, Zhang J, Kuster GM, Luptak I, et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2:e000184. doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfi MM, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 27.Reed AL, Tanaka A, Sorescu D, Liu H, Jeong EM, Sturdy M, et al. Diastolic dysfunction is associated with cardiac fibrosis in the senescence-accelerated mouse. Am J Physiol Heart Circ Physiol. 2011;301:H824–H831. doi: 10.1152/ajpheart.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, et al. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 30.Ciurzynski M, Bienias P, Irzyk K, Kostrubiec M, Szewczyk A, Demkow U, et al. Heart diastolic dysfunction in patients with systemic sclerosis. Arch Med Sci. 2014;10:445–454. doi: 10.5114/aoms.2014.43739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosmala W, Plaksej R, Przewlocka-Kosmala M, Kuliczkowska-Plaksej J, Bednarek-Tupikowska G, Mazurek W. Matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in premenopausal obese women: Relationship to cardiac function. Int J Obes (Lond) 2008;32:763–771. doi: 10.1038/sj.ijo.0803794. [DOI] [PubMed] [Google Scholar]
- 32.Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, et al. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol. 2012;52:1299–1307. doi: 10.1016/j.yjmcc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, et al. Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 36.Asrar ul Haq M, Mutha V, Lin T, Profitis K, Tuer Z, Lim K, et al. Left ventricular torsional dynamics post exercise for LV diastolic function assessment. Cardiovasc Ultrasound. 2014;12:8. doi: 10.1186/1476-7120-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN. Diastolic dysfunction: Improved understanding using emerging imaging techniques. Am Heart J. 2010;160:394–404. doi: 10.1016/j.ahj.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Garcia MJ, Palac RT, Malenka DJ, Terrell P, Plehn JF. Color M-mode Doppler flow propagation velocity is a relatively preload-independent index of left ventricular filling. J Am Soc Echocardiogr. 1999;12:129–137. doi: 10.1016/s0894-7317(99)70125-2. [DOI] [PubMed] [Google Scholar]
- 39.Dusch MN, Thadani SR, Dhillon GS, Hope MD. Diastolic function assessed by cardiac MRI using longitudinal left ventricular fractional shortening. Clin Imaging. 2014;38:666–668. doi: 10.1016/j.clinimag.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Vorovich E, French B, Ky B, Goldberg L, Fang JC, Sweitzer NK, et al. Biomarker predictors of cardiac hospitalization in chronic heart failure: A recurrent event analysis. J Card Fail. 2014;20:569–576. doi: 10.1016/j.cardfail.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, et al. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong EM, Brahmanandam V, Raicu M, Lee SY, Chung JH, Rutledge C, et al. Plasma myosin binding protein-C fragments and S-glutathionylation in diastolic heart failure (abstract) Circulation. 2012;126:A11366. [Google Scholar]
- 43.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 44.Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, et al. The Hong Kong diastolic heart failure study: A randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94:573–580. doi: 10.1136/hrt.2007.117978. [DOI] [PubMed] [Google Scholar]
- 45.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, et al. Diastolic dysfunction in heart failure with preserved systolic function: Need for objective evidence: Results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 46.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: Findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flather MD, Shibata MC, Coats AJ, van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 49.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 50.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228–1233. doi: 10.1016/s0735-1097(01)01116-0. [DOI] [PubMed] [Google Scholar]
- 51.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 52.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 53.Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 54.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, et al. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang XQ, Yamada S, Barry WH. Ranolazine inhibits an oxidative stress-induced increase in myocyte sodium and calcium loading during simulated-demand ischemia. J Cardiovasc Pharmacol. 2008;51:443–449. doi: 10.1097/FJC.0b013e318168e711. [DOI] [PubMed] [Google Scholar]
- 58.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: The RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Smith TW, Balligand JL, Kaye DM, Wiviott SD, Simmons WW, Han X, et al. The role of the NO pathway in the control of cardiac function. J Card Fail. 1996;2:S141–S147. doi: 10.1016/s1071-9164(96)80070-4. [DOI] [PubMed] [Google Scholar]
- 60.Carnicer R, Hale AB, Suffredini S, Liu X, Reilly S, Zhang MH, et al. Cardiomyocyte GTP cyclohydrolase 1 and tetrahydrobiopterin increase NOS1 activity and accelerate myocardial relaxation. Circ Res. 2012;111:718–727. doi: 10.1161/CIRCRESAHA.112.274464. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari Z, et al. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress: In vivo demonstration with magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2010;299:H985–H994. doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia N, Daiber A, Habermeier A, Closs EI, Thum T, Spanier G, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 63.Bartholome K, Lutz P, Bickel H. Determination of phenylalanine hydroxylase activity in patients with phenylketonuria and hyperphenylalaninemia. Pediatr Res. 1975;9:899–903. doi: 10.1203/00006450-197512000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Chung J, Jeong EM, Go Y, Gladstein S, Farzaneh-Far A, Lewandowski ED, et al. Mitochondria-targeted antioxidant ameliorates diet-induced diabetes and diastolic dysfunction (abstract) J Am Coll Cardiol. 2015;61:E597. [Google Scholar]
- 65.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Q, Harris N, Ren J, Han X. Mitochondria-targeted antioxidant prevents cardiac dysfunction induced by tafazzin gene knockdown in cardiac myocytes. Oxid Med Cell Longev. 2014;2014:654198. doi: 10.1155/2014/654198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]