Abstract
Biotin protein ligase (BPL) is widespread in the three domains of the life. The paradigm BPL is the Escherichia coli BirA protein, which also functions as a repressor for the biotin biosynthesis pathway. Here we report that Lactococcus lactis possesses two different orthologues of birA (birA1_LL and birA2_LL). Unlike the scenario in E. coli, L. lactis appears to be auxotrophic for biotin in that it lacks a full biotin biosynthesis pathway. In contrast, it retains two biotin transporter-encoding genes (bioY1_LL and bioY2_LL), suggesting the use of a scavenging strategy to obtain biotin from the environment. The in vivo function of the two L. lactis birA genes was judged by their abilities to complement the conditional lethal E. coli birA mutant. Thin-layer chromatography and mass spectroscopy assays demonstrated that these two recombinant BirA proteins catalyze the biotinylation reaction of the acceptor biotin carboxyl carrier protein (BCCP), through the expected biotinoyl-AMP intermediate. Gel shift assays were used to characterize bioY1_LL and BirA1_LL. We also determined the ability to uptake 3H-biotin by L. lactis. Taken together, our results deciphered a unique biotin scavenging pathway with redundant genes present in the probiotic bacterium L. lactis.
Biotin (vitamin H) is essential in the three domains of life. Biotin is a covalently-bound enzyme cofactor in central metabolism like the acetyl-CoA carboxylase (ACC) reaction required to form the fatty acid building block, malonyl-CoA1. Most bacteria synthesize biotin. However, some bacteria must scavenge biotin from the environment. BioY is a biotin transporter found in many bacteria2,3,4. Generally, BioY is considered to be the substrate-binding component (S component) of the Energy Coupling Factor transport family (ECF), an ATP-binding cassette transporter involved in the uptake of multiple micro-nutrients5,6. E. coli can either synthesize biotin or transport biotin using a non-BioY/ECF mechanism7. Interestingly, acquisition of biotin in Streptococcus suis, an animal pathogen, is solely dependent on the presence of the BioY transporter because it lacks a biotin synthesis pathway4. Hebbeln and coworkers reported the in vitro biochemistry of the tripartite biotin transporter BioMNY from the α-proteobacterium Rhodobacter2. Although the crystal structure of the BioY membrane protein of Lactococcus lactis was reported8, the regulated expression of bioY and biotin sensing mediated by the BirA gatekeeper protein remained unknown.
L. lactis IL1403 is a member of a group of low GC Gram-positive bacteria9. Generally, it is believed to stay dormant on plants, but grows within the gastrointestinal tract. Given the fact that L. lactis vigorously ferments lactose in milk, with production of ATP and lactic acid, L. lactis has been widely applied in the dairy industry, such as buttermilk and cheese production10. Given its long role in food fermentation, L. lactis has been generally recognized as safe (GRAS) status. The presence of L. lactis in the intestine of animals and humans significantly benefits the immune system and it is generally regarded as a probiotic bacterium11. It seemed unusual that L. lactis IL1403 with a small genome (2.37 Mb), which is estimated to be only half of the E. coli genome (4.64 Mb for MG1655)9, encodes numerous redundant (and/or duplicated) loci in the context of lipid metabolism as well as within the biotin utilization pathway. While unexpected, such an arrangement is not without precedent as a similar scenario was observed in Paracoccus12. This rare situation where redundant genes of biotin metabolism are present within the minimal genome of L. lactis might render some unknown physiological advantage, raising a possibility that it represents a relic of bacterial evolution and selection by its environmental niche13,14.
In this paper, we performed systematic functional analyses of the redundant genes in the context of biotin metabolism. We demonstrate in vivo evidence for biotin transport by L. lactis and also determined distinct functional assignments for the two BirA orthologues. Finally, we formulated a working model for biotin scavenging by L. lactis (Fig. 1). The atypical occurrence of two different biotin protein ligases might suggest its unique evolution history in adaption to its growing environment and/or ecological niche.
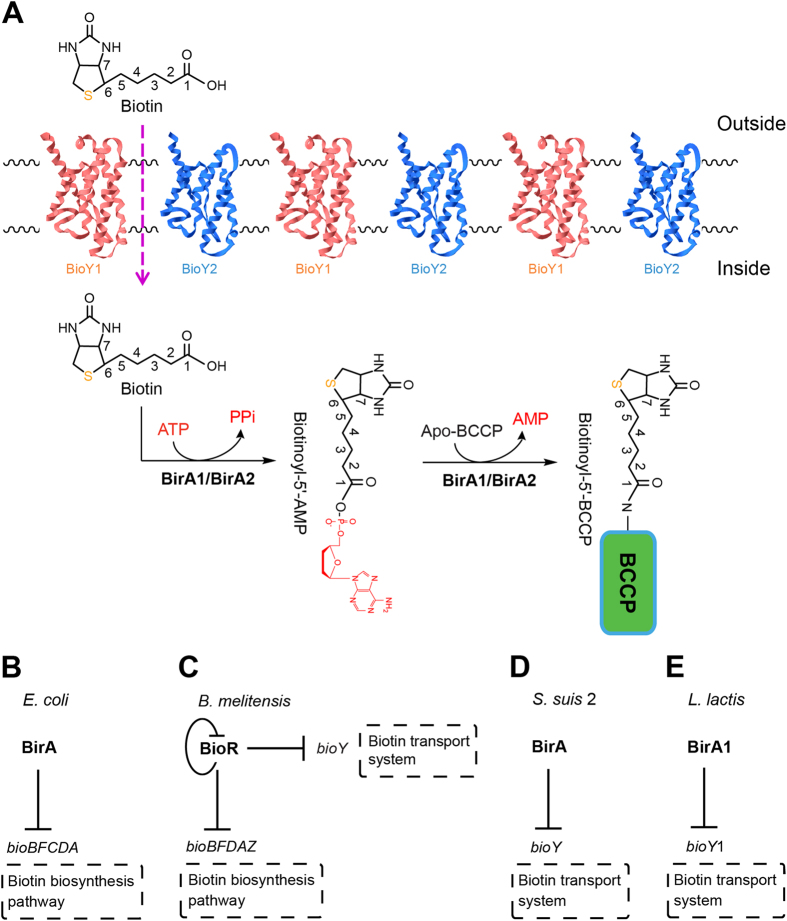
Figure 1. Current models for biotin uptake and BirA biotin protein ligase/repressor functions.
(A) Proposed biotin uptake and utilization pathway in L. lactis. The two half-reactions of BirA-mediated BCCP biotinylation are shown. (B) E. coli BirA represses the biotin synthesis pathway (C). B. melitensis BioR represses expression of the biotin synthesis pathway and the biotin transport system (D). Transcriptional repression of the bioY gene by the BirA of S. suis 2 (E). BirA1 represses expression of the bioY gene in L. lactis.
Results and Discussion
Redundancy of biotin metabolism genes in L. lactis
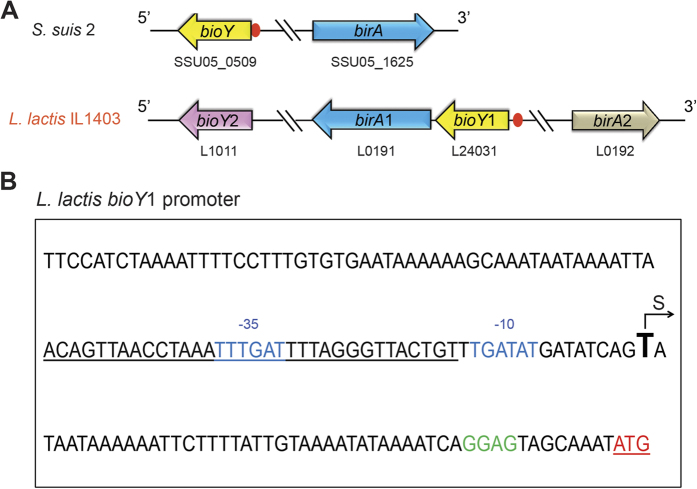
Similar to its closely-related cousin, the zoonotic pathogen Streptococcus suis15, L. lactis is also a low GC Gram-positive bacterium with a reduced genome (35.3% GC percentage, 2.37 Mb)9. However, it seemed unusual that the genetic organization of L. lactis features gene duplications and/or redundancy in the context of biotin metabolism (Table S1). Unlike S. suis that encodes only one birA gene and one bioY gene, L. lactis has two birA orthologues (called birA1 [L0191] and birA2 [L0192], Table S1) and two bioY orthologues (bioY1 [L24031] and bioY2 [L1011], respectively (Table S1). The birA2 gene encodes a putative “simple” biotin protein ligase (BPL) that lacks the N-terminal DNA binding motif found in the putative dual-functional birA1 (Figs 1 and 2). While the presence of multiple copies of birA and bioY homologues is atypical, two copies of birA have been found in Francisella16 and two different bioY orthologues are present in Paracoccus12. Apart from redundancies in birA and bioY, redundancy is also found within the fatty acid synthesis loci in L. lactis (Table S1). Three loci of fabG (3-oxoacyl-ACP reductase) exist in L. lactis, namely fabG1 (L0185), fabG2 (L27694), and fabG3 (L1530) (Table S1). Of note, Wang and Cronan reported that the fabG2 is not an active 3-oxoacyl-ACP reductase17, indicating a possible role of these fabG loci in sugar metabolism. Moreover, L. lactis has two loci (fabZ [L0188] and fabZ1 [L160425]) that encode a 3-hydroxyacyl-ACP dehydratase (Table S1). Two fatty acid degradation (fad) genes (fadA [L25946] fadD [L54546]) also are present in L. lactis, whereas the fad system is cryptic for Streptococcus (Table S1). These gene arrangements suggest an unexpected complexity of lipid metabolism (including biotin utilization) in L. lactis compared with its close relative, S. suis (Table S1).
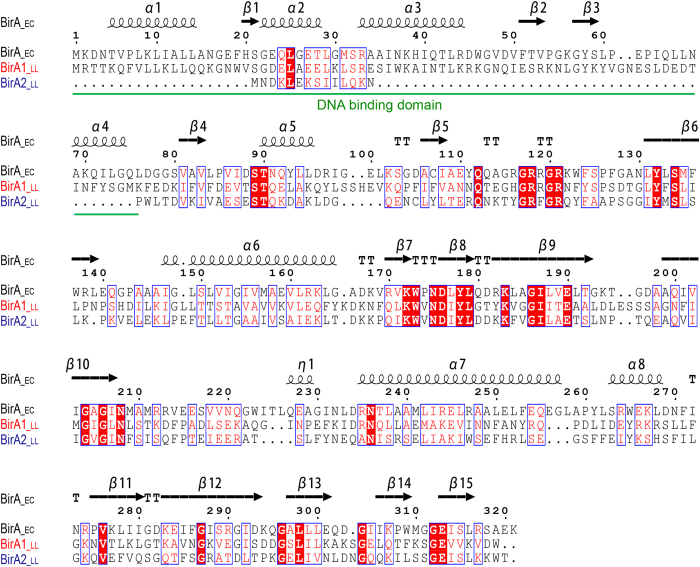
Figure 2. Sequence alignment of the L. lactis BirA homologues with the E. coli BirA.
The multiple alignment was performed using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html), and the final output was processed using the program ESPript 2.2 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). The well-studied BirA_ec protein was used from E. coli K-12 MG1655 (Accession no. AAC43075), and the two BirA homologues were derived from L. lactis IL1403. BirA1_LL (Accession no. NP_267937) is shown in red and BirA2_LL (Accession no. NP_268060) is indicated in blue. Identical residues are in white letters with red background, similar residues are in red letters with white background, varied residues are in black letters, and dots represent gaps. The predicted secondary structure of BirA is shown at the top of the alignment. α: α-helix; β: β-sheet; T: β-turns/coils. Designations: EC, E. coli; LL, L. lactis.
Characterization of the Two BirA homologues
BPL has been classified into two groups: the Group I BPLs lack an N-terminal DNA-binding motif whereas the Group II BPLs (BirAs) have an N-terminal DNA-binding domain4. The paradigm Group II BPL is the E. coli BirA and such enzymes also are found in the Archaea, suggesting that the group II version may be the ancestor of the BPL enzyme family. Based upon annotation, L. lactis IL1403 likely possesses two types of BPLs, which are referred to here on as BirA1_LL (323 residues, a putative Group II BPL) and BirA2_LL (250 aa, a putative Group I BPL). Multiple sequence alignments of the two BirA proteins showed that they exhibit 20.7% identity and 34.6% similarity compared to the E. coli BirA (Fig. 2).
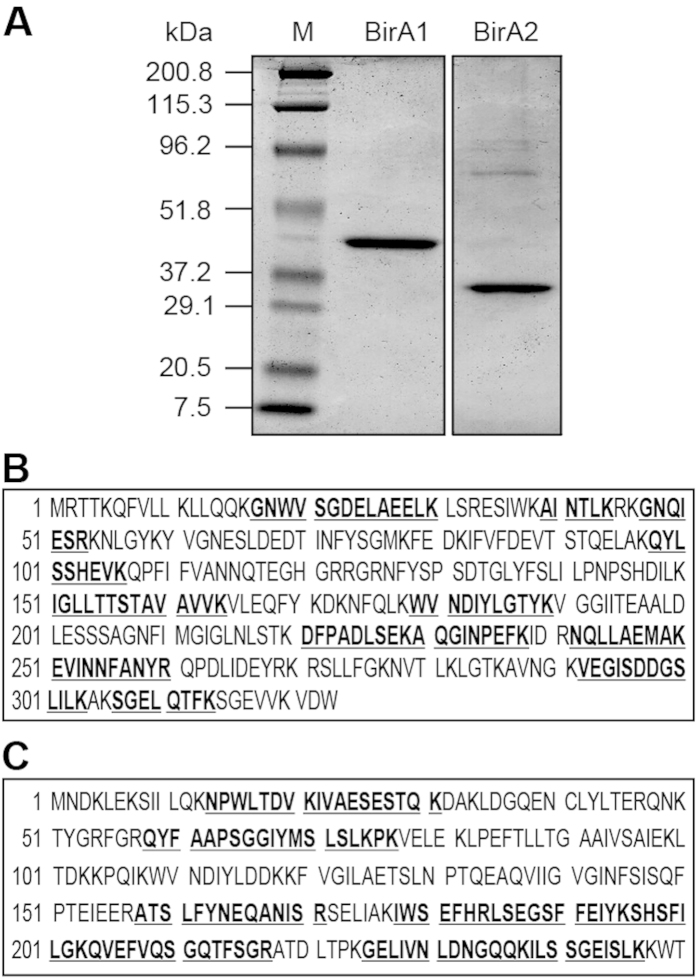
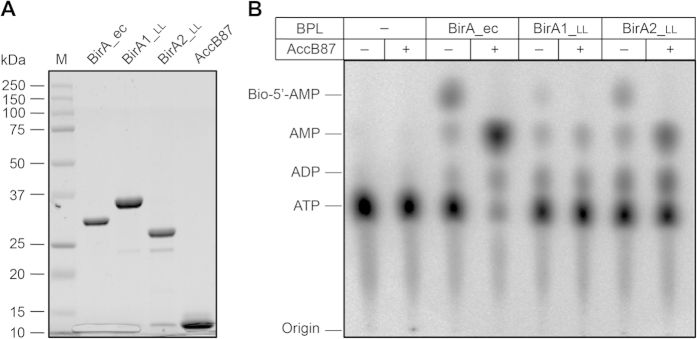
To test the BPL activity of the two L. lactis IL1403 BirA proteins, we purified the hexahistidine-tagged proteins to homogeneity. SDS-PAGE profiles of the purified recombinant proteins suggested that BirA1_LL is around 40 kDa, whereas the BirA2_LL is estimated to be about 30 kDa (Fig. 3A). To further verify their identity, MALDI-TOF was used to analyze digested polypeptide fragments. The MS results supported that the two purified, recombinant proteins are BirA1_LL (36.8% coverage) and BirA2_LL (45.6% coverage) (Fig. 3B,C).
Figure 3. Purification and MS identification of L. lactis BirA1 and BirA2.
(A) 12% SDS-PAGE profile of the two purified BirA proteins (BirA1 and BirA2). A pre-stained protein standard marker (M) was used in the first lane and marker sizes are indicated in kDa to the left of the image. Results for liquid chromatography quadrupole time-of-flight MS verification of the two recombinant proteins, BirA1 (in Panel B) and BirA2 (in Panel C). The peptide fragments that matched the database sequence are indicated in bold and underlined type. The coverage was 36.8% for BirA1, and 45.6% for BirA2, respectively.
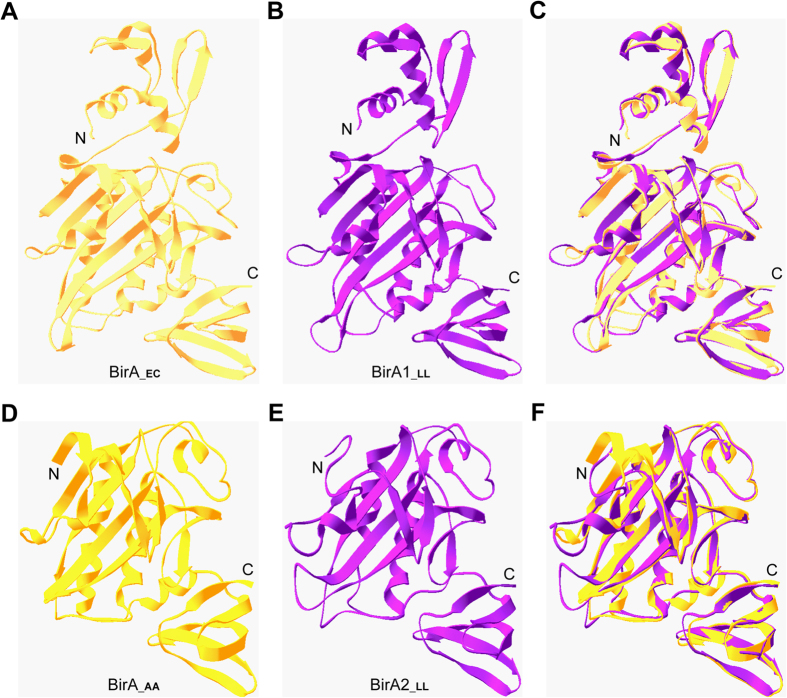
To better visualize the architectures of the two distinct forms of L. lactis BPLs, structural modelling was carried out. In similarity to that of the E. coli BirA (Fig. 4A)18, BirA1_LL also possesses the N-terminal DNA-binding motif and the C-terminal enzymatic domain (Fig. 4B,C), fitting the criteria for the Group II BPLs. The structure of the BirA2_LL was almost identical to that of the Aquifex aeolicus BirA, a member of Group I BPLs19 (Fig. 4D–F), supporting its classification as a Group I BPL.
Figure 4. Structural analyses of two BirA homologue proteins from L. lactis.
The E. coli BirA structure (in Panel A) and the modeled structure of L. lactis BirA1 (BirA1_LL) (in Panel B) exhibit strong structural similarity. (C) Structural superposition of BirA1_LL with BirA_EC. The BirA_EC structure (PDB: 1HXD) is in yellow, whereas that of BirA1_LL (with 28.6% identity to BirA_EC structure) is indicated in purple. An overall view of Aquifex aeolicus BirA protein (BirA_AA) structure (in Panel D) and the modeled structure of BirA2_LL protein (in Panel E) (F). Structure superposition of BirA2_LL with BirA_AA. The structure of BirA_AA (PDB: 3EFS) is in gold, whereas the modeled structure of BirA2_LL using BirA_AA as the template with 32.4% identity is highlighted in purple. Designations: AA, Aquifex aeolicus; LL, L. lactis; N, N-terminus; C, C-terminus.
Comparing the BPL activity of the two L. lactis BirA proteins
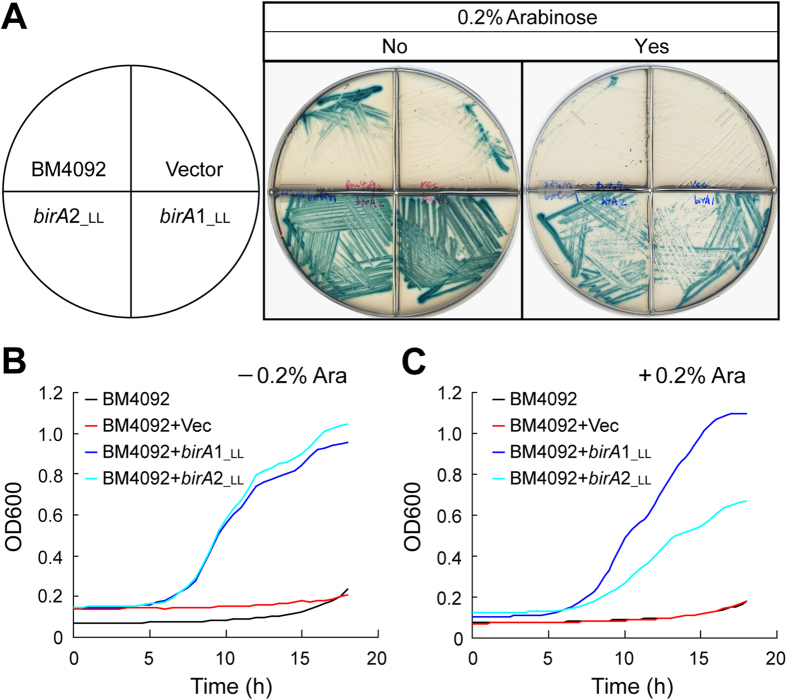
BPL activity was measured using the well-studied E. coli model together with in vitro enzymatic assays. The model E. coli strain for functional complementation of birA homologues is the birA1 mutant BM4092, which lacks the ability to synthesize biotin or to transport biotin with high affinity20. As expected, the BM4092 strain either with or without the empty vector pBAD24 did not grow on M9 agar plates supplemented with 25 nM of biotin (Fig. 5A). In contrast, the arabinose-induced expression (and even basal expression) of both birA1_LL and birA2_LL supported the growth of BM4092 under the non-permissive biotin levels (25 nM) (Fig. 5A). The bacterial growth curves of the BM4092 derivatives in liquid media also gave similar results to those obtained from the agar plates (Fig. 5B,C). Of note, overexpression of birA1_LL seemed slightly toxic for E. coli in that bacterial growth of the BM4092 strain supplemented with 0.2% arabinose was significantly inhibited when compared with growth conditions lacking the inducer, arabinose (Fig. 5C). Together, these data provided in vivo evidence that the two BirA orthologues of L. lactis (BirA1_LL and BirA2_LL) have BPL activity.
Figure 5. Both L. lactis birA homologues (LLbirA1_LL and LLbirA2_LL) can complement the E. coli birA Km mutant.
(A) L. lactis birA genes (birA1_LL and birA2_LL) can support growth of the E. coli birA1 Km mutant on minimal media supplemented with 25 nM biotin. BM4092 is an E. coli birA1 Km mutant strain carrying a bio-lacZ fusion. 0.5 μM X-gal was added into the media to monitor transcription of the bio-lacZ by BirA. Yes or No indicates whether the agar plate contains 0.2% arabinose. The inoculated plates were grown at 30 °C for ~20 hours. Representative plate results are shown. Designations: Ara, arabinose; Vec, the pBAD24 empty vector, LL, L. lactis. Growth curves of the BM4092 birA Km mutant complemented with either plasmid-borne L. lactis birA genes (either birA1_LL or birA2_LL) in liquid minimal media with (B) or without (C) the addition of 0.2% arabinose. 25 nM of biotin was supplemented into the M9 liquid media. Bacterial growth was measured by optical density at 600 nm, which is automatically recorded using a BioScreen C instrument. Each growth curve assay was carried out in triplicate and the average is shown in this plot32.
Subsequently, BPL function of the BirA orthologues was further compared using in vitro assays to assess biotinylation activity. In this reaction, three versions of BirA were included (BirA_ec, BirA1_LL and BirA2_LL), and the domain of the AccB protein targeted for biotinylation by BPLs (designated as AccB87 or BCCP87) was used as the substrate (Fig. 6A). In principle, the conserved lysine at position 122 (K122) of AccB87 should be biotinylated upon the presence of a functional BPL (Fig. 1A). The conversion of α-32P-labeled ATP and biotin to the intermediate product, biotinoyl-AMP, by the BPLs (Fig. 1A) was directly visualized using thin layer chromatography (TLC) (Fig. 6B). The TLC approach also provided indirect proof of the second ligase partial reaction that occurs upon introduction of the acceptor protein AccB87 (i.e., transferring of biotin from biotinoyl-5′-AMP to the AccB87 lysine) resulting in loss of the biotinoyl-5′-AMP intermediate and formation of AMP (Figs 1A and 6B). The increased consumption of ATP in the presence of AccB87 (most notably for BirA-ec) was due to the fact that in absence of the acceptor protein, biotinoyl-AMP remains tightly bound within the ligase active site so that only one molecule of biotinoyl-AMP was formed per molecule of ligase (i.e., biotinoyl-AMP synthesis is not catalytic). Biotin transfer to an acceptor protein allows for catalysis and continued conversion of ATP to biotinoyl-AMP. These results further confirmed BPL activity.
Figure 6. Assessment of BPL activity for the two BirA homologues (BirA1_LL and BirA2_LL) using TLC.
(A) SDS-PAGE profile of the affinity-purified BPL proteins and the AccB87substrate (B). TLC-based analyses of BPL activity. Minus means no addition of either BPL protein or AccB87 substrate protein and plus denotes addition of AccB87. Products of the BPL reaction were separated on TLC and visualized using phosphor-imaging. Abbreviations: M, protein marker (Bio-Rad); ec, E. coli; LL, L. lactis; BPL, biotin protein ligase.
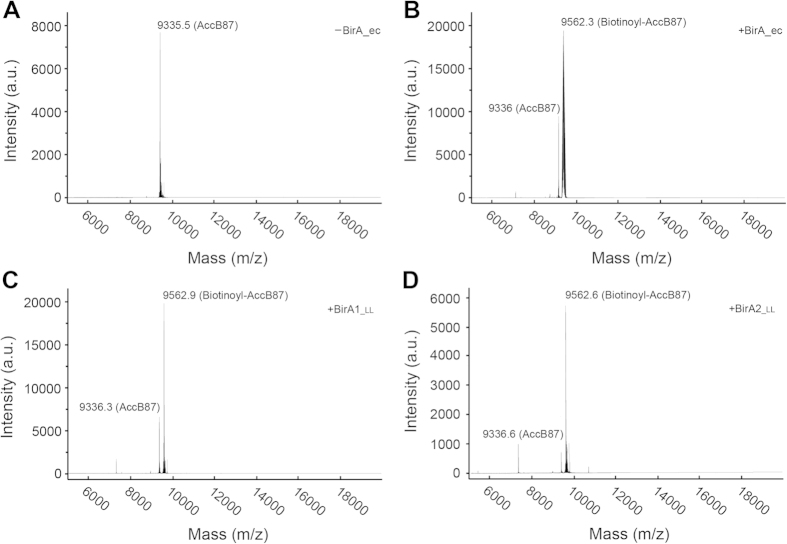
Our results demonstrate that both BirA proteins from L. lactis possess the ability to convert biotin and [α-32P]-ATP to the canonical biotinoyl-5′-AMP intermediate (Fig. 6B) and indirectly support transfer of the biotin moiety to the AccB87 acceptor protein (Fig. 6B). The most striking difference between BirA1_LL and BirA2_LL was that upon addition of the acceptor protein, the ratio of AMP to ATP in the BirA2_LL assay is appreciable higher than that seen in the BirA1_LL assay and BirA2_LL appears to form more of the biotinoyl-5′-AMP intermediate (Fig. 6B). This indicates that BirA2_LL catalyzes biotin attachment more quickly than BirA1_LL. The E. coli BirA consumed the majority of the α-32P-labeled ATP whereas no appreciable increase in ATP consumption was seen in the L. lactis BirA assays (Fig. 6B). This implies that the BirA_ec is the most active version amongst the three BPL enzymes under the conditions tested. Mass alteration of the AccB87 caused by biotinylation was directly measured using MALDI-TOF as previously described. In our assays, the mass for AccB87 was calculated to be 9335.5~9336.6 (Fig. 7A), and the mass for the biotinoyl-AccB87 was determined to be 9562.3~9562.9 (Fig. 7B–D). Collectively, the data illustrated that: 1) the two versions of L. lactis BirA (BirA1_LL and BirA2_LL) functioned as BPLs with weak activity; 2) BirA2_LL was more catalytically active relative to BirA1_LL.
Figure 7. MS-based identification of AccB87 biotinylation by L. lactis BirA1/BirA2.
(A) MALDI-TOF determination of the molecular weight of the un-biotinylated AccB87 polypeptide. The calculated mass for AccB87 is 9335.5~9336.6 and the expected mass for biotinoyl-AccB87 is 9562.3~9562.9. (B) The formation of the biotinylated AccB87 by E. coli BirA. (C) Biotinylation of AccB87 by L. lactis BirA1. (D) Biotinylation of AccB87 by L. lactis BirA2. Designations: minus denotes no addition of BirA enzyme, plus denotes addition of the BirA protein. Abbreviations: ec, E. coli; LL, L. lactis.
Binding of L. lactis BirA to the bioY genes
Relative to the scenario seen in the closely-relative cousin, S. suis, the genomic context of the bio loci is complex due to the presence of duplicated genes for birA and bioY (Fig. 8A). The putative BirA1 DNA binding site (ACA GTT AAC CTA AAT TTG ATT TTA GGG TTA CTG T) was detected in front of the bioY1 promoter region (Fig. 8B). According to the position of the transcriptional start site “T” predicted with the Neutral Network Program of Promoter Prediction (http://www.fruitfly.org/seq_tools/promoter.html), the BirA1-binding site appears to overlap the “−10” to “−35” promoter regions (Fig. 8B), suggesting the transcription of bioY1 might be negatively regulated by BirA1 in L. lactis.
Figure 8. Genetic organization of the birA and bioY loci and the L. lactis bioY1 promoter.
(A) Genetic organization of birA and bioY loci. The birA (referred to birA1) and bioY (referred to bioY1) loci are highlighted in blue and yellow, respectively. The red circle denotes the predicted BirA-binding site. The birA2 and bioY2 loci are indicated with silver and purple arrows. (B) The L. lactis bioY promoter “S” denotes the putative transcriptional start site and the red “ATG” is the translation initiation site. The letters “GGAG” in green refer to the ribosome binding site. The “−35” and “−10” regions are indicated in blue. The predicted BirA-binding site is underlined in black.
To test the function of the predicted BirA site (Figs 8A and 9A), electrophoresis mobility shift assays (EMSA) were conducted using the purified BirA1_LL and BirA2_LL (Fig. 9B–D). EMSAs confirmed that BirA1_LL effectively binds the bioY1 probe in a dose-dependent manner (Fig. 9B), whereas BirA2_LL, which lacks the putative N-terminal DNA binding motif, did not bind to the probe (Figs 2 and 9C). Interestingly, the S. suis bioY (bioY_SS) promoter has the ability to interact with the L. lactis BirA1_LL (Fig. 9D). A similar scenario was found in the case of S. suis BirA in that it also binds the L. lactis bioY promoter. Therefore, we anticipated that crosstalk would be present between the bioY and BirA of both S. suis and L. lactis. Given the fact that interaction occurs between the BirA1 and bioY1 promoter, while bioY2 lacks a predicted BirA1 binding site, it might be of interest to probe the physiological relevance of this regulation mechanism to biotin assimilation.
Figure 9. Binding of L. lactis BirA1 to the bioY promoter region.
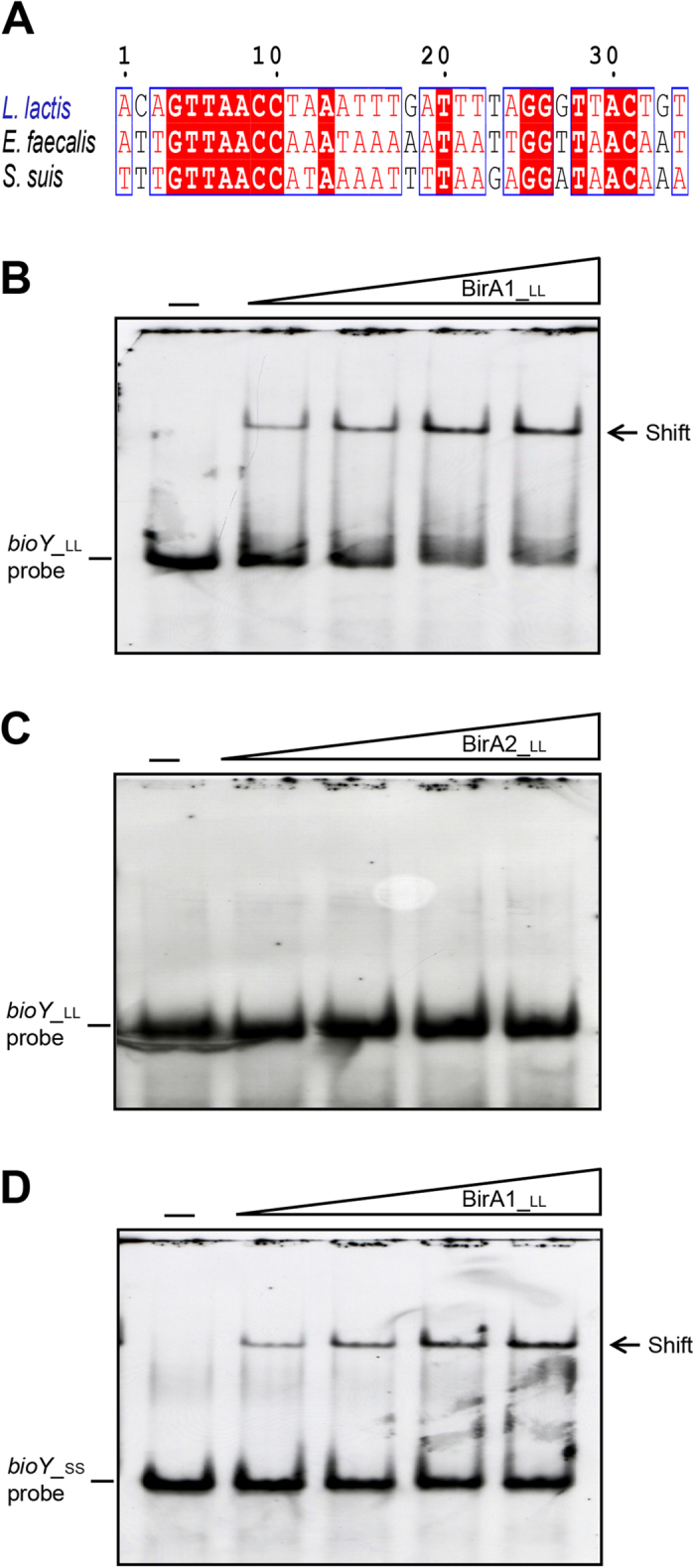
(A) Multiple sequence alignment of the predicted BirA-recognized palindromes localized in the bioY promoter regions Identical residues are in white letters with red background, similar residues are in red letters with white background, whereas non-conserved residues are in grey letters. Electrophoretic mobility shift assay (EMSA) of the binding of L. lactis BirA1 to the promoter regions of bioY from L. lactis (in Panel B) and the closely-related organism Streptococcus suis (in Panel D). (C) EMSA-based evidence that L. lactis BirA2 protein cannot bind to the promoter region of L. lactis bioY. The minus sign denotes no addition of either BirA1_LL or BirA2_LL protein, and the DIG-labeled probe shifted by BirA1_LL protein is indicated with an arrow. The concentrations of BirA1_LL (BirA2_LL) in the right four lanes of each panel were (from left to right) 3, 6, 9 and 12 pmol, respectively. The protein samples were incubated with 0.2 pmol of DIG-labeled probe in a total volume of 15 μl. To make biotinoyl-AMP, the ligand of BirA protein, 100 uM biotin plus 100 uM ATP was added into the gel shift system and incubated for ~1 hour. Designations LL and SS denote L. lactis and S. suis, respectively. BirA1_LL and BirA2_LL denote the two putative versions of L. lactis BirA protein, whereas bioY_LL and bioY_SS denote the bioY gene from L. lactis and S. suis, respectively. A representative graph from three independent gel shift assays is presented here. 7.5% native PAGE gels were used for the assays.
The ability of L. lactis to transport 3H-biotin
Structural modelling against a solved BioY structure8 suggested that both BioY1_LL (Fig. 10A) and BioY2_LL (Fig. 10B) consists of seven α-helices and exhibits appreciable similarity in their overall configuration. Given the fact that the L. lactis encoded two BioY transporters, we hypothesized that it would be capable of robust biotin transport. L. lactis cultures were grown in the presence of either limiting biotin or excess biotin (either 1 nM or 1 μM) for 4.5 hours, pelleted and washed with PBS to remove free biotin, and used for 3H-biotin transport assays (Fig. 10C,D). Relative transport of 3H-biotin by biotin depleted cultures (predicted to produce both BioY transporters) and biotin replete cultures (predicted to require single transporter) was similar over the intervals measured. Minor differences between the samples were only observed at the initial transport time point (Fig. 10D). In contrast, at one minute and later, uptake/accumulation of biotin is roughly equal (Fig. 10C). It seems likely that the increment of exogenous biotin tested did not significantly augment activity of biotin uptake.
Figure 10. Structural and functional analysis of the L. lactis IL1403 biotin transporters.
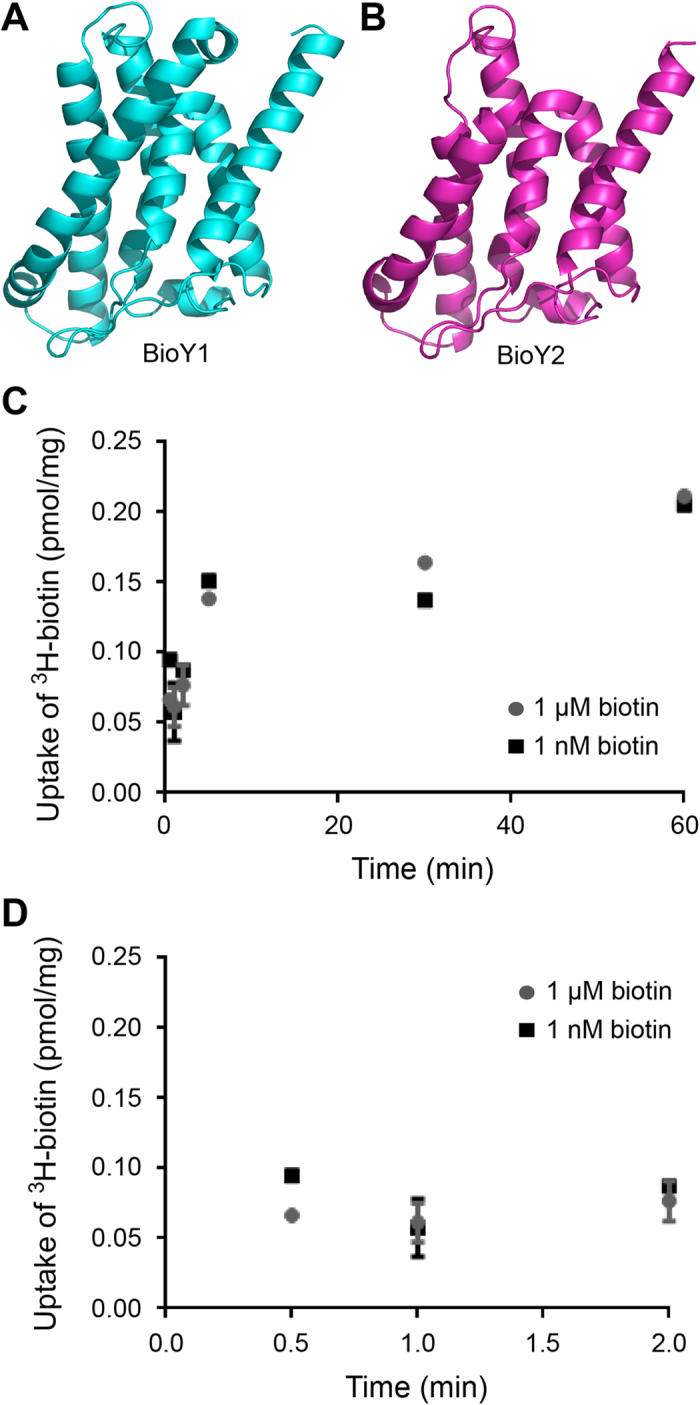
(A,B) Modelled structures of the BioY1 and BioY2 biotin transporter proteins of L. lactis IL1403. Using the S component structure (PDB: 4DVE) from a BioY ECF-type ABC transporter8 as the structural template, structural modeling of BioY1 and BioY2 was performed with the Swiss-Model program and the ribbon structure was produced with the software PyMol (C) Time trial analysis of transport of 3H-biotin by L. lactis grown under biotin replete (1 μM) or deplete (1 nM) conditions (D) Expanded curve of 3H-biotin transport by L. lactis at early time points. Bacteria were grown in the presence of either 1 nM or 1 μM biotin, pelleted and washed to remove external biotin, and then suspended in PBS plus 0.5% glucose for transport assays. For transport assays, samples were incubated with 0.5 μM 3H-biotin at 30 °C for 0.5, 1, 2, 5, 30, or 60 minutes. Reactions were halted via dilution in ice cold PBS and bacteria were collected on 0.45 μm filters. Filters were then mixed with scintillation fluid and DPM were counted using a Packard Scintillation counter. Average results for the time trials are shown in Panel C (time points less than 2 minutes are shown in Panel D).
Conclusions
The data reported here suggests a specific pathway for biotin utilization in the probiotic bacterium L. lactis (Fig. 1) differing with the closely related animal pathogen S. suis in the redundancy (and/or duplication events) of the birA and bioY loci (Fig. 8). L. lactis is anticipated to be a biotin auxotroph in that it lacks a full biotin biosynthesis pathway. Unlike the regulatory machinery for biotin uptake in S. suis in which BirA modulates the transcription of the bioY gene, L. lactis has two BioY transporters responsible for biotin uptake, and two BPL enzymes (BirA1_LL and BirA2_LL) catalyzing protein biotinylation. Among the two bioY genes (bioY1_LL and bioY 2_LL), it seems likely that transcription of bioY1_LL is regulated by BirA1_LL, whereas the expression of bioY2_LL is constitutive (Fig. 1). This unique mechanism might guarantee L. lactis possesses the ability to respond to fluctuating levels of biotin in the environment and/or the gastrointestinal tract.
To the best of our knowledge, regulation of bacterial biotin metabolism occurs by at least three diverse mechanisms represented by the E. coli BirA4, Agrobacterium BioR20, and Mycobacterium smegmatis BioQ21. The regulated transport of biotin is exemplified by BioR of Brucella22 plus Paracoccus12 and S. suis via BirA. The atypical occurrence of two distinct, functional biotin protein ligases in L. lactis adds to the breadth of unique examples for bacterial biotin utilization (Fig. 1). In the organism Francisella, we also noted the atypical presence of two different BirA orthologues16. However, we failed to detect any orthologue of the BioY transporter16, ruling out the possibility of regulated transport of biotin. Unlike the scenario with Francisella where the BirA bifunctional protein can cross-talk with the E. coli bio operon in vitro and in vivo, the L. lactis BirA1 cannot regulate expression of the E. coli bio operon (note: blue color is due to appreciable expression of the bio-lacZ fusion, Fig. 5). Of note, the L. lactis BirA can crosstalk with the S. suis bioY gene (Fig. 9D), and vice versa. Consistent with the scenario in E. coli, the accB gene (L0187) encoding the biotin-acceptor protein was detected in the genome of L. lactis (Table S1). The reduced BPL activities of the two individual BirA homologues from L. lactis compared to the BPL activity of the single E. coli BirA (Fig. 6) might argue that redundancy of BirALL is required to fulfill the need for protein biotinylation in L. lactis. The inability of L. lactis to synthesize biotin and the unique ecosystem of the gastrointestinal tract which is filled with a variety of nutritional elements and competing bacteria could select for the redundancy in biotin scavenging pathways or conversely, the gene organization might simply be a relic of bacterial evolution. Future work could explore the regulation of transporter production under different biotin conditions as well as differences in transporter efficiency.
Methods
Bacterial strains and growth conditions
The E. coli strains used included MG1655, BM4092 (Km mutant of birA)20, DH5α, and BL21 (DE3). The strains of L. lactis IL14039 and S. suis 215 were used for functional analyses. Luria Bertani (LB) and rich broth (RB) were used for the growth of E. coli and both Todd Hewitt Broth (THB) and minimal medium were used for the maintenance of L. lactis IL1403 and S. suis 223. When necessary, antibiotics were added as follows (in mg/liter): sodium ampicillin, 100; and kanamycin sulfate, 50.
Plasmids and genetic manipulations
The two birA genes (birA1 [L0191] and birA2 [L0192]) were amplified by PCR and cloned into the expression vector pET28(a) to create histidine-tagged proteins for affinity purification and the arabinose-inducible vector pBAD24 for gene complementation experiments as previously described24. The derivatives of pET28 were introduced into BL21 (DE3) for protein production, whereas the pBAD24 derivatives expressing birA were transformed into the Km mutant of birA (BM4092) for functional assay of birA25,26. All the acquired plasmids were validated by PCR and DNA sequencing.
Protein Purification
Expression, purification, and quantification of the truncated AccB (AccB87) apo acceptor protein was performed as previously described27. The recombinant BirA1_LL and BirA2_LL proteins were purified from 1-L LB cultures grown at 37 °C to an OD600 of 0.8 and protein production was initiated by the addition of 0.5 mM IPTG for 4 h at 30 °C. Cell lysis and protein purification were performed using the previously described protocols28. The purified proteins were dialyzed overnight in storage buffer containing 50 mM Tris-HCl [pH 8.0], 150 mM KCl, 10% glycerol and 0.1 mM DTT, concentrated using Millipore concentrators, flash frozen, and stored at −80 °C. The purity of all the protein samples was judged by separation on 12% SDS-PAGE gels and staining with Coomassie brilliant blue.
Liquid chromatography quadrupole time-of-flight mass spectrometry
A Waters Q-Tof API-US Quad-ToF mass spectrometer was used for the determination of the two L. lactis BirA orthologues (BirA1_LL & BirA2_LL). The protein band of interest was removed from the gel and digested with Trypsin (G-Biosciences St. Louis, MO). Finally, the resultant peptides were loaded on a Waters Atlantis C-18 column (0.03 mm particle, 0.075 mm × 150 mm) and the acquired data were subjected for further analyses by the ms/ms.
Bio-5′-AMP Synthesis Reactions
The assay for BirA-catalyzed in vitro protein biotinylation activity was performed as described previously28 with some modifications. Protein concentrations were determined using the extinction coefficients calculated from the protein sequence using the ExPASY Tools website. The assays contained 50 mM Tris-HCl (pH 8), 5 mM Tris-(2-carboxyethyl) phosphine, 5 mM MgCl2, 20 μM biotin, 5 μM ATP plus 16.5 nM [α-32P] ATP, 100 mM KCl and 2 μM BirA protein. Each of the reaction mixtures were incubated at 37 °C for 30 min. For each BirA protein tested, two identical tubes were used and at the end of the 30 min reaction AccB87 (50 μM) was added to one of each pair of tubes while the other tube was left untreated. The tubes were incubated for an additional 15 min at 37 °C. 1 μl of each reaction mixture was applied to a cellulose thin-layer chromatography plate of microcrystalline cellulose and the plates were developed in isobutyric acid-NH4OH-water (66:1:33)29. The thin-layer chromatograms were dried for 10 h, exposed to a phosphor-imaging plate and visualized using a Fujifilm FLA-3000 Phosphor Imager and Fujifilm Image Gauge software (version 3.4 for Mac OS).
Mass spectrometry
MALDI TOF/TOF mass spectrometer was used to measure the level of BirA-catalyzed biotinylation of AccB87. Reactions containing 100 μM AccB-87, 3 μM BirA, and 100 μM biotin, 1 mM ATP, 10 mM MgCl2, 100 mM KCl, 5 mM tris-(2-carboxyethyl) phosphine in 50 mM Tris-HCl, (pH 8), at 37 °C, were incubated for 16 hours. Prior to the low-resolution matrix-assisted laser desorption/ionization analyses, the mixtures were dialyzed in 25 mM ammonium acetate and lyophilized to dryness. Data processing was performed using the FlexAnalysis 3.3 software package (Bruker Daltonics). Spectra were smoothed and a baseline correction was applied using the built-in features of the software package
Electrophoretic mobility shift assays
Gel shift assays were performed to probe binding of BirA1_LL (and BirA2_LL) protein to the bioY promoters of L. lactis and S. suis 2 as previously described26,30,31. Two sets of DNA probes (bioY_LL and bioY_SS) were prepared by annealing two complementary oligonucleotides (bioY_LL-F: 5′-CAA ATA ATA AAA TTA ACA GTT AAC CTA AAT TTG ATT TTA GGG TTA CTG TTT GAT ATG-3′; bioY_LL-R: 5′-5′-CAT ATC AAA CAG TAA CCC TAA AAT CAA ATT TAG GTT AAC TGT TAA TTT TAT TAT TTG-3′). In the binding buffer (Roche), the purified BirA1_LL (and BirA2_LL) protein in a series of dilutions was mixed with the digoxigenin-labeled DNA probes (~0.2 pmol). If required, the biotinyl-5′-AMP ligand was added. The DNA/protein mixtures were separated on native 7% PAGE.
Bioinformatics analyses
Both orthologues of BirA and the BirA-binding sites were subjected to multiple sequence alignments using the program of ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html), and the final outputs were given with the program ESPript 2.2 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). The transcriptional start site was predicted using the Neutral Network Promoter Prediction server (http://www.fruitfly.org/seq_tools/promoter.html). Structural modelling was performed with the CPHmodels 3.2 Server (http://www.cbs.dtu.dk/services/CPHmodels) using appropriate structural templates: BirA_ec (PDB: 1HXD) for BirA1_LL; BirA of Aquifex aeolicus (PDB: 3EFS) for BirA2_LL; and the S component of ECF-type ABC transporter (PDB: 4DVE) is for two BioY orthologues of L. lactis.
Transport of 3H-biotin
L. lactis was grown overnight in THB. A one ml sample was pelleted, washed three times in PBS, and used to inoculate minimal medium supplemented with either 1 nM or 1 μM of biotin. Samples were grown for 4.5 h, pelleted, and washed three times with PBS to remove external biotin. Pellets were then suspended in PBS plus 0.5% glucose for transport assays6 and quantification of total protein in each sample using the Bio-Rad Protein assay. For transport assays, samples were incubated with 0.5 μM 3H-biotin at 30 °C for 0.5, 1, 2, 5, 30, or 60 minutes. Reactions were halted via dilution in ice cold PBS and bacteria were collected on 0.45 μm filters. Filters were then mixed with scintillation fluid and DPM were counted using a Packard Scintillation counter.
Additional Information
How to cite this article: Zhang, H. et al. Deciphering a unique biotin scavenging pathway with redundant genes in the probiotic bacterium Lactococcus lactis. Sci. Rep. 6, 25680; doi: 10.1038/srep25680 (2016).
Supplementary Material
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars (Grant No. LR15H190001), the National Natural Science Foundation of China (Grant No. 31570027), and the start-up package from Zhejiang University (Y.F.). Dr. Feng is a recipient of the “Young 1000 Talents” Award. We would like to thank Prof. John E. Cronan for critical reading.
Footnotes
Author Contributions Y.F. designed this project; Y.F., H.Z., Q.W., D.J.F., M.C., V.C., H.Y., P.L. and J.O.S. performed experiments and analyzed the data; Y.F. and D.J.F. contributed the reagents and tools; Y.F., D.J.F. and V.C. wrote this manuscript.
References
- Beckett D. Biotin sensing: universal influence of biotin status on transcription. Annu Rev Genet 41, 443–64 (2007). [DOI] [PubMed] [Google Scholar]
- Hebbeln P., Rodionov D. A., Alfandega A. & Eitinger T. Biotin uptake in prokaryotes by solute transporters with an optional ATP-binding cassette-containing module. Proc Natl Acad Sci USA 104, 2909–14 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen-Navarro K., Encarnacion S. & Dunn M. F. Biotin biosynthesis, transport and utilization in Rhizobia. FEMS Microbiol Lett 246, 159–65 (2005). [DOI] [PubMed] [Google Scholar]
- Rodionov D. A., Mironov A. A. & Gelfand M. S. Conservation of the biotin regulon and the BirA regulatory signal in Eubacteria and Archaea. Genome Res 12, 1507–16 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkens G. B. et al. The structural basis of modularity in ECF-type ABC transporters. Nat Struct Mol Biol 18, 755–60 (2011). [DOI] [PubMed] [Google Scholar]
- Fisher D. J., Fernandez R. E., Adams N. E. & Maurelli A. T. Uptake of biotin by Chlamydia Spp. through the use of a bacterial transporter (BioY) and a host-cell transporter (SMVT). PLoS One 7, e46052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenwirth F., Kirsch F. & Eitinger T. A versatile Escherichia coli strain for identification of biotin transporters and for biotin quantification. Bioengineered 5, 129–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsson R. P. et al. Structural divergence of paralogous S components from ECF-type ABC transporters. Proc Natl Acad Sci USA 109, 13990–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin A. et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11, 731–53 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalta E. & Montel M. C. Safety assessment of dairy microorganisms: the Lactococcus genus. Int J Food Microbiol 126, 271–3 (2008). [DOI] [PubMed] [Google Scholar]
- Perdigon G., Fuller R. & Raya R. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol 2, 27–42 (2001). [PubMed] [Google Scholar]
- Feng Y., Kumar R., Ravcheev D. A. & Zhang H. Paracoccus denitrificans possesses two BioR homologs having a role in regulation of biotin metabolism. Microbiology open 4, 644–59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann H., Starrenburg M. J., Molenaar D., Kleerebezem M. & van Hylckama Vlieg J. E. Microbial domestication signatures of Lactococcus lactis can be reproduced by experimental evolution. Genome Res 22, 115–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M. et al. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc Natl Acad Sci USA 103, 9274–9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. et al. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5, 477–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. et al. The atypical occurrence of two Biotin protein ligases in Francisella novicida is due to distinct roles in virulence and biotin metabolism. MBio 6, e00591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. & Cronan J. E. Only one of the two annotated Lactococcus lactis fabG genes encodes a functional beta-ketoacyl-acyl carrier protein reductase. Biochemistry 43, 11782–9 (2004). [DOI] [PubMed] [Google Scholar]
- Weaver L. H., Kwon K., Beckett D. & Matthews B. W. Corepressor-induced organization and assembly of the biotin repressor: a model for allosteric activation of a transcriptional regulator. Proc Natl Acad Sci USA 98, 6045–50 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. J., Coulson J., Baillie R. & Campopiano D. J. Biotinylation in the hyperthermophile Aquifex aeolicus. Eur J Biochem 270, 1277–87 (2003). [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhang H. & Cronan J. E. Profligate biotin synthesis in α-Proteobacteria - A develoing or degenerating regulatory system? Mol Microbiol 88, 77–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q. et al. Mycobacterium smegmatis BioQ defines a new regulatory network for biotin metabolism. Mol Microbiol 94, 1006–1023 (2014). [DOI] [PubMed] [Google Scholar]
- Feng Y., Xu J., Zhang H., Chen Z. & Srinivas S. Brucella BioR regulator defines a complex regulatory mechanism for bacterial biotin metabolism. J Bacteriol 195, 3451–67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. Two novel regulators of N-acetyl-galactosamine utilization pathway and distinct roles in bacterial infections. Microbiology open 4, 983–1000 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. F. & Campbell A. M. Use of bio-lac fusion strains to study regulation of biotin biosynthesis in Escherichia coli. J Bacteriol 143, 789–800 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J. & Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177, 4121–30 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. & Cronan J. E. Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters. Mol Microbiol 80, 195–218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravartty V. & Cronan J. E. Altered regulation of Escherichia coli biotin biosynthesis in birA superrepressor mutant strains. J Bacteriol 194, 1113–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravartty V. & Cronan J. E. The wing of a winged helix-turn-helix transcription factor organizes the active site of BirA, a bifunctional repressor/ligase. J Biol Chem 288, 36029–39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O. & Eisenberg M. A. Biotinyl 5′-adenylate: corepressor role in the regulation of the biotin genes of Escherichia coli K-12. Proc Natl Acad Sci USA 76, 5592–5 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. & Cronan J. E. A new member of the Escherichia coli fad regulon: transcriptional regulation of fadM (ybaW). J Bacteriol 191, 6320–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. & Cronan J. E. Overlapping repressor binding sites result in additive regulation of Escherichia coli FadH by FadR and ArcA. J Bacteriol 192, 4289–99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. & Cronan J. E. The Vibrio cholerae fatty acid regulatory protein, FadR, represses transcription of plsB, the gene encoding the first enzyme of membrane phospholipid biosynthesis. Mol Microbiol 81, 1020–33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.