Abstract
Primary dysmenorrhea (PDM), a significant public health problem for adolescents and young women, is characterized by painful menstrual cramps. Recent neuroimaging studies have revealed that brain functional and structural abnormalities are related to the pathomechanism of PDM. However, it is not clear whether there are white matter (WM) alterations in PDM. We analyzed diffusion tensor imaging data from 35 patients and 35 healthy controls (HCs) matched for age and handedness. Tract-based spatial statistics and probabilistic tractography were used to measure integrity of WM microstructure. Compared to HCs, patients had increased fractional anisotropy (FA) along with decreased mean diffusivity (MD) and radial diffusivity (RD) in the corpus callosum (CC), superior longitudinal fasciculus (LF), corona radiata (CR), internal capsule (IC) and external capsule (EC). The FA of the splenium CC and right IC positively correlated with PDM duration while FA of the right anterior CR positively correlated with PDM severity in patient group. These WM tracts were found to show connections to other brain regions implicated in sensoimotor, affective, cognitive and pain processing functions through tractography. These findings provide preliminary evidence for WM microstructure alterations in PDM, which is potentially valuable for understanding pathomechanism of PDM.
As a significant public health problem for adolescents and young women, primary dysmenorrhea (PDM) is characterized by painful menstrual cramps of uterine origin in the absence of pelvic pathology and enhanced pain sensitivity1,2,3. PDM is prevalent in 20% to 90% of female adolescents4, has a negative effect on quality of life for women, and results in tremendous health care costs5,6. Previous studies have proposed that PDM is associated with prostaglandins and painleukotrienes, which may play a role in modulating hyperalgesia and inflammatory pain and causing uterine contractions4,6. However, the clear mechanism underlying PDM remains to be determined.
Recent functional brain imaging studies have provided evidence to support the idea that abnormal brain functions may contribute to developing or maintaining PDM7,8. For example, PDM patients had abnormalities of cerebral metabolism in the nociceptive and pain modulatory pathways, including increased metabolism in the prefrontal cortex (PFC), orbitofrontal cortex (OFC), thalamus and decreased metabolism in the sensorimotor cortex7. Given thermal noxious stimulation, PDM patients exhibited altered regional activations linked to the hypothalamic–pituitary–adrenal (HPA) axis and central sensitization8. Meanwhile, structural brain imaging studies have enabled us to characterize alterations of brain anatomical substrates in PDM9,10,11. Tu and colleagues found that PDM patients had increased volumes of gray matter (GM) were mainly located in the anterior/posterior cingulate cortex (ACC/PCC), hippocampus (HIPP), hypothalamus and midbrain, As well as decreased ones were located in the PFC, OFC, insula and somatosensory cortex10. In a previous study, we reported increased cortical thickness in the OFC, insula, primary/secondary sensory area (SI/SII), superior temporal cortex, precuneus and PCC, as well as decreased subcortical volumes in the caudate, thalamus and amygdala in PDM patients9. Moreover, changes of GM were reported to be associated with the states of dysmenorrhea (the dysmenorrhea pain and pain-free states)11. These aforementioned studies suggest that PDM is accompanied by central nervous system (CNS) abnormalities which may predispose to chronic pain.
To better understand structural and/or functional characteristics, the microstructural properties of white matter (WM) should be investigated. Investigating relationships between certain disorder and changes of WM microstructure could further provide potential value for understanding the pathomechanism of the disorder. Diffusion tensor imaging (DTI) is a novel technique to assess WM microstructure and/or connectivity in vivo12,13. DTI metrics include fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD). FA reflects WM integrity or directionality of the molecular motion of water14,15. MD is associated with edema and inflammation16. RD possibly reflects changes in membrane permeability and myelination17. AD is related to diffusion along tracts. FA and other diffusivity measures have been used to examine changes in WM microstructural pathways involved in pain processing18. However, little is known regarding whether there are altered WM microstructures in PDM patients.
In this study, we tried to evaluate changes of WM and reorganization of the WM connectivity in PDM patients using tract-based spatial statistics (TBSS)19 and probabilistic tractography20, and to determine whether parameters of the abnormalities in the WM tracts correlated with clinical characteristics of PDM. Based on previous studies, we hypothesized that alterations of WM would be found in the tracts associated with pain processing, sensory, motor, affection and cognition. We also expected that clinical characteristics would correlate with parameters of tracts that are related to interoception and affective processing of pain. In addition, psychosocial factors, such as depression and anxiety, are known to be associated with menstrual pain21,22 and exert their role in pain processing in other pain disorders23,24,25,26. Thereby, we further hypothesized that psychosocial factors might influence on alterations of WM in PDM.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of all subjects are shown in Table 1. Demographic characteristics between 35 PDM patients and 35 healthy controls (HCs) were not significantly different, including age, body mass index (BMI), onset of menses and length of menstrual phase (P > 0.05). However, there were significantly higher McGill pain questionnaire (MPQ)27,28, Cox retrospective symptom scale (RSS)29, self-rating anxiety scale (SAS)30 and self-rating depression scale (SDS)31 in PDM patients compared with HCs (P < 0.005).
Table 1. Comparison of demographics and clinical symptoms between PDM patients and healthy controls.
| Items | PDM (Mean ± SD) | HCs (Mean ± SD) | p-value |
|---|---|---|---|
| Age(year) | 22.6 ± 1.1 | 22.3 ± 1.8 | – |
| Body mass index | 21.8 ± 1.8 | 21.7 ± 1.6 | – |
| Onset of menses (year) | 12.5 ± 1.2 | 12.7 ± 1.3 | – |
| Length of menstrual phase (day) | 3–7 | 3–7 | – |
| Duration of dysmenorrhea (year) | 6.3 ± 2.5 | – | – |
| MPQ | 22.0 ± 9.6 | 0 | <0.001 |
| RSS | 34.6 ± 16.8 | 5.7 ± 2.1 | <0.001 |
| SAS | 30.1 ± 5.9 | 25.2 ± 6.6 | <0.005 |
| SDS | 31.6 ± 8.5 | 25.4 ± 5.5 | <0.001 |
Abbreviations: SD, standard deviation; PDM, primary dysmenorrheal; HCs, healthy controls; RSS, Cox retrospective symptom scale; MPQ, McGill pain questionnaire; SAS, self-rating anxiety scale; SDS, self-rating depression scale.
White matter microstructure alterations
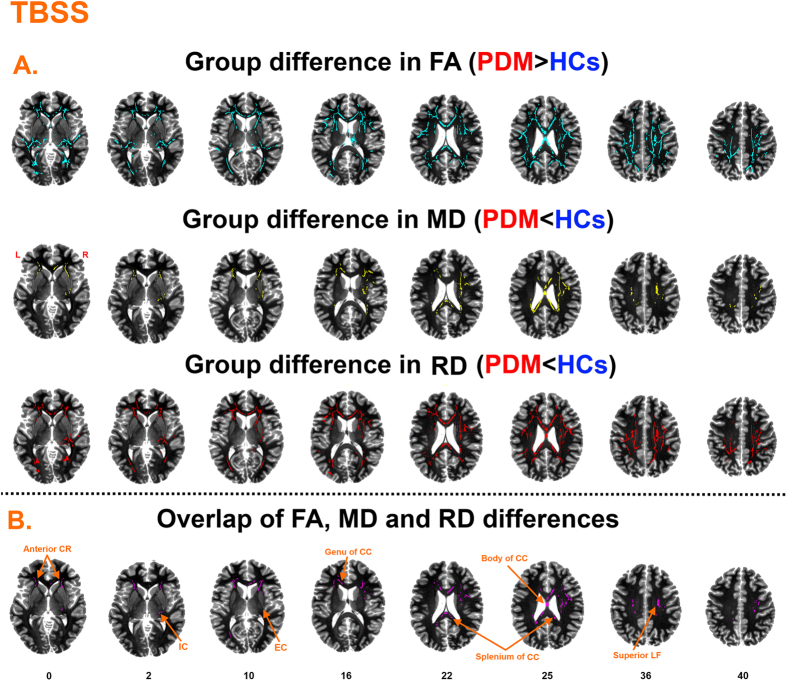
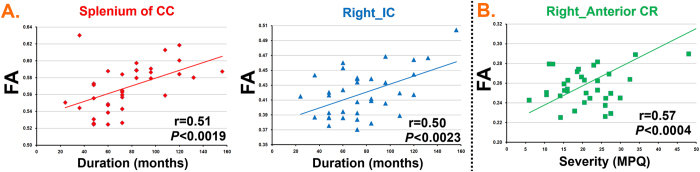
Compared with HCs, PDM patients had significantly increased FA, decreased MD and RD in many regions shown in Fig. 1A. Specifically, PDM patients had increased FA mainly in tracts of the corpus callosum (CC) (genu, body and splenium), fornix, bilateral internal capsule (IC), bilateral external capsule (EC), corona radiata (CR), bilateral posterior thalamic radiation, bilateral sagittal stratum, right cingulum and bilateral superior longitudinal fasciculus (LF). Decreased MD and RD in PDM patients was mainly obsered in the bilateral CC, right IC, bilateral CR, right EC and right superior LF. The overlapping regions exhibiting increased FA and decreased MD and RD included the CC (genu, body and splenium), right superior LF, bilateral anterior CR, right IC and right EC (Fig. 1B and Table 2). In these regions, FA of overlapping regions correlated with PDM clinical characteristics including duration and severity (MPQ). In detail, the FA of splenium CC and right IC positively correlated with PDM duration (Fig. 2A). The FA of the right anterior CR positively correlated with PDM severity (Fig. 2B). Results were considered significant when passing P < 0.05/8 = 0.00625.
Figure 1. TBSS findings.
(A) Regions showing increased FA (Fig. 2A first row), decrease MD (Fig. 2A second row) and decreased RD (Fig. 2A third row) in PDM patients. (B) Overlapping regions including the anterior corona radiata (anterior CR), internal capsule (lC), corpus callosum (genu, body and splenium of CC), external capsule (EC), superior longitudinal fasciculus (superior LF).
Table 2. Main locations of white-matter tracts showing differences of FA, MD and/or RD in PDM patients compared to healthy controls.
| White-matter tracts | none |
anxiety and depression |
||||
|---|---|---|---|---|---|---|
| FA | MD | RD | FA | MD | RD | |
| Genu of corpus callosum | 1137 | 371 | 1107 | 1021 | 838 | |
| Body of corpus callosum | 2852 | 1246 | 2713 | 2842 | 219 | 2517 |
| Splenium of corpus callosum | 605 | 627 | 646 | 406 | 163 | 573 |
| Internal capsule R | 791 | 122 | 570 | 740 | 167 | |
| Internal capsule L | 833 | 191 | 722 | 342 | ||
| Anterior corona radiata R | 1463 | 542 | 1357 | 1360 | 1184 | |
| Anterior corona radiata L | 1497 | 538 | 1398 | 1430 | 1291 | |
| Superior corona radiata R | 924 | 717 | 1120 | 955 | 918 | |
| Superior corona radiata L | 521 | 191 | 463 | 523 | 353 | |
| Posterior corona radiata R | 459 | 392 | 581 | 538 | 217 | 350 |
| Posterior corona radiata L | 419 | 293 | 421 | 419 | 361 | |
| Posterior thalamic radiation R | 555 | 345 | 527 | |||
| Posterior thalamic radiation L | 449 | 113 | 378 | 413 | ||
| Sagittal stratum R | 252 | 207 | 221 | |||
| Sagittal stratum L | 317 | |||||
| External capsule R | 116 | 226 | 326 | 213 | ||
| External capsule L | 142 | 305 | ||||
| Cingulum (cingulate gyrus) R | 145 | 118 | ||||
| Fornix R | 151 | |||||
| Fornix L | 187 | |||||
| Superior longitudinal fasciculus R | 921 | 253 | 845 | 824 | 471 | |
| Superior longitudinal fasciculus L | 774 | 595 | 681 | 339 | ||
The labels of the columns indicated the factors used as covariates.
Abbreviations: FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; L, Left; R, Right.
Figure 2. Overlapping regions correlated with clinical characteristics of primary dysmenorrhea (PDM).
(A) The FA of splenium of corpus callosum (CC) and right internal capsule (lC) positively correlated with PDM duration. (B) The FA of right anterior corona radiata (CR) positively correlated with PDM severity.
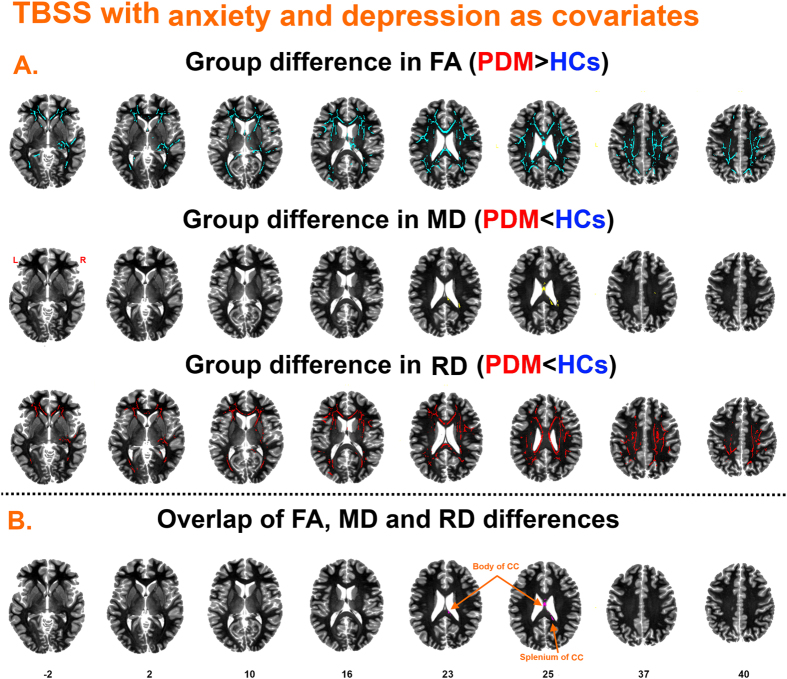
TBSS results with anxiety and depression as covariates of no interest are shown in Fig. 3 and Table 2. The FA and RD maps showed moderate changes, while the MD maps showed almost no changes. Overlapping significant regions for FA, MD and RD were found in the CC (body and splenium).
Figure 3. TBSS findings with self-rating anxiety scale (SAS) and self-rating depression scale (SDS) as covariates.
(A) Regions showing increased FA (Fig. 3A first row), decreased MD (Fig. 3A second row) and decreased RD (Fig. 3A third row) in PDM patients compared to healthy controls. (B) Overlapping regions including the body and splenium of corpus callosum (CC).
Probabilistic tractography
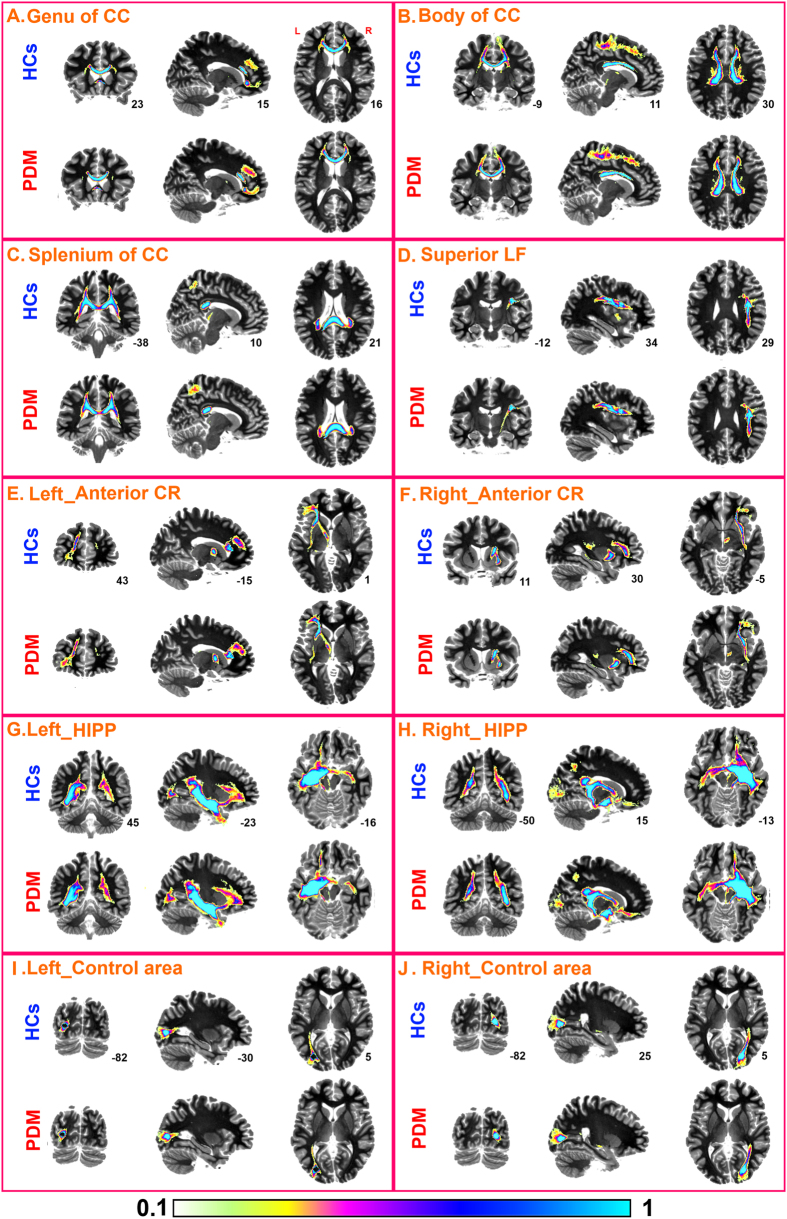
Tractography analysis was performed in the patient and control groups respectively, using the identified group-differentiating regions (genu, body, splenium of CC, right superior LF and anterior CR) as seed regions (Fig. 4). Through visual inspection, PDM patients appeared to have denser connections between the genu of CC and medial prefrontal cortex (MPFC) (Fig. 4A); between the body of CC and supplementary motor cortex, somatosensory cortex, middle cingulate cortex (MCC) and thalamus (Fig. 4B); and between the splenium of CC and precuneus (Fig. 4C) compared to HCs. And denser connections in PDM patients were also noted between the superior LF and putamen, insula, hippocampus and amygdala (Fig. 4D); and between anterior CR and MPFC, lateral prefrontal cortex and ACC (Fig. 4E,F).
Figure 4. Probabilistic tractography of seed regions in both PDM patients and healthy controls, including the genu, body, splenium of corpus callosum (CC), the superior longitudinal fasciculus (superior LF), anterior corona radiata (CR), hippocampus (HIPP) and two control areas in visual cortex.
Furthermore, we also investigated the WM tracts through HIPP, as it is well documented that HIPP is involved in PDM10. Compared to HCs, PDM patients had denser connections between the HIPP and MPFC, amygdala and OFC (Fig. 4E,F).
Finally, we selected two control regions in the visual cortex that is likely not related to PDM. We used these two regions as seed regions and performed the tractography analysis. Overall the connectivity patterns of these tracts were largely comparable between the two groups (Fig. 4I,J).
Discussion
To the best of our knowledge, this was the first study to investigate WM microstructure alterations in PDM patients by diffusion-weighted MRI. We found that a) PDM patients had increased FA, decreased MD and RD in various WM tracts compared to HCs, mainly including the CC, superior LF, anterior CR, IC and EC; b) the FA of the splenium CC and right IC positively correlated with PDM duration, and the FA of the right anterior CR positively correlated with PDM severity; c) there were abnormal connectivity patterns of the genu, body and splenium of CC, superior LF, anterior CR and HIPP with other brain regions implicated in sensory, motor, cognitive, affective and pain processing; c) the WM microstructure alterations might be partly related to psychosocial factors in PDM.
The first finding in the current study was that PDM patients had increased FA in the main tracts including the fornix, IC, EC, CR, CC, superior LF and thalamic radiation and the tracts extended to the OFC, PFC, insula, parietal cortex, cingulate cortex and basal ganglia. In most of the previous pain studies, decreased FA was reported to be associated with chronic pain, such as temporomandibular disorder32, fibromyalgia33, back pain34 and complex regional pain syndrome35. However, increased FA-related findings have been observed in other pain conditions36,37. For example, Chen et al. found that patients with irritable bowel syndrome (IBS) had increased FA in the fornix, EC, IC, insula, thalamus, cingulate cortex and SI36. Recently, we found increased FA in the EC, IC, CC, LF and CR37 in patients with postprandial distress syndrome. Moreover, it was reported that repeated juggling induced used-dependent plastic changes in the WM with increased FA38. Taken together, our findings could indicate that via plastically learning process from nociceptive input with menstruation, PDM patients might have increased myelination, increased number of myelination fibers or increased ratio between longitudinal vs. oblique aligned myelinated fibers in WM tracts. And the altered WM microstructures could be related to specific characteristic of PDM rather than the chronic painful state, which should be further evaluated in future studies.
In this study, PDM patients were found to have increased FA in the genu, body and slpenium of CC along with lower MD and RD, and had altered connectivity of the subregions of CC to the MPFC, supplementary motor cortex, somatosensory cortex, middle anterior/middle cingulate cortex, thalamus and precuneus in this study. CC, the largest commissural white matter bundle in brain, is topographically organized with its genu connecting orbitofrontal and frontal cortices, while its body and splenium connecting temporal, parietal and occipital regions39,40. Playing an important role in interhemispheric communication, CC is also critical to the evolution of structures and functions of cerebral cortex41. Neuroimaging studies have identified that PDM patients have dysfunctional nociceptive pathways and pain modulatory pathway and sensorimotor regions including the PFC, OFC, thalamus, insula and sensorimotor cortex7,10. Our findings might suggest that PDM patients have abnormalities in integrating pain modulation and sensory information which could relate to the disrupted structural connectivity arising from white matter microstructural anomalies in the CC. Furthermore, it has been revealed that abnormal interhemispheric transfer in the CC contributes to heightening pain perception and strengthening augmented pain processing42, which could be an interpretation of enhanced responses to noxious stimuli in PDM condition. On the other hand, fibers in splenium of CC connect regions of the parietal cortex involved in somatosensory information processing43. The positive correlation between FA of the splenium of CC and PDM duration in our study could reflect abnormal processing of somatosensory and pain as a consequence of extended periods of PDM.
Another finding of this study is that PDM patients showed altered WM microstructure in the superior LF. Superior LF is a complex brain association fiber system connecting the front and the back of the cerebrum including frontal, parietal and occipital cortex. It originates from the frontal cortex, passes through the operculum and ends in the posterior area of the lateral sulcus where numerous neurons radiate into the occipital cortex while others turn downward and forward around the putamen and radiate to anterior temporal cortex44. WM changes in the superior LF have been reported in pain-related disorders, implicated in the cognitive-affective dimension of pain, attention and somatosensory input37,45,46. In our study, PDM patients had denser connections between the superior LF and brain regions including hippocampus, amygdala, putamen and insula. Our findings may suggest that altered WM microstructures in the superior LF are likely involved in abnormalities of cognitive and emotional processing in PDM patients.
PDM patients had WM microstructure alterations in the IC. IC conveys information from primary and supplementary motor areas, frontopontine and thalamic peduncles to brain stem and cerebellar regions, and from thalamus to prefrontal cortex47. The damage in the posterior limb of IC, adjacent to the sensory thalamus, can produce ataxia48. Stimulation of the posterior limb of IC also may evoke paresthesias49. In addition, PDM patients had WM microstructure alterations in the EC adjacent to the posterior insula in this study. Craig provides a model of interoceptive input such that interoceptive information regarding the physiological condition of the body is projected to the anterior insula via the posterior insula50. The involvement of the posterior insula in visceral regulation and in homeostatic regulation is well elaborated51. EC also conveys information pertaining to the emotional component of pain perception46. Thereby, we speculate that WM microstructure alterations in the IC and EC might be implicated in pain and abnormal sensation arising from uterine, which could yield abnormal hypometabolisms in the sensorimotor and/or emotional regions7. Considering the positive correlation between the IC and PDM duration, we further speculate that WM microstructure alterations in the IC might be driven by extended periods of PDM.
We found WM microstructure alterations in the anterior CR in PDM patients. Anterior CR includes thalamic projections from the internal capsule to the cortex, and is a part of limbic-thalamo-cortical circuitry which is implicated in emotional regulation (Drevets et al., 2008). It has been reported that during menstruation women with PDM are more agitated and have poorer mood states, which are strongly associated with PDM52,53. Tu et al suggested that disinhibition of thalamo-orbitofrontal-prefrontal networks could contribute to the generation of pain and increased pain sensitivity in PDM patients, possibly by increasing negative emotion7. Additionally, anterior CR connects the ACC and striatum54. Altered gray matters in the ACC and striatum have been associated with PDM9,10. Abnormal activation or structure in the ACC is often found in experimental pain and chronic pain, and is related to affective aspects of pain processing55. Striatum is also enrolled in motor and emotional processing56,57,58. Thereby, our findings might indicate that PDM patients have abnormal processing of emotion which could associate with WM changes in the anterior CR.
PDM patients had abnormal connectivity between the HIPP and brain regions including the MPFC, amygdala and OFC in this study. HIPP is known to be a prime target for memory and learned behavior. However, it is also involved in inhibitory feedback to the HPA axis by stress59. Amygdala has an important direct influence on the emotional dimension of pain as well as fear, anxiety, and depression. There is a classic fear learning circuit related to amygdala and hippocampus60. HIPP and MPFC cooperate during anxiety processing as well61. Thereby, our finding might suggest that PDM may be sensitive to stress or negative emotion. Abnormal hippocampal structural connectivity might be hypothesized as a generating mechanism in PDM.
Increasingly, psychosocial factors such as anxiety, depression and catastrophizing have proven to be important contributors to patients with pain disorders23,24,25,26. As early as the late 1970s, the relationship between psychological factors and severity of dysmenorrhea was reported62. Our results with anxiety and depression as covariates showed that the tracts showing WM microstructural alterations only included the body and splenium of CC (Fig. 3). Our findings support that psychosocial factors partially influence on WM microstructure in PDM.
The present study presented some limitations which should be worth mentioning: a) WM changes near a structure are not necessarily attributable to axonal changes. These are huge axonal bundles that cross large stretches of brain, and abnormal axonal segment may just be passing through the neighborhood. Without T1 results to correlate volume/density with local white matter changes, we really cannot verify whether these FA changes are relevant to the described brain regions. Multimodal fusion of brain imaging data should be used in the future to further assess neural mechanisms of PDM. b) The number of collected diffusion directions was 30 in this study. Neuroimaging data with more number of diffusion directions should be collected to retest the current results. c) The DTI data were collected from PDM patients, who were not in the menstrual cycle. It has been found that PDM-related changes of GM structure were related with the dysmenorrhea pain or pain-free states11. So, our future study can be focused more on the question: whether there are differences of WM between the different sates of the PDM patients.
Conclusion
In summary, to the best of our knowledge, we provide the first evidence that PDM patients have white matter microstructure alterations, abnormal structure connectivity and that the PDM clinical characteristics were related to altered white matter tracts. To a certain extent, the psychology factors also disrupted pattern of white matter microstructure in PDM patients. These findings might contribute to our understanding of the neural mechanism of PDM. However, the interaction between the altered white matter microstructures and PDM should be more comprehensively investigated in future longitudinal studies and in a larger population of PDM patients.
Methods
All research procedures of the present study were conducted in accordance with the Declaration of Helsinki and were approved by the West China Hospital Subcommittee on Human Studies. Informed consent was obtained from all subjects before participation. And all methods were carried out in accordance with the approved guidelines.
Subjects
35 right-handed PDM patients and 35 age-matched right-handed healthy females were recruited in this study. All the subjects were university students from Chengdu University of Traditional Chinese Medicine or West China School of Medicine of Sichuan University. Patients were included if they met all of the following inclusion criteria: a) regular menstrual cycle (28 ± 3days) and menstruation lasting 3 to 7 days; b) moderate or severe dysmenorrhea (based on numeric rating scales (NRSs), greater than 4 points7 on a visual analogue scale in which 0 means no pain and 10 means worst pain imaginable); c) attacking at least 4 months in the past 6 months and being nulliparous. Exclusion criteria for all the subjects included: a) secondary dysmenorrhea induced by endometriosis or pelvic inflammatory disease; b) recurrent pelvic or lower abdominal pain; c) gastrointestinal diseases including recurrent gastritis, gastric or duodenal ulcer; d) chronic pain; e) being pregnant or intending to become pregnant during the course of the trial; f) having history or evidence of serious diseases, neurological or psychiatric diseases; g) any contraindication of MRI scanning; h) receiving oral contraceptives within 6 months prior to this study; i) smokers; j) heavy drinker or alcohol/drug addicts; k) receiving NSAIDs (including aspirin) or analgesics within 24 h before MRI scanning. In addition, each patient underwent a basic evaluation with pelvic ultrasound to further ensure to exclude PDM patients caused by endometriosis, uterine myomas, endometrial polyps, pelvic inflammatory disease, and other gynecological problems. Healthy female controls also underwent the same basic evaluation mentioned above.
Clinical assessment
McGill pain questionnaire27,28 and Cox retrospective symptom scale29 were applied to assess the lower abdominal menstrual pain and associated symptoms of each PDM patient in past three months. self-rating anxiety30 and self-rating depression31 scales were then adopted to evaluate subject’s anxiety and depression status. Other baseline data including age, body mass, height, length of menstrual cycle, length of menstrual phase and duration of dysmenorrhea were recorded as well. All the related assessments were completed before scanning, similar to the previous study from Tu et al.7,10.
Scanning protocol
All the subjects underwent scanning in a 3T Siemens scanner (Allegra, Siemens Medical System, Erlangen, Germany) at the Huaxi MR Research Center, West China Hospital of Sichuan University, Chengdu, China. Two Diffusion-weighted sequences with single-shot echo planar imaging in alignment with the anterior–posterior commissural plane were acquired with the following parameters: repetition time (TR) = 6800 ms, echo time (TE) = 93 ms, field of view (FOV) = 240 mm × 240 mm, matrix = 128 × 128, 1.875 mm × 1.875 mm in-plane resolution, slice in thickness = 3 mm, 45 continuous axial slices with no gap, b = 1,000 seconds/mm2, diffusion sensitizing gradients applied along 30 non-collinear isotropic directions, with two no diffusion weighting image (b0 = 0 seconds/mm2).
Demographic and clinical characteristics analysis
Continuous variables of demographic, clinical symptom severity, and emotional measures were tested by an independent 2-sample t-test. Statistical significance was set to P < 0.05 (two-tailed test).
DTI image analysis
Because DTI data may be disturbed by cardiac pulsatility63, signal dropout, motion artifacts and/or other artifacts, all the DTI image volumes were visually checked before preprocessing to screen noisy images that could bias the results. We also inspected data for volumes containing large signal variation or ghosting.
The diffusion image processing was performed using the Oxford Centre for Functional MRI of the Brain’s (FMRIB) Software Library (FSL version 4.1.6, http://www.fmrib.ox.ac.uk/fsl)64. Preprocessing included eddy current and motion artifact correction using the FSL diffusion toolbox (FDT)65. The mean DTI images were calculated from the two preprocessed DTI acquisitions for each subject for a greater signal-to-noise ratio. The preprocessed images were then fit with a diffusion tensor model using DTIFIT in the FMRIB diffusion toolbox after individual brain masks were created from the b0 image of each subject using the by brain extraction tool66. FA, MD, RD and AD maps were generated from the duffusion tensor model.
Voxelwise statistical analysis of FA data was carried out by TBSS19. Individual FA images underwent nonlinear registration to a standard space (a 1-mm isotropic FA image (FMRIB58_FA)) using the non-linear registration tool FNIRT. A mean FA image stemmed from all the subjects’ images were created and thinned to represent the center of major WM tracts common to all subjects, forming a mean WM skeleton with an FA threshold of >0.25. Each subject’s aligned FA map was projected onto the nearest relevant tract center of the mean FA skeleton by searching perpendicular to the local skeleton structure, which circumvents misalignment during registration. One skeleton was created for analysis of group differences that included all the subjects. Combing FA, MD, RD and AD would probably provide more information about the neural mechanism of PDM. We thereby assessed the other DTI metrics similar to the analysis steps of FA.
FSL’s permutation-based non-parametric testing (Randomise v2.1) was then adopted to compare FA between the patient and HCs with 10,000 times. Multiple comparisons across voxels were corrected using the threshold-free cluster enhancement (TFCE) method at P < 0.05, with a cluster size of >100 voxels67. Other metric images were also examined using TBSS, related to MD, RD and AD. The WM tracts with significant difference were identified by the JHU ICBM-DTI-81 white-matter label atlas68.
To investigate relationships between parameters of WM tracts and clinical characteristics, Pearson correlation analysis was adopted to examine relationships between PDM clinical characteristics (duration and severity) and FA of the WM tracts showing between-group differences in the overlapping map. The significance level was set at P < 0.05/N for multiple tests (Bonferroni-corrected, where N is the number of WM tracts tested).
We selected seed regions including the WM tracts showing between-group differences in DTI measures, HIPP and visual cortex as control areas. Bilateral HIPP were defined from the Harvard–Oxford Structural Probability Atlas distributed with the FSL neuroimaging analysis software package. Seed regions of HIPP was thresholded at 75% to yield a conservative anatomical representation. Seed regions of visual cortex were spheres centered on MNI coordinates of sphere: ±27, −80, 6 and radius = 4 mm, that were related to PDM. Probabilistic tractography was then adopted to examine connectivity of these seed regions, using FDT. By fitting a multifiber diffusion model, the probability distribution on direction of 1 or more fiber populations at each voxel was assessed for the pathways through regions of fiber crossings69. The connectivity distribution images, in which each voxel represented the probability of the connection to the seed voxel, were built up by producing 5,000 streamline samples from each seed voxel with parameters of curvature threshold at 0.2 and step length at 0.5 mm. Seed masks were binary significant clusters identified with TBSS analysis. The connectivity distribution of each subject was thresholded at 2,500 to remove spurious connections37,70,71. To visualize the group findings, each of the subject’s tracts were binarized and overlaid on a standard brain to produce a probabilistic map of the pathways for patients and HCs, respectively.
In order to investigate the effects of psychosocial factors on DTI-derived metrics, we carried out TBSS between the two groups with anxiety and depression as covariates. Multiple comparisons were also corrected using the TFCE at P < 0.05.
Additional Information
How to cite this article: Liu, P. et al. White matter microstructure alterations in primary dysmenorrhea assessed by diffusion tensor imaging. Sci. Rep. 6, 25836; doi: 10.1038/srep25836 (2016).
Acknowledgments
This study was supported by the Project for the National Key Basic Research and Development Program (973) under Grant Nos 2012CB518501 and 2014CB543203, the National Natural Science Foundation of China under Grant Nos 81471738, 81303060, 81271644, 81471811, 61401346, the Fundamental Research Funds for the Central Universities, NIH 1R01EB006841, R01EB005846 and P20GM103472.
Footnotes
Author Contributions P.L., F.Y., G.W. and Y.L. performed the data analysis and made the original finding. J.S., L.J., X.Y. and W.Q. recorded the MRIs and provided clinical data. P.L. wrote the draft. Q.Y. and V.D.C. revised the paper. All authors participated in data interpretation and in writing the final paper.
References
- Harlow S. D. & Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol 103, 1134–1142 (1996). [DOI] [PubMed] [Google Scholar]
- Coco A. S. Primary dysmenorrhea. Am Fam Physician 60, 489–496 (1999). [PubMed] [Google Scholar]
- Iacovides S., Avidon I. & Baker F. C. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update, 21, 762–778 (2015). [DOI] [PubMed] [Google Scholar]
- French L. Dysmenorrhea. Am Fam Physician 71, 285–291 (2005). [PubMed] [Google Scholar]
- Bettendorf B., Shay S. & Tu F. Dysmenorrhea: contemporary perspectives. Obstet Gynecol Surv 63, 597–603 (2008). [DOI] [PubMed] [Google Scholar]
- Harel Z. A contemporary approach to dysmenorrhea in adolescents. Pediatric Drugs 4, 797–805 (2002). [DOI] [PubMed] [Google Scholar]
- Tu C. H. et al. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage 47, 28–35 (2009). [DOI] [PubMed] [Google Scholar]
- Vincent K. et al. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 152, 1966–1975 (2011). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. Eur J Pain 20, 512–520 (2016). [DOI] [PubMed] [Google Scholar]
- Tu C. H. et al. Brain morphological changes associated with cyclic menstrual pain. Pain 150, 462–468 (2010). [DOI] [PubMed] [Google Scholar]
- Tu C. H. et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain 154, 1718–1724 (2013). [DOI] [PubMed] [Google Scholar]
- Nucifora P. G., Verma R., Lee S.-K. & Melhem E. R. Diffusion-tensor mr imaging and tractography: exploring brain microstructure and connectivity. Radiology 245, 367–384 (2007). [DOI] [PubMed] [Google Scholar]
- Le Bihan D. et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13, 534–546 (2001). [DOI] [PubMed] [Google Scholar]
- Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol 45, 169–184 (2003). [DOI] [PubMed] [Google Scholar]
- Basser P. J. & Jones D. K. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed 15, 456–467 (2002). [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15, 435–455 (2002). [DOI] [PubMed] [Google Scholar]
- Apkarian A. V., Bushnell M. C., Treede R. D. & Zubieta J. K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9, 463–484 (2005). [DOI] [PubMed] [Google Scholar]
- Davis K. D. & Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8, 518–534 (2013). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 (2006). [DOI] [PubMed] [Google Scholar]
- Behrens T. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6, 750–757 (2003). [DOI] [PubMed] [Google Scholar]
- Gagua T., Tkeshelashvili B., Gagua D. & Mchedlishvili N. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. J Pediatric Adolescent Gynecol 26, 350–354 (2013). [DOI] [PubMed] [Google Scholar]
- Alonso C. & Coe C. L. Disruptions of social relationships accentuate the association between emotional distress and menstrual pain in young women. Health Psychol 20, 411 (2001). [PubMed] [Google Scholar]
- Linton S. J. A review of psychological risk factors in back and neck pain. Spine 25, 1148–1156 (2000). [DOI] [PubMed] [Google Scholar]
- Linton S. J. Occupational psychological factors increase the risk for back pain: a systematic review. J Occup Rehabil 11, 53–66 (2001). [DOI] [PubMed] [Google Scholar]
- McGrath P. A. Psychological aspects of pain perception. Archf Oral Biol 39, S55–S62 (1994). [DOI] [PubMed] [Google Scholar]
- Schreiber K. L. et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain 154, 660–668 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droz J. & Howard F. M. Use of the Short-Form McGill Pain Questionnaire as a diagnostic tool in women with chronic pelvic pain. J Minim Invasive Gynecol 18, 211–217 (2011). [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain 30, 191–197 (1987). [DOI] [PubMed] [Google Scholar]
- Cox D. J. & Meyer R. G. Behavioral treatment parameters with primary dysmenorrhea. J Behav Med 1, 297–310 (1978). [DOI] [PubMed] [Google Scholar]
- Zung W. W. A rating instrument for anxiety disorders. Psychosomatics 12, 371–379 (1971). [DOI] [PubMed] [Google Scholar]
- Zung W. W. K., Richards C. B. & Short M. J. Self-rating depression scale in an outpatient clinic: further validation of the SDS. Arch Gen Psychiatry 13, 508 (1965). [DOI] [PubMed] [Google Scholar]
- Moayedi M. et al. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain 153, 1467–1477 (2012). [DOI] [PubMed] [Google Scholar]
- Lutz J. et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion‐tensor and volumetric imaging study. Arthritis Rheum 58, 3960–3969 (2008). [DOI] [PubMed] [Google Scholar]
- Mansour A. R. et al. Brain white matter structural properties predict transition to chronic pain. Pain 154, 2160–2168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha P. Y. et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60, 570–581 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y.-W., Blankstein U., Diamant N. E. & Davis K. D. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res 1392, 121–131 (2011). [DOI] [PubMed] [Google Scholar]
- Zhou G. et al. White-matter microstructural changes in functional dyspepsia: a diffusion tensor imaging study. Am J Gastroenterol 108, 260–269 (2012). [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M. C., Behrens T. E. & Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci 12, 1370–1371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe O. et al. Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr 28, 533–539 (2004). [DOI] [PubMed] [Google Scholar]
- Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat Rec 119, 119–135 (1954). [DOI] [PubMed] [Google Scholar]
- Schulte T., Sullivan E. V., Müller-Oehring E., Adalsteinsson E. & Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex 15, 1384–1392 (2005). [DOI] [PubMed] [Google Scholar]
- Kim D. J. et al. Altered White matter integrity in the corpus callosum in fibromyalgia patients identified by tract‐based spatial statistical analysis. Arthritis Rheumatol 66, 3190–3199 (2014). [DOI] [PubMed] [Google Scholar]
- Caminiti R. et al. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci 33, 14501–14511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. et al. White matter integrity in physically fit older adults. Neuroimage 82, 510–516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza D. D., Hodaie M. & Davis K. D. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain 155, 37–44 (2014). [DOI] [PubMed] [Google Scholar]
- Lieberman G. et al. White matter involvement in chronic musculoskeletal pain. J Pain 15, 1110–1119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. V., Zahr N. M., Rohlfing T. & Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia 48, 4155–4163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J. D., Rosene D. L. & Pandya D. N. Motor projections to the basis pontis in rhesus monkey. J Comp Neurol 478, 248–268 (2004). [DOI] [PubMed] [Google Scholar]
- Adams J. E., Hosobuchi Y. & Fields H. L. Stimulation of internal capsule for relief of chronic pain. J Neurosurg 41, 740–744 (1974). [DOI] [PubMed] [Google Scholar]
- Craig A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- Mayer E. A., Naliboff B. D. & Craig A. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 131, 1925–1942 (2006). [DOI] [PubMed] [Google Scholar]
- Iacovides S., Baker F. C., Avidon I. & Bentley A. Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. J Pain 14, 1066–1076 (2013). [DOI] [PubMed] [Google Scholar]
- Dorn L. D. et al. Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health 44, 237–243 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L. M., Van Zijl P. C. & Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology 230, 77–87 (2004). [DOI] [PubMed] [Google Scholar]
- Rainville P., Duncan G., Price D., Carrier B. & Bushnell M. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 (1997). [DOI] [PubMed] [Google Scholar]
- Alexander G. E., DeLong M. R. & Strick P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9, 357–381 (1986). [DOI] [PubMed] [Google Scholar]
- Nakano K., Kayahara T., Tsutsumi T. & Ushiro H. Neural circuits and functional organization of the striatum. J Neurol 247, V1–V15 (2000). [DOI] [PubMed] [Google Scholar]
- Marchand W. R. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct 215, 73–96 (2010). [DOI] [PubMed] [Google Scholar]
- Rodrigues S. M., LeDoux J. E. & Sapolsky R. M. The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32, 289–313 (2009). [DOI] [PubMed] [Google Scholar]
- Neugebauer V., Li W., Bird G. C. & Han J. S. The amygdala and persistent pain. Neuroscientist 10, 221–234 (2004). [DOI] [PubMed] [Google Scholar]
- Adhikari A., Topiwala M. A. & Gordon J. A. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A. et al. Dysmenorrhea in adolescents and young adults: prevalence, related factors and limitations in daily living. Acta Med Port 24, 383–388 (2011). [PubMed] [Google Scholar]
- Jones D. K. & Leemans A. Diffusion tensor imaging. Methods Mol Biol 711, 127–144 (2011). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004). [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M. & Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002). [DOI] [PubMed] [Google Scholar]
- Smith S. M. Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. & Nichols T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009). [DOI] [PubMed] [Google Scholar]
- Mori S. et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T., Berg H. J., Jbabdi S., Rushworth M. & Woolrich M. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34, 144–155 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó N. et al. White matter microstructural alterations in migraine: A diffusion-weighted MRI study. Pain 153, 651–656 (2012). [DOI] [PubMed] [Google Scholar]
- Westerhausen R., Gruner R., Specht K. & Hugdahl K. Functional relevance of interindividual differences in temporal lobe callosal pathways: a DTI tractography study. Cereb Cortex 19, 1322–1329 (2009). [DOI] [PubMed] [Google Scholar]