SUMMARY
Dientamoeba fragilis is a protozoan parasite of the human bowel, commonly reported throughout the world in association with gastrointestinal symptoms. Despite its initial discovery over 100 years ago, arguably, we know less about this peculiar organism than any other pathogenic or potentially pathogenic protozoan that infects humans. The details of its life cycle and mode of transmission are not completely known, and its potential as a human pathogen is debated within the scientific community. Recently, several major advances have been made with respect to this organism's life cycle and molecular biology. While many questions remain unanswered, these and other recent advances have given rise to some intriguing new leads, which will pave the way for future research. This review encompasses a large body of knowledge generated on various aspects of D. fragilis over the last century, together with an update on the most recent developments. This includes an update on the latest diagnostic techniques and treatments, the clinical aspects of dientamoebiasis, the development of an animal model, the description of a D. fragilis cyst stage, and the sequencing of the first D. fragilis transcriptome.
INTRODUCTION
Dientamoeba fragilis is a single-celled protozoan parasite largely ignored by medicine as a cause of human gastrointestinal (GI) disease and is often described as a “neglected parasite.” Despite regular, continuous reports emerging over the last 100 years that describe an association between D. fragilis and human GI disorders, including diarrhea, it is still often ignored as a pathogen, and routine testing may not be routinely conducted by diagnostic laboratories. Indeed, knowledge on the basic biology of this species is scant at best. As a result, we remain ignorant of this parasite's host distribution, its life cycle, and many other aspects of its biology. However, recent developments have been made in the field of D. fragilis research, which are changing this profile. The design and development of new, modern diagnostic tests for D. fragilis have been associated with a major increase in the rate of detection of this parasite in cases of GI disease. This in turn has resulted in a reassessment of historical knowledge on D. fragilis, particularly its potential role as a human pathogen. This review focuses on D. fragilis as a cause of human disease and on its diagnosis and treatment and provides an update on our current understanding of its biology and life cycle.
HISTORICAL ASPECTS
The distinguished English protozoologist Charles Wenyon is credited with the discovery of D. fragilis in 1909 after examining his own parasitological stool preparations. However, it was not until 1918 that Margaret Jepps and Clifford Dobell described the parasite in the scientific literature (1). As this protozoan was very different from the amoebae that were known to occur in the human bowel at that time, they reported that “it differs in some respects so conspicuously from the others that it appears to us necessary to place it not only in a new species, but even in a new genus” (1).
Jepps and Dobell (1) described D. fragilis as a binucleate amoeba between 8 and 10 μm in diameter. Due to the fragile nature of the organism, in the form of rapid morphological degeneration once passed outside the human body, the name D. fragilis was given to this new parasite. While D. fragilis was considered to be an amoeboid organism, it was not long until Dobell challenged the validity of this nomenclature. Through many experiments, he concluded that the nuclear apparatus of D. fragilis was flagellate-like and that its method of nuclear division was not characteristic of an amoeba but was more like that of a flagellate (2). He postulated that D. fragilis was a flagellate and undertook several experiments to induce the organism to express a flagellum, all of which were unsuccessful. Despite these initial findings, Dobell documented the similarities between D. fragilis and the amoeboflagellate Histomonas meleagridis (a pathogen of poultry and birds) (3). Dobell subsequently concluded that Dientamoeba was a flagellate, which somewhere along its evolutionary development had permanently lost its flagella. Dobell's hypothesis that Dientamoeba was indeed an “unflagellated” flagellate was shown to be correct, as later researchers verified the close relationship between D. fragilis and the other flagellates, especially H. meleagridis (4–7). His assumptions at the time were not entirely correct, as it seems that D. fragilis has not permanently lost its flagella, just the ability to express them externally, as flagellum-like structures have been visualized via transmission electron microscopy (8).
Both Dobell and Jepps initially thought that D. fragilis was nonpathogenic in spite of noting six of seven patients who suffered from dysentery or chronic diarrhea and finding only one sample from asymptomatic patients after screening numerous healthy persons (1). It was not long until other researchers started to question the pathogenicity of D. fragilis. In 1919, a year after D. fragilis was first described in the literature, Kofoid et al. reported D. fragilis in military officers from the United States who suffered from bowel complaints (9). The following year, another study found D. fragilis in three symptomatic children in the Philippines (10), and later, D. fragilis was implicated as a cause of diarrhea in an adult male from England (11). Thus, in the space of 4 years following the discovery of D. fragilis, controversy surrounding its pathogenicity arose and persists to this day.
TAXONOMY
Jepps and Dobell were the first to not only describe D. fragilis in the literature but also to assign it a taxonomic position (1). In 1918, three Entamoeba species were known to occur in the human bowel: the nonpathogenic species Entamoeba coli and Entamoeba nana (now known as Endolimax nana) and the pathogenic species Entamoeba histolytica. While this new protozoan was placed in the family Entamoebidae, Jepps and Dobell (1) argued that as it had a binucleated form and no cyst stage, it not only was a new species but also warranted the formation of a new genus. They demonstrated that once outside the human body, this organism became “fragile” and degenerated rapidly. Subsequently, the name Dientamoeba fragilis was given and remains in use today.
Dobell would continue his research on this organism for many years to come. Using only microscopy and culture techniques, Dobell came to recognize the close structural similarities between D. fragilis and H. meleagridis, in particular the dividing stages of these two organisms (2). Notably, Dobell observed that the nuclei, chromosomes, and centrodesmus were similar between the two organisms. He also recognized the differences between other amoebae and D. fragilis, such as the predominately binucleate form of trophozoites; the distinct nuclear structure; the extranuclear spindle, which is present in dividing organisms; the apparent absence of cysts from the life cycle; and similarities with other flagellates. Having collected this information, Dobell postulated that D. fragilis was a flagellate that had somehow lost its flagella permanently. Wenrich also documented the similarities between D. fragilis and H. meleagridis and found that both organisms shared many flagellate characteristics (12). On the basis of the above-mentioned scientific data and because D. fragilis was significantly different from other amoebae, in 1953, D. fragilis was reclassified and placed into the family Dientamoebidae along with Histomonas (13).
The advent of electron microscopy enabled studies that would substantiate the hypothesis that D. fragilis was indeed closely related to the flagellates described by Dobell and Wenrich. Bird et al. (14) reported a series of electron micrographs that illustrated the fine structure of uni- and binucleate trophozoites of D. fragilis. The demonstration of a persistent internuclear spindle of microtubules in the binucleate stage supported Dobell's assumptions. Also, the well-developed parabasal filament in both uninucleated and binucleated trophozoites of D. fragilis substantiated its close affinity with H. meleagridis.
Dwyer used gel diffusion and quantitative fluorescent-antibody methods to analyze the antigenic relationships among Dientamoeba, Histomonas, Trichomonas, and Entamoeba (15–17). These results demonstrated that Dientamoeba, Histomonas, and Trichomonas shared many closely related antigens with each other and far fewer with Entamoeba. Two years later, immunoelectrophoresis techniques were employed to analyze the antigenic relationships among these same species, and the close antigenic relationship among Dientamoeba, Histomonas, and Trichomonas was confirmed once more (18). It was also evident that Dientamoeba shared an antigenic basis with Histomonas while being distinct antigenically from Entamoeba histolytica and Entamoeba invadens (18).
In 1974, by using electron microscopy, a further taxonomic revision of Dientamoeba occurred. Several similarities between Dientamoeba and Histomonas were noted, especially with regard to the parabasal apparatus. It was also evident from the electron micrographs that there were many structural differences between Dientamoeba and Entamoeba. On the basis of these findings, D. fragilis was placed in the order Trichomonadida and the family Monocercomonadidae, subfamily Dientamoebidae (19). In 1980, Levine et al. reclassified Dientamoeba in the order Trichomonadida (20).
Molecular techniques were first used in 1996 to determine the taxonomic position of Dientamoeba. Molecular phylogenies were constructed based on the complete small-subunit (SSU) rRNA sequences of D. fragilis (21). The D. fragilis SSU rRNA gene was shown to have a G+C content that was low compared to those of other trichomonads and to contain ∼100 extra nucleotides. All phylogenic constructions showed that D. fragilis was closely related to trichomonads (21). Additional molecular studies using the SSU rRNA gene loci were unable to resolve the phylogenetic position of D. fragilis in comparison to other parabasalids (22). When analysis of the SSU rRNA gene of H. meleagridis was undertaken (6), the sequence data also showed a reduced G+C content and an increased sequence length, similar to what was observed for D. fragilis.
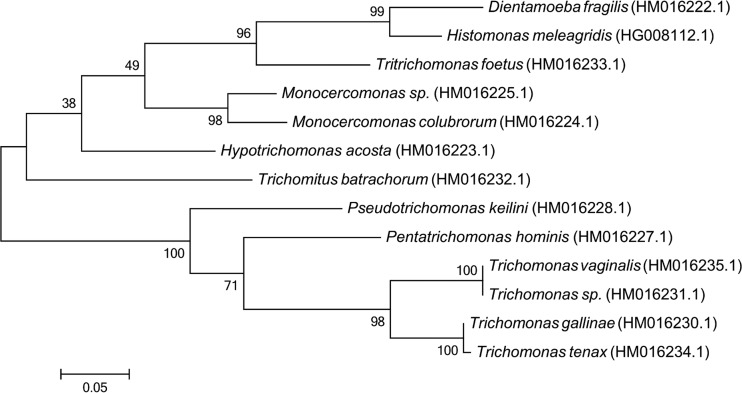
Phylogenetic studies confirmed a close relationship between D. fragilis and H. meleagridis and indicated that both organisms share a recent common ancestor, which probably exhibited a more complex cytoskeletal structure. This idea was supported by the phylogenetic studies of Gerbod et al. (6), who suggested that the morphology of both species probably arose through a secondary loss or reduction of some cytoskeletal structures. Recent phylogenetic studies of the Parabasalia using protein sequences have also confirmed the close relationship of these two organisms (as sister groups) (23, 24) (Fig. 1).
FIG 1.
Molecular phylogenetic analysis of RNA polymerase II largest-subunit protein sequences from various trichomonads, including D. fragilis. The GenBank accession number for each sequence is shown in parentheses. The evolutionary history was inferred by using the maximum likelihood method based on the Jones, Taylor, Thornton (JTT) matrix-based model (199). The percentage of trees in which the associated taxon cluster together is shown next to the branches. Evolutionary analyses were conducted with MEGA6 (200). The robustness of the tree was assessed by using the bootstrap method with 1,000 replicates.
A major critical taxonomic revision of the Parabasalia (25) is yet to be completely acknowledged. A recent revised classification scheme for D. fragilis (Table 1) is based on the above-mentioned reports and indicates that D. fragilis belongs to the Dientamoebidae family of the newly revised class Tritrichomonadidae and the order Tritrichomonadida of the Parabasalia (25), which contains the previously recognized families Tritrichomonadidae, Monocercomonadidae, and Simplicimonadidae. The new Dientamoebidae family contains four genera, Dientamoeba, Histomonas, Parahistomonas, and Protrichomonas, based on criteria such as the number of nuclei, presence of flagella, and absence of a costa. Recent electron microscopy studies of cultured trophozoites (7) and cysts produced by rodents (8) have enhanced our knowledge in this area, particularly in the recognition of flagellum-like structures in the cyst. Cysts possess a well-developed cyst wall that encloses an amoeboid-shaped cell, and the cyst diameter is typically within the range of 4 to 6 μm. The cysts contain one or two nuclei, and the nuclear membrane is often not visible, suggesting that karyogamy may be occurring during cyst development. Hydrogenosomes and a basal body structure are present in D. fragilis cysts, which also possess an axostyle, flagellar axonemes, pelta, and a costa (8). In contrast, the pelta and flagella are absent in trophozoites (7). The presence of a costa is not associated with an undulating membrane in either the cyst or the trophozoite. The trophozoites move by the crawling action associated with cytoplasmic streaming of pseudopodia.
TABLE 1.
Modern classification of D. fragilis
| Taxonomic rank | Classification |
|---|---|
| Kingdom | Excavata |
| Subkingdom | Metamonada |
| Phylum | Parabasalia |
| Class | Tritrichomonadidae |
| Order | Trichomonadida |
| Family | Dientamoebidae |
| Genus | Dientamoeba |
| Species | Dientamoeba fragilis (Jepps and Dobell, 1918) |
GENETIC DIVERSITY
Based on current literature, there are two major D. fragilis genotypes, genotype 1 and genotype 2 (also known as the Bi/PA strain), with genotype 1 being the most common subtype (26–28). Originally, the distinction between these genotypes was made based on differences between their 18S rRNA sequences (29–31), although these differences are minor (2 to 4%) (30, 32). In a recent study (32), the actin and elongation factor 1α genes for both D. fragilis genotypes were compared, and it was found that the differences in these genes were also very minimal (∼3%), suggesting that the two genotypes diverged recently. As such, genetic diversity appears to be limited in D. fragilis, although very few genes and isolates have been examined so far.
Several reports indicate that diversity may exist (or does exist) in genes other than those studied previously. Barratt et al. (33) describe phenotypic differences between the in vitro growth characteristics of different clinical D. fragilis isolates and suggested that they might have a genetic basis. All D. fragilis isolates included in the study by Barratt et al. (33) were confirmed to be genotype 1 isolates, so any potential genetic diversity must exist in other genes. Windsor et al. (34) identified the multicopy internal transcribed spacer (ITS) genes as a source of genetic diversity within individual D. fragilis isolates, which is unusual among protozoa. This observation eventually led to the development of a C-profiling technique, which could be used to distinguish between isolates (35, 36). In another report, Hussein et al. (37) detected diversity in the 18S RNA genes of isolates derived from patients with irritable bowel syndrome (IBS) by using PCR with high-resolution melt curve analysis. While those authors noted differences between isolates, they did not include samples from asymptomatic individuals (37). As such, it is unknown whether this technique might be useful for differentiating between virulent and avirulent strains of D. fragilis.
Dientamoeba fragilis strains that infect animals may also be distinct from human strains. According to Caccio et al. (38), two populations of D. fragilis were identified in a population of swine based on 8 single nucleotide polymorphisms (SNPs) observed following sequencing of PCR products amplified from the SSU rRNA gene. Given this small number of differences, however, these parasites were still considered to belong to the D. fragilis genotype 1 SSU subtype. Other investigators also demonstrated that there is very little diversity in the SSU rRNA genes of D. fragilis isolates (32). Subsequently, the search for diversity should include examination of other genes. This is in line with current theories of molecular evolution that link times of evolutionary divergence with the number of mutations found in coding and noncoding regions of the genome.
Many enteric protozoa exhibit extensive genetic diversity in genes other than the rRNA genes in the absence of morphological variation. Indeed, protozoa that were originally thought to be one species have subsequently been found to comprise two or more new species. Entamoeba histolytica and Entamoeba dispar represent one such example. These organisms are morphologically identical and exhibit remarkable similarity at their SSU rRNA gene loci (∼97%). In contrast, significant genetic differences have been identified at other loci, enough to justify their separation into distinct species (39, 40). This is yet to be established for D. fragilis but may have important clinical and epidemiological implications. The identification of D. fragilis in individuals without clinical disease raises the question of whether multiple lineages of D. fragilis exist, some of which may not be associated with disease. Asymptomatic carriage of pathogenic E. histolytica was known for many years, and it was not until 1993 that E. dispar was identified as a separate, nonpathogenic species (40). Since no population-style studies have yet been conducted on D. fragilis at this time, it remains to be seen whether D. fragilis exists as a species complex.
Over 6,000 novel nucleotide sequences were made available with the publication of the D. fragilis trophozoite transcriptome. These data will facilitate the selection of novel targets for exploring the genetic diversity of D. fragilis (41). The transcriptome was sequenced from a cultured isolate of D. fragilis SSU genotype 1, originally obtained from a patient with GI symptoms from Sydney, Australia (33). The transcriptome is described as sharing many features present in the genome and transcriptomes of Trichomonas vaginalis (41–43). More than one-third of D. fragilis contigs received hits to T. vaginalis proteins with E values of <1.00E−49, reflecting the close relationship between these organisms in spite of their morphological differences. Trophozoites of D. fragilis were also described as being metabolically similar to those of T. vaginalis (41). Like T. vaginalis, D. fragilis has also undergone a massive expansion in its repertoire of BspA-like leucine-rich repeats (44) and actin family genes (43), among several others (41).
Several transcripts possessing homology to cytotoxic cysteine proteases of T. vaginalis were identified in the transcriptome and discussed in reference to the pathogenic potential of D. fragilis (41, 45). As the pathogenic nature of D. fragilis has been debated since its discovery (26), the identification of potential virulence factors for future investigation is applicable to this debate. It has been argued that differences in clinical outcomes reported for D. fragilis infection may be a reflection of genetic diversity between D. fragilis populations (32). It is suggested that as a first port of call, examination of genes encoding cysteine proteases and other potential virulence factors would be of great value, as diversity in these genes might explain differences in clinical outcomes (26, 32). This is not unprecedented, as differences in cysteine protease genes between pathogenic and nonpathogenic Entamoeba spp. have been noted and have been implicated as one factor among many that may account for differences in virulence (39). Furthermore, genetic diversity in cysteine protease genes between isolates of Tritrichomonas foetus infecting disparate host species (cattle and cats) has been reported (46). Consequently, the cysteine protease genes might represent a useful target for the identification of genetic diversity among D. fragilis isolates in future studies.
Before the completion of the D. fragilis transcriptome, only five protein-encoding gene sequences and 62 nucleotide sequences were available in public databases, with the vast majority being derived from the rRNA genes. These rRNA gene sequences have been studied closely and are the targets for the majority of PCR and real-time PCR (RT-PCR) tests that are currently available for D. fragilis (30, 31, 47–51). The availability of the D. fragilis transcriptome means that >6,000 novel sequences are available for public reference (41). These data will lay the foundations for future molecular studies on D. fragilis, including the search for genetic diversity.
MORPHOLOGICAL CHARACTERISTICS
Trophozoites
D. fragilis possesses a pleomorphic trophozoite stage (Fig. 2), ranging in size from 4 μm to 20 μm, with most in the range of 5 μm to 15 μm (12, 14, 52, 53). Larger forms with sizes upwards of 20 to 40 μm are sometimes seen in culture (54). Dientamoeba trophozoites are typically binucleate, with up to 20% of forms being uninucleate (2), although this percentage can vary considerably (53). Nuclear pleomorphism is quite common, with the nucleus size varying in relation to the rest of the cell (55). In permanently stained smears, peripheral chromatin is absent, and the nuclear membrane is delicate. The karyosome is fragmented and contains chromatin granules often appearing as chromatin packets (19). The D. fragilis cytoplasm is often finely granular and often contains vacuoles and food inclusions along with ingested microorganisms (12).
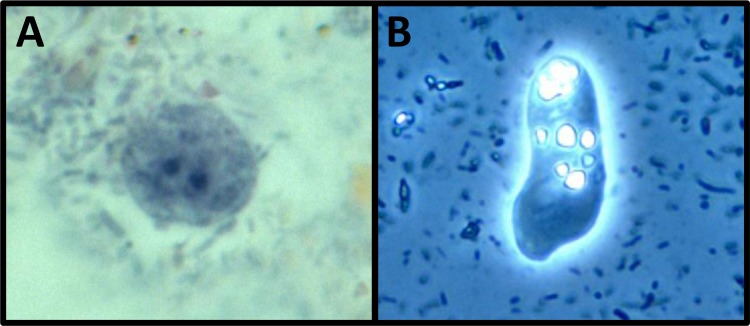
FIG 2.
Pleomorphic trophozoites of D. fragilis. (A) Binucleate trophozoite of D. fragilis (stained with a modified iron-hematoxylin stain) (magnification, ×1,000); (B) live D. fragilis trophozoite displaying motility (viewed under phase-contrast microscopy) (magnification, ×400).
Motility demonstrating characteristic fan-shaped pseudopodia with irregular lobes and indentations may be seen in freshly passed specimens or culture media (56, 57) (see File S1 in the supplemental material). This motility is temperature dependent, and trophozoites become less motile with cooler temperatures (2, 58).
Scanning electron microscopy of D. fragilis trophozoites derived from xenic culture systems showed two main types of cell populations based on the structure of cell surfaces: ruffled and smooth cell types (Fig. 3) (7). Ruffled cells accounted for up to 90% of the cell population; however, time interval experiments showed an increase in the number of smooth cells at 72 h. There was no statistically significant difference in size between the two morphological forms. While ruffled cells are observed in other trichomonads, it is unknown what significance this change in trophozoite surface structure represents (59). While the smooth cell types observed were initially postulated to represent a pseudocyst-like stage, experiments to induce pseudocyst formation using adverse environmental growth conditions failed (7). However, as D. fragilis does not possess undulating membranes or external flagella like other trichomonads, investigation of the formation of pseudocysts is difficult.
FIG 3.
Different cell surface structures observed in D. fragilis trophozoites grown in a xenic culture system. (A and C) Smooth (A) and ruffled (C) cell structures observed by scanning electron microscopy. Ruffled cells accounted for up to 90% of the cell population, although time interval experiments showed an increase in the number of smooth cells at 72 h. (B) Cells with a slightly textured surface are also observed and may represent an intermediate between the smooth and ruffled forms.
Using scanning electron microscopy on cultured D. fragilis trophozoites, Banik et al. (7) also described for the first time an endogenous D. fragilis virus. This is not unprecedented, as many protozoa are hosts of their own endogenous viruses, including Trichomonas vaginalis, which hosts a double-stranded RNA virus (60). The significance of this is currently unknown, although studies on T. vaginalis suggest that its presence or absence in T. vaginalis isolates may influence virulence (61).
Precysts
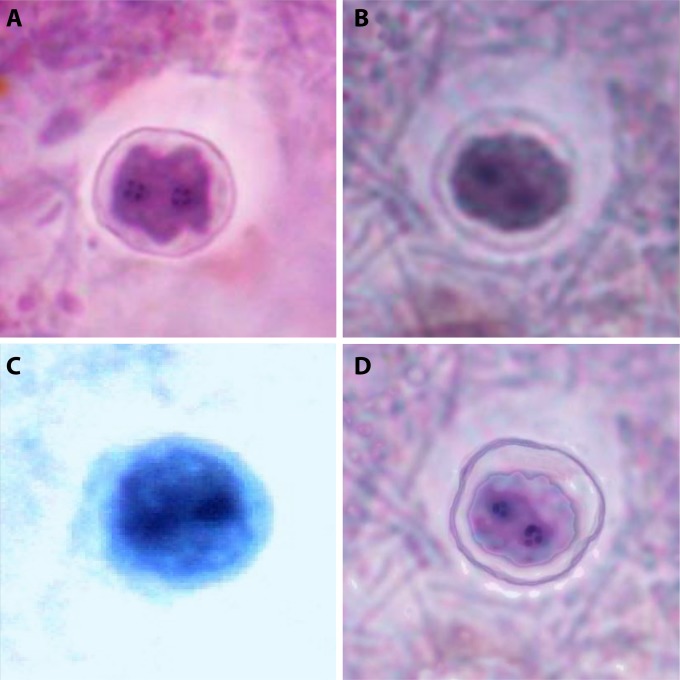
Putative precystic forms of D. fragilis were described recently by Stark et al. (62). However, a thorough examination of historical publications regarding this parasite indicates that precystic forms were described in the literature several times previously. At the time, these reports were dismissed as a misrepresentation of degenerative individuals (2). The initial description of D. fragilis in 1918 indicated that “extremely minute forms (3.5 μm) are not infrequent” (1). Dobell subsequently described forms that he referred to as “dwarfs, with diameters of only 3 to 4 μm [that were] also found in cultures. They appear to be formed by rapid division, without intermediate growth, of normal individuals” (2). In 1923, precystic forms were described in North America (63), and in 1926, Kudo described precystic forms as “small spherical amoeba without food particles” that were ~4 µm in diameter (64). Wenrich conducted a study on the morphological characteristics of Dientamoeba and described what he believed was a precystic form. Like other researchers, Wenrich described these precystic forms as small, ranging from 3.5 to 5 μm in diameter, including both uninucleate and binucleate forms with a finely granular and uniform cytoplasm that exhibits intense staining (65). These forms, described many years before, were morphologically identical to those described by Stark et al. (62) (Fig. 4).
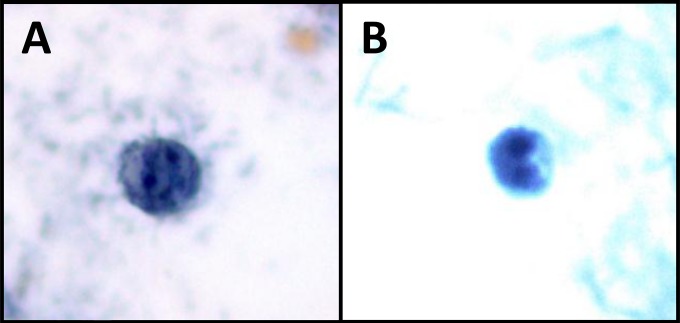
FIG 4.
Precystic forms from human clinical stool samples (stained with a modified iron-hematoxylin stain) (magnification, ×1,000). Precystic forms are typically smaller (3.5 to 5 μm in diameter) and stain more densely than trophozoites.
Several researchers dismissed these findings (2, 12), with Dobell being the most vocal opponent. He stated that it was “inconceivable that D. fragilis would have a cyst stage,” while “Histomonas—its closest relative also has no cysts” (2). Recently, cyst-like stages of H. meleagridis have been reported (66, 67). These forms are similar to those described by Stark et al. (62) for D. fragilis: completely spherical compact structures with a size of 4 μm. These cyst-like forms were thought to represent the initial stages of true cyst formation that can withstand harsh environmental conditions (67, 68), although the infectivity of these structures is yet to be confirmed.
Cysts
Despite recent reports that cyst forms of D. fragilis have never been reported in humans, it is now understood that this is incorrect (69). Jepps and Dobell were the first to postulate that D. fragilis may have a cyst stage that occurred in an animal host and that humans were an accidental host in which cyst formation did not occur (1). However, after studying this organism for 20 years, Dobell concluded that D. fragilis did not produce cysts (2). In 1923, the American protozoologist Charles Kofoid described a cyst stage of Dientamoeba (63). Then, in 1928, a South American researcher also described cyst forms in Argentina (70). Then, 20 years later, cyst forms in several patients from Germany were described (71). In contrast, many researchers have not reported this stage in the life cycle of Dientamoeba (72), including Dobell, who was unable to detect cyst forms from patient samples. He searched one individual 42 times over a 10-month period. Another case was monitored for 5 weeks, and stool samples were examined a total of 12 times, to no avail (1).
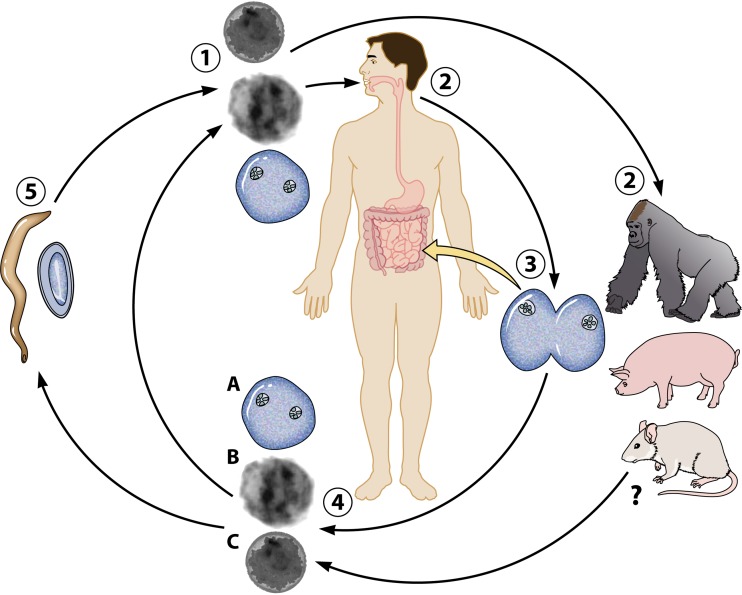
The successful establishment of a rodent model to study D. fragilis infection and transmission underpinned the characterization of the D. fragilis cyst stage. Following oral inoculation of mice with cultured trophozoites, the D. fragilis cyst forms were identified in the feces of infected animals (Fig. 5) (8). It was not until 2014 that D. fragilis cysts from human specimens were reported in the literature again. This recent study confirmed the detection of cysts in human clinical samples in two separate laboratories from different global locations (the United States and Australia) (62). These cysts are morphologically similar to the cysts reported in rodents (Fig. 6) (62). Due to the scant number of cysts encountered in this cohort, only light microscopy was used in this study, and unfortunately, electron microscopy could not be undertaken for definitive confirmation of the cyst forms. Further definitive studies utilizing in situ hybridization are required to allow correlation between the human and animal structures. It should be noted that D. fragilis cyst forms were incredibly rare in these human samples. A total of 547 slides were examined, which were collected over a 2-year period, and only a few cysts were identified (62). The frequency in which cysts are found in human clinical samples leads us to believe that they may not be the predominant transmissible stage in humans and may actually be an aberrant form in this host.
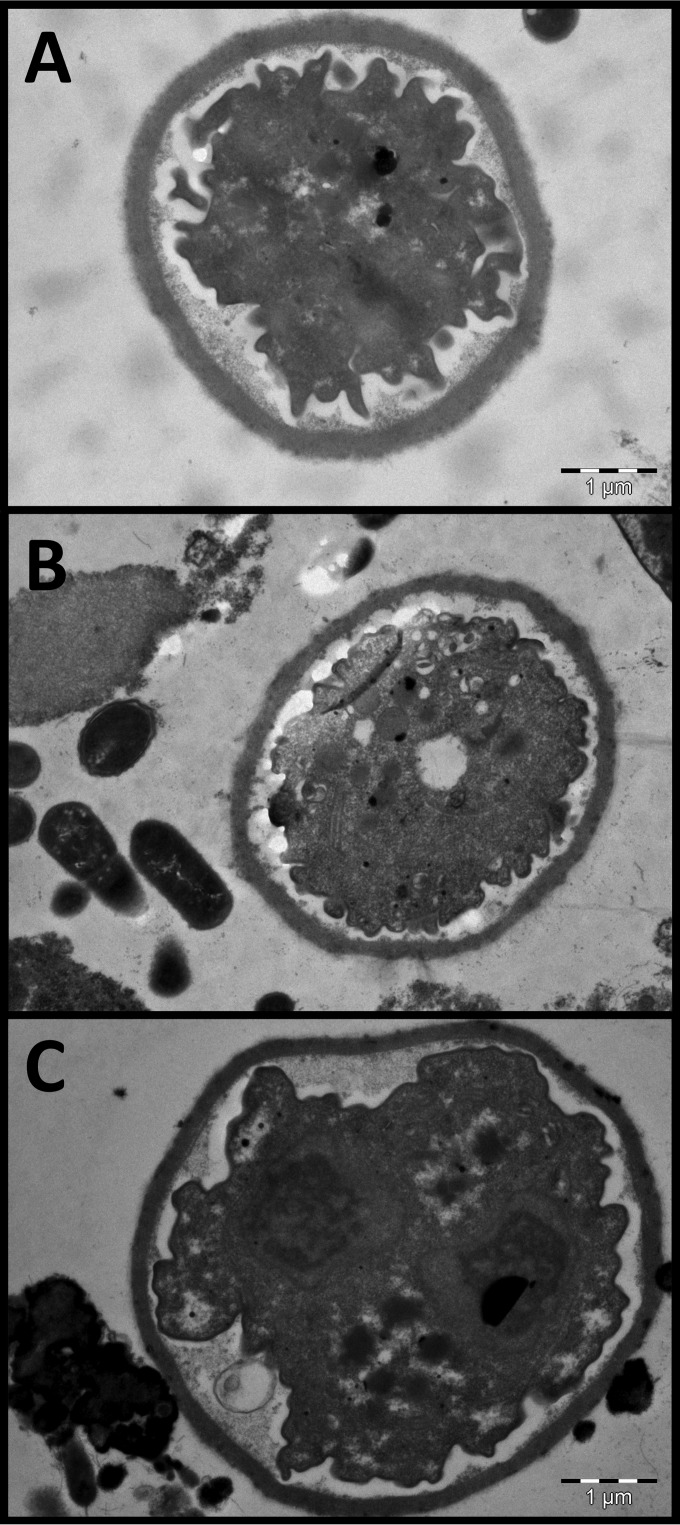
FIG 5.
Transmission electron micrographs of D. fragilis cysts identified in rodent studies of D. fragilis infection. Dientamoeba cysts are 4 to 6 μm in diameter and possess a distinct cyst wall and a clearly visible peritrophic space.
FIG 6.
Binucleate cysts of D. fragilis identified in rodent studies (A and B) and from human clinical stool samples (C and D) (stained with a modified iron-hematoxylin stain) (magnification, ×1,000).
DIAGNOSTIC TECHNIQUES
Microscopy
One hundred years have passed since the discovery of D. fragilis, and from that time, there have been few advances in the techniques used to diagnose infections with this parasite. Definitive diagnosis is based primarily on microscopy utilizing permanent stains of fixed fecal smears (69). However, newer molecular detection techniques, such as real-time PCR, are becoming the methods of choice for clinical diagnostic laboratories, although these tests are not employed routinely by most diagnostic laboratories.
In wet preparations, D. fragilis appears as a nonspecific rounded mass, and the characteristic nuclear structure cannot be visualized in saline or even with the use of iodine preparations (73). The trophozoite morphology degenerates rapidly, and as such, prompt fixation of the specimen is necessary (2, 74). Therefore, detection of D. fragilis using microscopy is solely associated with the use of a fixation step followed by permanent staining. Many different stains and fixatives have been used successfully with D. fragilis. Suitable fixatives include mercury-based compounds (11, 75), sodium acetate-acetic acid-formalin (SAF) (74), phenol alcohol-formalin (76), modified Schaudinn's fixative (77), and thimerosal (Merthiolate)-iodine-formalin (78). A wide variety of permanent stains have been used to detect D. fragilis, including Mayer's hemalum and Lawless' stain (27), with the most common stains still in use today being iron-hematoxylin and trichrome stains (79). In our own experience, SAF used in conjunction with modified iron-hematoxylin provides the best combination for staining and fixation, compared to other combinations that we have trialed over the years. It should be noted that our group has had little experience with trichrome and fecal fixatives containing polyvinyl alcohol (PVA), and many laboratories in the United States use this combination with good effect. The main disadvantages of the use of permanent stains are that they require a highly trained microscopist to read the stains and are time-consuming compared to newer technologies.
An alternative to trichrome or hematoxylin staining is the use of the Chlorazol black stain. Based on our experience, SAF also works best in combination with this stain. van Gool et al. (80) described Chlorazol stain used on patient stool samples collected over three consecutive days. The test was highly effective, relatively easy, and fast to perform, and those authors had no issues in detecting D. fragilis. A recent study used modified Field's stain to differentiate D. fragilis from Blastocystis in patient samples cultured in Loeffler's medium (81). This modified stain provided greater contrast between the two organisms and was simpler and quicker to use than Giemsa and iron-hematoxylin stains. However, as this stain was only evaluated on cultured parasites, its performance on clinical stool specimens requires evaluation. Regardless, these results look promising (81).
As with other enteric protozoa, D. fragilis trophozoites are shed intermittently, and daily shedding is highly variable (31, 80). This necessitates the examination of multiple stool specimens for optimal diagnosis. Hiatt et al. (82) examined the sensitivity of examining one stool specimen compared to multiple specimens. It was found that collecting multiple stool samples increased the percentage of positive results by 31.1% for D. fragilis. These data indicate that even in symptomatic patients, the examination of a single stool specimen could miss a large number of D. fragilis infections (82). As a result, collection of three specimens on consecutive days is recommended for the diagnosis of D. fragilis infection if permanently stained smears are used.
Culture
Parasite culture techniques have been used for nearly 90 years to detect D. fragilis. A wide variety of culture systems have been used, including Boeck and Drbohlav's medium (83), Robinson medium (84), Dobell and Laidlaw's medium (85), Cleveland-Collier medium (86), Balamuth's medium (87), and TYGM-9 (88). Dobell and Svensson were the first to grow “monoprotist” D. fragilis cultures in 1929, as all previous attempts had resulted in overgrowth with Blastocystis. They used a diphasic medium devised by Dobell, which was comprised of an inspissated horse serum slant overlaid with diluted egg whites in Ringer's solution and supplemented with rice. He also reported that cultures grew best at 41°C, a temperature much higher than one would expect an intestinal protist of humans to grow (2).
A comprehensive modern evaluation of several culture media, including modified Boeck and Drbohlav's medium, TYGM-9, modified Loeffler's slope medium, Robinson's medium, medium 199, Trichosel, and Tritrichomonas foetus medium, was carried out at different temperatures and under different atmospheric conditions for the growth of Dientamoeba (33). It was found that biphasic Loeffler's medium in a microaerophilic atmosphere was the optimal combination for growing D. fragilis. This report also noted that D. fragilis grew optimally at a temperature of 42°C. To further optimize the biphasic media reported above, Munasinghe et al. (89) undertook experiments supplementing the medium with essential growth nutrients, including cholesterol, iron, and lipids. Different liquid overlays for this biphasic medium were also evaluated. When a new liquid overlay comprised of Earle's balanced salt solution supplemented with cholesterol and ferric ammonium citrate in conjunction with Loeffler's slope medium was used, a 2-fold increase in the number of trophozoites grown was observed compared to the original Loeffler slope medium described by Barratt et al. (33, 89).
Compared to permanent stains, a number of studies have demonstrated that culture techniques are sensitive. Sawangjaroen et al. (58) found that for the diagnosis of dientamoebiasis, culture was significantly more sensitive than microscopy. A more recent study using modified Robinson medium showed a dramatic increase in the rate of detection of D. fragilis compared to traditional microscopy techniques (90). Stark et al. (49) compared PCR and microscopy against two xenic culture methods. It was found that the use of modified Boeck and Drbohlav's medium was superior to microscopy, while the use of TGYM-9 medium was less sensitive than microscopy.
It should be noted that cultivation of luminal parasitic protists is difficult, time-consuming, and often unsuccessful (91). As such, these specialized culture techniques are usually restricted to specialist, research, or reference parasitology laboratories and are not routinely offered by diagnostic laboratories. Dientamoeba has been reported to be difficult to establish in long-term culture; however, short-term cultures are relatively easy to establish before dying out (91). In our experience, D. fragilis isolates can be continuously passaged for several years with no major issues. One major disadvantage of culture systems is that the success of establishing initial cultures is temperature and time dependent. As such, stool specimens need to be inoculated promptly (12, 57). Also, rates of D. fragilis recovery are adversely affected if specimens have been refrigerated, and this greatly reduces the sensitivity of culture methods (58).
All the culture methods that have been used to grow D. fragilis are xenic culture systems. These are systems in which the parasite is grown in the presence of the bacterial flora derived from a patient's stool. Attempts to grow D. fragilis in axenic culture systems have all failed (92, 93), including attempts by our group, which has so far failed to axenize D. fragilis cultures despite extensive efforts over several years. The unavailability of an axenic culture system has contributed to the slow progress of D. fragilis research. Other parasites such as E. histolytica, Giardia, and T. vaginalis have all been grown in axenic systems, allowing closer study of the organisms to be undertaken without the interference of the bacteria associated with xenic systems.
Immunoassays
Immunofluorescence microscopy using commercially available monoclonal antibodies, several commercial enzyme immunoassays (EIAs), and immunochromatographic tests (ICTs) are available for the detection of antigens for Cryptosporidium, Giardia intestinalis, and E. histolytica in stool samples (94). However, no such tests are commercially available for D. fragilis. The development of user-friendly tests such as these for D. fragilis might encourage the adoption of routine D. fragilis testing by more laboratories.
Chan et al. (92) developed an indirect fluorescent-antibody assay to detect D. fragilis in preserved fecal samples. A total of 155 specimens were tested, 42 with no parasites, 9 with D. fragilis, and 104 with various other protozoa. There were no false-positive readings and no cross-reactivity with the other protozoa, although two of the nine positive samples gave doubtful results. The authors of this study concluded that this was due to the low numbers of trophozoites in the samples. This method showed promise and indicates that other diagnostic tests such as enzyme immunoassays could be developed for this parasite.
PCR
Molecular biology techniques now offer a diagnostic alternative to traditional methods such as microscopy. Identification of D. fragilis as the causative agent is also important for patient management, as specific treatment is often required given the potential for chronic infections to occur. Available PCR techniques enable rapid identification of Dientamoeba directly from clinical samples, with results potentially being available in several hours. These PCR techniques have been used for the detection of a wide variety of protozoan parasites from clinical samples (94). Several PCR assays have now been described for D. fragilis and are listed in Table 2, with a list of commercially available assays included in Table 3. It should be noted that these tests are currently not FDA approved in the United States; however, the Genetic Signatures assay is currently undergoing the registration process.
TABLE 2.
PCR primers and probes used in conventional, nested, and real-time PCR assays for amplification of D. fragilis DNAd
| Assay and gene target | Amplicon size | Primer or probe | Sequence | Reference |
|---|---|---|---|---|
| Conventional PCR | ||||
| 18S rRNA | 1.7 kb | TRD5a | 5′-GATACTTGGTTGATCCTGCCAAGG-3′ | 30 |
| TRD3a | 5′-GATCCAACGGCAGGTTCACCTACC-3′ | |||
| 18S rRNA | 850 bp | DF400 | 5′-TATCGGAGGTGGTAATGACC-3′ | 30 |
| DF1250 | 5′-CATCTTCCTCCTGCTTAGACG-3′ | |||
| 18S rRNA | 364 bp | DFpn_1f | 5′-GCCAAGGAAGCACACTATGG-3′ | 155 |
| DFpn_364r | 5′-GTAAGTTTCGCGCCTGCT-3′ | |||
| 18S rRNA | 662 bp | DF1 | 5′-CTCATAATCTACTTGGAACCAATT-3′ | 123 |
| DF4 | 5′-CCCCGATTATTCTCTTTGATATT-3′ | |||
| ITS1-5.8S | 300 bp | ssu2 | 5′-GGAATCCCTTGTAAATGCGT-3′ | 35 |
| 1su1 | 5′-AGTTCAGCGGGTCTTCCTG-3′ | |||
| ITS1 | 300 bp | ssu2 | 5′-GGAATCCCTTGTAAATGCGT-3′ | 35 |
| 5.8s1 | 5′-TGTGAGGAGCCAAGACATCC-3′ | |||
| Nested PCR | ||||
| 18S rRNA | 662 bp | DF1b | 5′-CTCATAATCTACTTGGAACCAATT-3′ | 38 |
| DF4Ib | 5′-CCCCGATTATTCTCTTTGATATT-3′ | |||
| 18S rRNA | 366 bp | DF322Forc | 5′-GAGAAGGCGCCTGAGAGATA-3′ | 38 |
| DF687Revc | 5′-TTCATACTGCGCTAAATCATT-3′ | |||
| 18S rRNA | 850 bp | DF400b | 5′-TATCGGAGGTGGTAATGACC-3′ | 98 |
| DF1250b | 5′-CATCTTCCTCCTGCTTAGACG-3′ | |||
| 18S rRNA | 403 bp | DFF2c | 5′-CGGGGATAGATCTATTTCATGGC-3′ | 98 |
| DFR2c | 5′-CCAACGGCCATGCACCACC-3′ | |||
| ITS1 | ~540 bpe | Ssu2b | 5′-GGAATCCCTTGTAAATGCGT-3′ | 38 |
| DF-ITSREVb | 5′-GCGGGTCTTCCTATATAAACAAGAACC-3′ | |||
| ITS1 | 380 bp | Df-ITSnesForc | 5′-ATACGTCCCTGCCCTTTGTA-3′ | 38 |
| ITSnesRevc | 5′-GCAATGTGCATTCAAAGATCGAAC-3′ | |||
| Real-time PCR | ||||
| 18S rRNA | 78 bp | DF3 | 5′-GTTGAATACGTCCCTGCCCTTT-3′ | 95 |
| DF4 | 5′-TGATCCAATGATTTCACCGAGTCA-3′ | |||
| TaqMan probe | 5′-FAM-CACACCGCCCGTCGCTCCTACCG-TAMRA-3′ | |||
| 18S rRNA | 101 bp | 5DMB | 5′-GGCGAAAGCATCTATCAAGTGTAAT-3′ | 96 |
| 3DMB | 5′-CGGCATCGTTTAAGGTAGGAAC-3′ | |||
| TaqMan probe | 5′-FAM-ACCCGGGTCTCTGATCCGGTTGG-TAMRA-3′ | |||
| 18S rRNA | 662 bp | DF1 | 5′-CTCATAATCTACTTGGAACCAATT-3′ | 37 |
| DF4 | 5′-CCCCGATTATTCTCTTTGATATT-3′ | |||
| 5.8S rRNA | 98 bp | Df-124F | 5′-CAACTTGGCTCTTTA-3′ | 51 |
| Df-221R | 5′-TGCATTCAAAGATCGAACTTATCAC-3′ | |||
| Df-172revT | 5′-FAM-CAATTCTAGCCGCTTAT-3′-MGB |
Generic trichomonad primer.
Outer nested primer.
Inner nested primer.
NA, not applicable; FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
The amplicon size for ITS1 is variable.
TABLE 3.
Commercially available PCR assays that incorporate a D. fragilis target
| Assay | Assay type | Target | Company |
|---|---|---|---|
| EasyScreen enteric parasite detection kit | Multiplex PCR using 3-base technology | 18S rRNA gene | Genetic Signatures |
| Gastrointestinal parasite | Multiplex PCR | 18S rRNA gene | AusDiagnostics |
| Rida Gene Dientamoeba fragilis | Real-time PCR | 18S rRNA gene | R-Biopharm |
| G-DiaFrag | Real-time PCR | 5.8S rRNA gene | Diagenode |
| LightMixModular Dientamoeba | Real-time PCR | 5.8S rRNA gene | Roche Diagnostics |
Based on the literature, Peek et al. (31) were the first to develop a conventional PCR assay that amplified the SSU rRNA gene to detect D. fragilis. While the analytical detection limit of this PCR assay was ∼0.1 D. fragilis trophozoites per reaction, the clinical sensitivity and specificity of the assay were not determined (31). Another conventional PCR assay and an RT-PCR assay based on the SSU rRNA gene of D. fragilis were developed soon after (30, 95). To determine the sensitivity of these assays, the SSU rRNA gene was cloned and amplified. The detection limits were ∼100 plasmid copies (∼1 D. fragilis trophozoite) for conventional PCR and 1 plasmid copy of the SSU rRNA gene (∼0.01 D. fragilis trophozoites) for the RT-PCR assay (95). When the molecular assays were compared to microscopy for the detection of D. fragilis, both PCRs were 100% specific, with conventional PCR having a sensitivity of 88.9% and RT-PCR having a sensitivity of 100% (95). This RT-PCR assay was later shown to cross-react with T. vaginalis, and subsequent studies have shown that it cross-reacts with other trichomonads, thus making its use in clinical microbiology laboratories for the specific detection of D. fragilis questionable at best (D. Stark, D. Chan, J. Barratt, and J. Ellis, unpublished observations, 2016).
Given the conserved nature of the SSU rRNA gene among trichomonads, an alternate RT-PCR assay targeting a 98-bp fragment of the 5.8S rRNA gene was developed (51). This PCR showed 100% specificity for D. fragilis when tested against a range of protozoa, helminths, bacteria, and yeasts. However, this assay was tested against only one other trichomonad (T. vaginalis), and further specificity experiments should be performed. This study also reported that D. fragilis DNA could be detected in refrigerated feces for up to 8 weeks. It was also noted that intermittent shedding of D. fragilis had less influence on false negativity rates when PCR was used for diagnosis than when microscopy was used. This suggests that while intact parasites may be shed inconsistently in consecutive stool specimens, parasite DNA is more consistently detectable during an infection. De Canale et al. (96) also developed a real-time assay for the detection of D. fragilis and compared it to a conventional PCR assay. The RT-PCR assay showed 100% sensitivity and specificity and did not cross-react with clinical samples containing Chilomastix, T. vaginalis, or Trichomonas hominis. Like the assay reported by Verweij et al. (51), D. fragilis DNA was detectable in samples refrigerated for up to 8 weeks.
In addition to RT-PCR, several nested PCR assays have also been reported in the literature as either a complement to RT-PCR or a confirmatory assay in clinical surveys of D. fragilis in patients (97, 98). In a study by Caccio et al. (38) that surveyed for the presence of D. fragilis in pigs, two assays, targeting either the 18S rRNA or the more variable ITS1 gene of D. fragilis, were developed. This study reported issues with the assay amplifying the ITS1 gene, which cross-reacted with flagellates from different vertebrate classes. However, the assay that amplified the SSU rRNA gene distinguished between two subpopulations of D. fragilis based on 8 SNPs observed upon sequencing. This assay was further employed in a clinical survey of D. fragilis infections in Brazil (97). Additionally, a nested PCR targeting the small subunit was developed in a health care center in Tabriz, Iran, and tested on patients displaying GI disease (98).
Although D. fragilis is not commonly tested for in all clinical laboratories, there are now several commercial kits/assays available for the detection of D. fragilis, either included in a multiplex format or as an individual RT-PCR (Table 3). The Ridagene kit was recently used in a prevalence study in Portuguese children (99). Both the AusDiagnostics and genetic signature assays have been evaluated, and this work was reported, with both assays showing good sensitivity and specificity compared to an in-house PCR (50, 100). It should be noted that while some of these kits have local Conformité Européenne (CE) marking in Europe and Therapeutic Goods Administration (TGA) approval in Australia, no current D. fragilis molecular assay has Food and Drug Administration (FDA) registration. As such, these assays can be used only as research tools in the United States and not for routine diagnostics. Currently, commercial kits have an advantage over in-house protocols in that they do not require extensive and complex optimization or validation. Additionally, these assays can be semiautomated by using liquid-handling robots and automated front-end extraction systems to allow high throughput (100).
New diagnostic tools are steadily becoming available for the detection of Dientamoeba, although they are currently in limited use. Indeed, many laboratories still rely on the same methods that were available at the turn of the last century. Where many advances have been made in the detection of other pathogenic parasites, D. fragilis has been neglected, and newer diagnostic methods would be a welcome addition for both laboratories and physicians. Given the increased sensitivity and specificity of these molecular assays, where possible, these methods should be used in clinical laboratories for optimal detection of the parasite. When PCR is not available, multiple permanently stained smears from suitably fixed fecal specimens should be utilized.
CLINICAL ASPECTS AND EPIDEMIOLOGY
Since its initial description in the scientific literature (1), D. fragilis has been reported in all human-inhabited continents. The prevalence of D. fragilis varies between 0.4% and 71% depending on the cohort and the diagnostic method employed (26, 79, 101), generally with a higher prevalence in developed countries (102), a correlation not usually observed for bowel protozoa. Jepps and Dobell (1) initially concluded that D. fragilis was nonpathogenic based on its “mode of nutrition” and a case of asymptomatic carriage that was described in the earliest report on D. fragilis (1). Soon thereafter, Jepps described 10 cases of D. fragilis infection from a war hospital in 971 soldiers (2). This led to increased interest and subsequent reporting of the parasite throughout the world (75). A list of major studies reporting the locations and prevalences of cases of dientamoebiasis is provided in Table 4. The clinical presentation of infection ranges from asymptomatic carriage to various GI symptoms, including altered bowel motions, abdominal pain, and diarrhea, often in association with eosinophilia, which is reported in up to 50% of patients (47, 103, 104).
TABLE 4.
Prevalence of D. fragilis described in various studiesb
| Reference | Prevalence (%) | Sample type | No. of patients | Technique(s) | Country or region |
|---|---|---|---|---|---|
| 52 | 36.25 | Fecal specimens from inmates in a mental asylum | 80 | LM | Holland |
| 112 | 2.4 | Fecal specimens from patients examined in a parasitology laboratory | 14,203 | LM | USA |
| 141 | 20.1 | Fecal specimens from GI outpatients | 1,114 | LM | Israel |
| 201 | ND | Fecal specimens from patients with Ascaris lumbricoides | NA | LM | Thailand |
| 74 | ∼4.2 | Fecal specimens submitted for parasitological examination | 43,029 | LM | Canada |
| 202 | 9.6 | Fecal specimens containing Entamoeba histolytica/E. dispar | 125 | Cultivation | Mexico City, Mexico |
| 203 | 1.1 | Fecal specimens from schoolchildren | 94 | Cultivation | Durban, South Africa |
| 138 | 52 | Fecal specimens from adult members of a semicommunal group | 81 | LM | Los Angeles, CA, USA |
| 138 | 21.1 | Fecal specimens from children attending dental and general pediatric clinics | 104 | LM | Los Angeles, CA, USA |
| 204 | 8.6 | Fecal specimens from children in day care centers | 900 | LM | Toronto, Canada |
| 4 | Fecal specimens from adult staff at day care centers | 146 | LM | Toronto, Canada | |
| 132 | 1.3 | Fecal specimens from homosexual men | 150 | LM | San Francisco, CA, USA |
| 78 | 16.8 | Fecal specimens examined during an outbreak of GI complaints in a residential community | 125 | LM | French's Forest, Sydney, Australia |
| 133 | 1.1 | Fecal specimens from homosexual men with diarrhea | 274 | LM | Chicago, IL, USA |
| 205 | 21 | Fecal specimens from indigenous people | 242 | LM | Irian Jaya, Indonesia |
| 125 | 3 | Fecal specimens from patients with GI disorders | 1,350 | LM | Christchurch, New Zealand |
| 117 | 82.9 | Fecal specimens from children infected with any GI protozoa | 123 | LM | Germany |
| 206 | 3 | Fecal specimens from children in rural communities | 266 | LM | Honduras |
| 207 | 2 | Fecal specimens from subjects with light to moderate dehydration and diarrhea | 100 | LM | Dominican Republic |
| 58 | 1.5 | Fecal specimens from patients with diarrhea | 260 | Cultivation | Brisbane, Australia |
| 134 | 25.6 | Fecal specimens from HIV-infected patients without diarrhea | 82 | LM | Buenos Aires, Argentina |
| 208 | 2.3 | Fecal specimens from pediatric refugees | 87 | LM | USA |
| 135 | 91 | Sera from healthy children | 189 | IFA | Canada |
| 209 | ∼8 | People with GI complaints in the Netherlands | NA | NA | Netherlands |
| 210 | 2.1 | Fecal specimens from HIV-negative patients | 48 | LM | San Pedro Sula, Honduras |
| 119 | 5.1 | Fecal specimens submitted for routine microbiological analysis | 857 | LM | Oman |
| 211 | 5.5 | Fecal specimens submitted to a university hospital in Tunisia | 27,053 | LM | Sfax, Tunisia |
| 212 | 3 | Fecal specimens from HIV-positive patients | 34 | LM | North Brazil |
| 213 | 11.3 | Fecal specimens from patients with various GI complaints | 151 | LM | Italy |
| 121 | 8.8 | Fecal specimens from patients admitted to a Turkish university hospital | 400 | LM | Celal Bayar University, Turkey |
| 127 | 0.9 | Fecal specimens from patients with diarrhea | 6,750 | LM | Sydney, Australia |
| 214 | 0.82 | Fecal specimens from sanitary workers | 241 | LM | Malatya, Turkey |
| 123 | 6.3 | Fecal specimens from patients expected to harbor a parasitic bowel infection | 448 | LM and TFT | Brussels, Belgium |
| 161 | 3.7 | Fecal specimens from patients with various GI complaints | 3,139 | LM | Italy |
| 215 | 3.4 | Fecal specimens from patients with various GI complaints | 1,141 | LM | Italy |
| 216 | 4.1 | Fecal specimens from patients with various GI complaints | 1,989 | LM | Italy |
| 217 | 2 | Fecal specimens from children and neonates at Ibn-Sina hospital | 350 | LM | Ibn-Sina hospital, Surt, Libya |
| 218 | 2.7 | Fecal specimens from people in an aboriginal community | 112 | LM | Salta, Argentina |
| 219 | 2.7 | Fecal specimens submitted to a Turkish university hospital | 770 | LM | Turkey |
| 220 | 8.9 | Fecal specimens from patients presumed to be infected with intestinal parasites | 168 | LM | Egypt |
| 220 | 29.8 | Fecal specimens from patients presumed to be infected with intestinal parasites | 168 | Cultivation | Egypt |
| 221 | 0.8 | Fecal specimens from HIV-negative MSM | 628 | LM | Sydney, Australia |
| 0.3 | Fecal specimens from HIV-infected MSM | 618 | LM | Sydney, Australia | |
| 1.1 | Fecal specimens from non-MSM patients | 622 | LM | Sydney, Australia | |
| 151 | 11.7 | Fecal specimens from patients suspected of harboring intestinal parasites | 103 | LM | Denmark |
| 48 | 32 | Fecal specimens from patients with GI complaints | 397 | Combination LM and qPCR | Zwolle, The Netherlands |
| 222 | 0.2 | Fecal specimens from schoolchildren | 2,975 | LM | Van province, Turkey |
| 223 | 14.6 | Fecal specimens from people attending complementary health practices between 2002 and 2004 | 3,719 | LM | British Isles |
| 16.9 | Fecal specimens from people attending complementary health practices between 2005 and 2007 | 2,491 | LM | British Isles | |
| 106 | 5.2 | Fecal specimens from patients with GI complaints | 750 | qPCR | Sydney, Australia |
| 50 | 5.5 | Fecal specimens submitted to the Department of Microbiology at St. Vincent's Hospital, Sydney | 472 | Tandem multiplex PCR | Sydney, Australia |
| 224 | 1.6 | Fecal specimens from patients with digestive disorders | 8,313 | LM | Catalonia, Spain |
| 156 | 21.4 | Fecal specimens from patients with clinical suspicion of intestinal parasitosis | 491 | qPCR | Parma, Italy |
| 225 | 3.5 | Fecal specimens from patients with IBS-associated diarrhea | 171 | LM | Karachi, Pakistan |
| 4 | Fecal specimens from patients with IBS-associated diarrhea | 171 | Cultivation | Karachi, Pakistan | |
| 4 | Fecal specimens from patients with IBS-associated diarrhea | 171 | PCR | Karachi, Pakistan | |
| 226 | 0.04 | Fecal specimens from patients experiencing GI discomfort | 2,604 | LM (wet mount and TS) | Rocky Mountain region, USA |
| 227 | 0.9 | Stool specimens from locals and immigrants submitted for microbiological examination | 1,503 | GS smears | Reggio Emilia, Italy |
| 228 | NA | Stool specimens from children (aged 15 yr or younger) containing D. fragilis | 41 | PCR and IH-stained smears | Sydney, Australia |
| 229 | 0.6 | Stool specimens from children | 1,181a | NL, sedimentation techniques, modified TS, and acid-fast TS | Malatya, Turkey |
| 230 | 23 | Stool specimens from children aged 4–16 yr referred to a secondary medical center | 220 | Standard laboratory testing | Netherlands |
| 224 | 1.6 | Stool specimens from outpatients with digestive abnormalities | 8,313 | Modified ZN technique | Catalonia, Spain |
| 231 | 0.9 | Stool specimens from administrators and workers in sanitary and nonsanitary institutions | 2,443a | NL, sedimentation techniques, modified TS, and acid-fast TS | Turkey |
| 232 | 15.5 | Stool samples of food handlers | 8,502a | LM | Tunisia |
| 233 | 5 | Stool samples from internationally adopted children | 1,042 | Wheatley's modified TS smears | USA |
| 234 | 1.3 | Stool samples from children with diarrhea | 225 | Modified Ehrlich ZN method | Turkey |
| 235 | 1.2 | Stool specimens from patients with HIV/AIDS | 82 | Simplified IH technique | Brazil |
| 236 | 2.2 | Stool specimens from patients with IBS | 45 | PCR | Mexico |
| 236 | 26.7 | Stool specimens from healthy controls | 45 | PCR | Mexico |
| 237 | 8.8 | Stool samples from patients with GI complaints, patients attending Al-Nuseirate Refugee Camp Clinic | 319 | IH-stained fecal smears | Gaza Strip |
| 238 | 9 | Stool specimens submitted to Hacettepe University Faculty of Medicine Parasitology Laboratory | 85,707 | Examination of stained fecal smears | Turkey |
| 239 | 62 | Stool specimens from pediatric patients presenting with GI symptoms | 163 | Multiplex qPCR | Netherlands |
| 240 | 0 | Stool specimens from patients with IBS | 55 | TS and culture | Turkey |
| 240 | 0 | Stool specimens from patients with gastroenteritis | 80 | TS and culture | Turkey |
| 240 | 0 | Stools from healthy volunteers | 50 | TS and culture | Turkey |
| 101 | 43 | Stool specimens submitted to Statens Serum Institut in Denmark | 22,000a | qPCR | Denmark |
| 98 | 2.3 | Stool specimens collected from various laboratories | 1,000 | Nested PCR | Tabriz, northwest Iran |
Refers to the number of stool specimens and not individual patients.
IFA, indirect immunofluorescence assay; ND, not disclosed; NA, not applicable; TFT, triple-feces test protocol; LM, light microscopy; qPCR, quantitative real-time PCR; IH, iron-hematoxylin; TS, trichrome stain; GS, Giemsa stain; NL, native Lugol; ZN, Ziehl-Neelsen; MSM, men who have sex with men.
Immediately following its first description (1), the pathogenicity of D. fragilis was placed under scrutiny. Haughwout and Horrilleno (10) reported D. fragilis in three Filipino children suffering from GI complaints. Soon afterwards, Gittings and Waltz (105) described case reports of two children with GI complaints infected with D. fragilis, who improved clinically following treatment. Since then, hundreds of studies and case reports have provided support for D. fragilis as a potential pathogen (26, 27, 106). Based on an overwhelming majority of reports, patients harboring D. fragilis commonly suffer from diarrhea and abdominal pain, which can be of an acute or chronic nature. Some case studies have also shown that Dientamoeba may be implicated in cases of colitis, but the association is weak (26, 27, 79). Another weak association between D. fragilis and IBS has also been described (107); however, a statistically significant association has not been reported, and as such, the organism most likely plays no role in this syndrome (108).
Wenrich et al. (55) reported the detection of D. fragilis in 4.3% of stool specimens from 1,060 university students in the United States. Diarrhea and abdominal pain were the major symptoms recorded. Hakansson (56) described his own personal experience with D. fragilis, having obtained a D. fragilis infection himself. He described the presence of recurrent GI symptoms for 2 weeks; following treatment with carbarsone, he reported complete resolution of symptoms and eradication of D. fragilis based on posttreatment stool examinations. Hakansson later described a group of patients (n = 12) infected with D. fragilis, half of whom were suffering from GI complaints (57). These infected patients were treated with carbarsone, resulting in the clearance of D. fragilis and improved clinical outcomes (57). Sapero and Johnson (109) described the detection of D. fragilis in a group of U.S. Navy personnel who were returning from military service in Asia. Dientamoeba was found in 26% of their stool specimens. Of this group, 27% had GI complaints (109). Hood (110) also demonstrated that elimination of D. fragilis from a patient's stool using arsenicals or oxyquinoline compounds usually cured patients of GI symptoms.
Like Hakansson (56), Wenrich (12) described his personal experiences with D. fragilis after infecting himself with the organism on two separate occasions. Both of these infections were chronic (2 months and 2 years) and spontaneously resolved with no treatment. Wenrich (12) described himself as having regular bouts of diarrhea as a result of both infections, which gradually abated over time. Soon thereafter, Knoll and Howell (111) reported a study of six patients with D. fragilis (three children and three adults) who had acute and chronic GI symptoms for up to 1.5 years. Treatment of these patients with carbarsone led to the eradication of the parasite and clinical improvement. Based on these observations, Knoll and Howell (111) proposed that D. fragilis was pathogenic.
More than 2 decades later, Kean and Malloch (112) reported an examination of 20,917 stool specimens submitted to the Cornell University Medical College, New York, NY, over a period of 6 years. Dientamoeba was detected in 2.4% of these cases, and 100 of these cases were described as “pure” infections, where D. fragilis was the only potentially pathogenic organism identified. Abdominal pain, diarrhea, and nausea were the most common clinical signs experienced by these 100 patients, who were mostly U.S. citizens who had not traveled outside the country. Kean and Malloch (112) found that D. fragilis disappeared from patient stool specimens following treatment with antiprotozoals and that this resulted in the resolution of clinical symptoms. In a similarly large study (74), D. fragilis was found in 4.2% of 43,029 patients from 1970 to 1974 in Ontario, Canada. Higher rates of infection were found in females than in males, with nearly half occurring in patients <20 years old. The most common symptoms included diarrhea, abdominal pain, and loose stools (74).
In the Parasitology Division of the Clinical Laboratories at the University of California, Los Angeles, stool samples from 695 children were examined for ova and parasites between 1976 and 1978 (113). Dientamoeba was recovered from 65 children (9.4%). A retrospective analysis involving 35 children was then undertaken. It was shown that 91% of them had GI complaints, including abdominal pain, diarrhea, and anorexia. The bowel movements of these children varied from frequent or daily to episodes of intermittent diarrhea. Increased peripheral eosinophil counts were also noted for 50% of children with D. fragilis. Following treatment, clinical signs were reduced in severity or were completely absent upon follow-up, leading those investigators to the conclusion that D. fragilis is pathogenic in children (113). Soon afterwards, Spencer et al. (114) carried out a retrospective analysis involving 50 patients with pure D. fragilis infections. Symptoms including abdominal pain, diarrhea, and nausea were present in most subjects. Twenty of these patients suffered from chronic complaints, which had been present from 6 months to 18 years, with 17 patients having symptoms for over 2 years. Eosinophilia was found in 53% of patients with chronic symptoms (114). In another report by Spencer et al. (115), stool samples from 104 pediatric patients were examined, and D. fragilis was found in 21% of patients. Diarrhea and abdominal pain were the most common symptoms in patients infected with D. fragilis (115). Two years later, Turner (116) conducted a study that concluded that D. fragilis and Giardia lamblia cause very similar spectra of disease based on clinical data collected from patients infected with either organism.
In a German study by Preiss et al. (117), in 123 pediatric patients infected with intestinal protozoa, D. fragilis was found in 102 cases. Acute and recurrent diarrhea were found to be the most common symptoms in children infected with Dientamoeba, and one-third of these cases demonstrated peripheral blood eosinophilia. Antiprotozoal therapy, which led to the eradication of the parasite, also resolved GI symptoms in these patients. These researchers reported that 21% (21/102) of cases had immunoglobulin G and/or immunoglobulin M levels that exceeded age-dependent reference ranges. Five patients also had abnormal transaminase levels. According to an American study by Grendon et al. (118), 237 cases of D. fragilis infection were reported between 1985 and 1986 at the Washington State Public Health Laboratory. Nearly 80% of patients reported symptoms associated with their D. fragilis infection, with clinical manifestations of diarrhea or loose stools (118). In a study by Windsor et al. (119), 857 fecal specimens submitted over a 6-month period from Oman were examined. Dientamoeba fragilis was identified in 4.1% of 857 stool specimens and was the most commonly found enteropathogen. Of those subjects diagnosed with dientamoebiasis, 83% had abdominal pain, 50% had diarrhea, and some experienced chronic symptoms lasting up to 2 years (119). A Swedish study of 87 patients retrospectively diagnosed with D. fragilis infection found the highest incidence in pre-school-aged boys, with most patients being symptomatic (120).
In a study of 400 patients carried out in Turkey, D. fragilis was found to be more prevalent (8.8% versus 8.6%) and associated with more symptoms than G. intestinalis (121). A study carried out from 2002 to 2003 on 1,989 outpatients attending a day care center in central Italy found that Dientamoeba was more than twice as prevalent as Giardia (4.1% compared to 1.8%) (122). Those patients infected with D. fragilis were more likely to have clinical symptoms than those infected with Giardia. Vandenberg et al. (123) found Dientamoeba (6.3%) and Giardia (7.1%) at similar prevalences, with the symptoms most frequently encountered with D. fragilis being abdominal pain and diarrhea (69.2% and 61.5%, respectively). However, patients with D. fragilis infection were less likely to report nausea and/or vomiting, anorexia, and weight loss.
A recent study of Portuguese children between 2011 and 2013 found D. fragilis in 8.5% of children hospitalized with acute GI symptoms. Dientamoeba was associated with diarrhea, fever, vomiting, and abdominal pain in this patient group (99). Ogren et al. (124) found an association between D. fragilis and GI symptoms in children aged 1 to 10 years from the county of Jönköping, Sweden.
In Australasia, the prevalence of infection varies dramatically, ranging from 1.5% in Queensland (58) to 2.2% in New Zealand (125) and 16.8% in suburban New South Wales (78), while the D. fragilis prevalence rate in Australian Aboriginal children from rural areas was 5.0% (126). A more recent prospective study of 6,750 outpatients from Sydney found that D. fragilis was detected in 0.9% of patients when permanently stained smears were used for diagnosis. Gastrointestinal symptoms were present in most patients, with over 60% of patients presenting with chronic symptoms (127).
In a report by Walker et al. (78), a prevalence of 16.8% was found in suburban Sydney (78). However, that study included subjects who were living in an area where many households were unsewered, and the high incidence of D. fragilis infection was also correlated with a high incidence of infection by Blastocystis and other bowel parasites in this group. A similarly high incidence of D. fragilis carriage was reported in a study from Israel, where 201,750 stool specimens were examined between 1960 and 1969. A D. fragilis detection rate of 15.2% was reported for these samples (128).
Dientamoeba fragilis has also been reported in association with allergic colitis. Cuffari et al. (104) reported a case of eosinophilic colitis associated with D. fragilis in a female 4-year-old child. The child presented with a 3-year history of chronic diarrhea. A colonoscopy was performed, and biopsy specimens were taken. Areas within the lamina propria showed eosinophilic infiltrates, and a biopsy specimen from the descending colon showed >50 eosinophils per high-power field. Isolated eosinophils were also observed infiltrating the glandular and surface epithelia. A diagnosis of eosinophilic colitis was made on the basis of histopathology, and stool samples for identification of ova, cysts, and parasites were collected from the patient. Dientamoeba trophozoites were detected in the patient's stool samples. She was treated with iodoquinol and promptly became asymptomatic and remained so after follow-up for a number of years (104).
Another case report of colitis associated with D. fragilis was described, involving a Burmese woman who presented with ulcerative colitis (129). The patient was hospitalized, and sigmoidoscopy revealed multiple punctate aphthous ulcers with mild to moderate erythematous, nonfriable, intervening mucosa. Stool cultures were negative for bacterial enteropathogens. After 1 week of hospitalization, sigmoidoscopy was ordered, and biopsy specimens were taken. The biopsy specimens revealed shallow ulceration with evidence of acute and chronic inflammation. When aspirates from mucosal ulcerations were fixed and stained with trichrome, many D. fragilis trophozoites were seen. The patient was treated with diiodohydroxyquin and metronidazole and subsequently made a complete recovery. Based on the clinical, radiological, endoscopic, and histological findings, the authors of this study concluded that D. fragilis was the cause of this case of invasive colitis (129). Another similar case of ulcerative colitis associated with D. fragilis in a 9-year-old boy was documented in Canada (130). These case reports suggest that D. fragilis may be associated with colitis in certain individuals, although the small number of cases described means that this requires further investigation. An Australian study by Borody et al. (107) also provides support for an association between D. fragilis and IBS. However, other more exhaustive studies using larger cohorts have shown no association between D. fragilis carriage and IBS (108).
It has been well documented that higher rates of gut protozoal infection have been reported among men who have sex with men (MSM) in developed countries (131, 132). However, this phenomenon is not apparent for D. fragilis. A study of enteric protozoa in MSM over a 2.5-year period found that 48.5% of patients harbored one or more intestinal protozoa. D. fragilis, however, made up only 1% of the protozoa found, compared to the E. histolytica-E. dispar complex, which accounted for 26% (133). Another study from the San Francisco Bay area in the United States reported a prevalence of potentially pathogenic enteric protozoa of 47% among male homosexual patients. E. histolytica-E. dispar complex isolates were found in 36% of patients, and D. fragilis was found in only 1.3% of patients (132). The rates of D. fragilis carriage reported in these studies are comparable to those reported for heterosexual groups. One study in Argentina suggested that the incidence of D. fragilis infections may be higher in immunocompromised patients (134). In all other studies conducted, immunosuppression does not seem to be a contributing factor for infection with D. fragilis, although there have been few reports on this topic (26).
There has been only one study on the seroprevalence of D. fragilis. Chan et al. (135) used an indirect immunofluorescence assay and found that of 189 randomized serum samples from children and young adults aged between 6 months and 19 years from Canada, 91% were seropositive for D. fragilis antibodies. This study suggests that D. fragilis infection is common in Canada; however, those researchers did not raise the issue of cross-reactivity, and the 91% positivity rate could be due in part to this phenomenon.
Not surprisingly, higher rates of D. fragilis infection are often seen where sanitation and hygiene levels are poor. This is seen in studies performed on disadvantaged groups and communities (136, 137). For example, the pioneering investigator S. L. Brug reported a remarkably high incidence of D. fragilis carriage (36.25%) in Holland among inmates in a mental asylum (52). Similarly, high prevalences were reported in the United States in ∼300 members of a semicommunal religious group. Dientamoeba fragilis was detected in this community at an incidence of 53%. Over 81% of D. fragilis-infected patients had GI complaints, most commonly recurrent or chronic diarrhea, and substandard hygiene practices were evident among this group. In accordance with cultural beliefs, toilet paper was not used after defecation; bare hands were used to wash the anal area with soapy water. Hand washing before meals was not a common practice, and meals were often eaten without the aid of cutlery (138).
There is an overwhelming body of evidence, dating back several decades, indicating that treatments that eliminate D. fragilis result in significant clinical improvement of patients experiencing GI symptoms (11, 12, 26, 53, 55–57, 74, 103, 104, 111, 112, 114, 117, 125, 129, 139–143). This suggests that D. fragilis plays some role in the development of GI disease. However, some reports from northern Europe support a very different trend. In a recent publication from Denmark (101), a very high incidence of D. fragilis infection (43%) was reported after performing a quantitative PCR (qPCR) survey involving a large number of stool samples (n = 22,484) submitted by subjects for investigation of intestinal parasitosis. In this same report (101), the authors refer to the unpublished work of a colleague (L. R. Krogsgaard et al., unpublished data) who observed a similarly high incidence of D. fragilis infection among healthy Danish adults. As these authors state, “this is a staggeringly high proportion, particularly for healthy individuals.” This is, in fact, comparable to the incidences reported by Brug (52) for inmates in a Dutch mental asylum (incidence of 36.25%) and by Millet et al. (138) for members of a semicommunal group that partook in substandard hygiene practices (incidence of 53%). In a study from the Netherlands (Holland) by de Wit et al. (144), D. fragilis was detected more frequently in the stool specimens of healthy controls (14.6%) than in the stool specimens of subjects suffering from GI complaints (10.3%). A recent large case-controlled comparison study comprising 1,515 symptomatic patients and 1,195 healthy controls detected D. fragilis in 390 symptomatic patients at a prevalence of 25.7% and in 446 individuals in the control group at a prevalence of 37.3% (145). This study found that D. fragilis was more commonly found in healthy nonsymptomatic groups than in symptomatic patients. Another study by de Jong et al. (146) found higher rates of D. fragilis detection in healthy controls than in pediatric patients presenting with chronic abdominal pain. Dientamoeba was found at astonishingly high prevalences of 50.6% in controls and 43.2% in the study group. These authors found no differences in symptoms of children with and those without D. fragilis infection, and no relationship between clinical and microbiological responses after treatment was found. This retrospective study suggested no association between chronic abdominal pain and D. fragilis infection. Another Danish study found high rates of D. fragilis carriage of between 35% to 41% in primary care patients diagnosed with IBS (147). All of these studies reporting a high prevalence of D. fragilis infection used the same diagnostic method, a real-time PCR method described previously by Verweij et al. (51). Subsequently, it seems that further testing and evaluation of this assay are warranted to determine if these extraordinarily high rates of infection are a true indication of the prevalence of D. fragilis in Denmark or are artifacts of the test.
In another recent study from Denmark, Petersen et al. (148) suggest that people harboring D. fragilis are less likely to suffer from inflammatory bowel disease (IBD) and relate this to the hygiene theory. In that study, healthy control groups had a higher incidence of D. fragilis than did those with IBD. This is in direct contrast to previous reports that implicated D. fragilis as a cause of such complaints and reported resolution of symptoms following antiprotozoal therapy (104, 129, 130). In another Danish study, D. fragilis was associated with a low frequency of defecation in patients with IBS (149), which is in direct contrast to the vast majority of reports that suggest that fecal urgency and diarrhea (two typical IBS symptoms) are associated with D. fragilis carriage. The study by Engsbro et al. (149) also employed real-time PCR and reported a similarly high incidence of D. fragilis compared to that reported in a previous Danish study (101). In contrast to the reports by Engsbro et al. (149, 150), Borody et al. (107) demonstrated that antimicrobial therapy that eradicates D. fragilis led to the resolution of IBS-like symptoms.
These reports from northern Europe (101, 149) of remarkably high D. fragilis carriage (especially in healthy individuals [Krogsgaard et al., unpublished]) are perplexing, especially considering that the test subjects were residing in wealthy, developed nations where hygiene and sanitation are excellent (151). These reports are also in direct contrast to the overwhelming majority of reports from a large number of research groups that suggest that at the very least, individuals carrying D. fragilis are more predisposed to GI symptoms such as abdominal pain and diarrhea than the general population (for peer-reviewed articles that strongly support this point, see references 26, 27, 79, 102, 106, and 152). Furthermore, there is an overwhelming body of evidence dating back several decades, from numerous investigators, indicating that treatments that eliminate D. fragilis result in marked clinical improvement (11, 12, 26, 53, 55–57, 74, 103, 104, 106, 111, 112, 114, 117, 125, 129, 139–143).
Several reports implicate the human pinworm, Enterobius vermicularis, as a probable vector of D. fragilis transmission (72, 153–155). This is not an unreasonable hypothesis based on the evidence at hand. However, a D. fragilis incidence of 43% in Danish patients (and a similar incidence in healthy individuals) implies that at least 43% of the Danish population would also be infected with, or has previously been infected with, pinworm. This hypothesis, of course, assumes that the pinworm is the bona fide vector of D. fragilis and that every pinworm isolate in Denmark is harboring D. fragilis. Given the consistently high D. fragilis incidence reported in this location in healthy and ill subjects, it would be interesting (and seems necessary) to explore the incidence of pinworm infection across a similarly large group of Danish patients.
It is also quite possible that the sensitivity of the real-time PCR assay employed for routine screening (101) resulted in more cases being detected in this study than in other studies. However, real-time PCR surveys have been carried out in other developed nations, and such high incidences have not been reported elsewhere (49, 156). It is also possible that this is a localized phenomenon, potentially attributable to local climate or the possible existence of a resilient, highly transmissible strain of D. fragilis that is endemic to the region. Regardless, this unusual localized phenomenon certainly requires closer examination.
TRANSMISSION
Despite the recent description of a D. fragilis cyst stage, the details surrounding D. fragilis transmission remain unclear (69). Dientamoeba fragilis trophozoites are thought to be fragile and unable to survive for extended periods outside their host. Initially, the absence of a cyst stage meant that the transmission strategy employed by D. fragilis remained elusive for many years (69). Several theories were proposed to address this problem, the most prominent being that transmission occurred via the ova of a helminth (69, 72). Recent advances in D. fragilis cultivation (33, 89), animal studies (8, 38), microscopic and electron microscopic studies (7), and molecular screening (154, 155) have contributed to our current understanding of D. fragilis transmission. However, despite these advances, the mode of transmission employed by D. fragilis remains elusive and is still a topic of debate.
Cysts or Pinworm?
The hypothesis that D. fragilis may possess a helminth vector is plausible, as the closest known relative of D. fragilis, H. meleagridis, has a helminth vector (69). The helminth vector theory is also supported by the work of Ockert (157–159), who reportedly infected himself with D. fragilis by ingesting ova of the human pinworm, Enterobius vermicularis. Yang and Scholten (74) and Girginkardesler et al. (153) later reported a strong association between the incidence of E. vermicularis and D. fragilis infection, providing indirect support for the helminth vector hypothesis.
Menghi and colleagues (160) were the first to investigate the role of the pinworm in the life cycle of D. fragilis using molecular techniques. Pinworm ova were purified from stool specimens of D. fragilis-pinworm-coinfected individuals, and DNA on the external surface of these ova was removed by DNase treatment. Finally, DNA extracted from these ova was tested with a D. fragilis-specific PCR assay. These experiments failed to detect D. fragilis DNA within these ova (160). Similarly, reports by Stark et al. (36, 106) identified no association between the occurrence of pinworm and D. fragilis infection. However, pinworm infections can spontaneously resolve, and D. fragilis infections may be chronic, providing one explanation as to why carriers of D. fragilis may not always be infected with pinworm (27).
The role of E. vermicularis ova as the vector of D. fragilis transmission was placed under further scrutiny with the recent description of the D. fragilis cyst stage in experimentally infected rodents (8) and later in human specimens (62). The rodents were confirmed to be parasite free by microscopy and D. fragilis free by PCR prior to experimental infection with D. fragilis. The structures identified as D. fragilis cysts were never detected in control mice from the same supplier. While cultured trophozoites were infectious to mice, the same cultured trophozoites could not induce an infection in rats. Instead, stool from mice containing cysts was used to successfully induce infection in rats. While this does not rule out the possibility that E. vermicularis can transmit D. fragilis via its ova, this study indicates that E. vermicularis is not required for the transmission of D. fragilis.
Also pertinent to this debate is the fact that D. fragilis is capable of infecting pigs, having been detected in a population of farmed swine (38, 161). Humans are the only known host of E. vermicularis. Consequently, it seems unlikely that pinworms would be responsible for the maintenance of D. fragilis infection in a swine population, unless the pigs are coming into regular contact with E. vermicularis ova excreted by humans who are coinfected with both parasites. Of course, this does not rule out the possibility that pinworm ova can transmit D. fragilis, although, as with the rodent experiments, this suggests that E. vermicularis is not an absolute requirement for D. fragilis transmission.
The role of E. vermicularis as a vector of D. fragilis transmission is supported by two recent studies that detected D. fragilis DNA in DNA extracted from surface-sterilized E. vermicularis ova (154, 155). Hypochlorite was used to ensure that the external surfaces of these ova were DNA free prior to DNA extraction (154, 155). In both studies, molecular testing confirmed the presence of D. fragilis DNA within a large proportion of ova from patients infected with both parasites as well as patients with an unknown D. fragilis infection status (154, 155). As Roser et al. (155) propose, the presence of D. fragilis DNA inside these ova does not indicate that viable D. fragilis is present. It was suggested that animal studies would be useful to determine whether these ova are capable of inducing D. fragilis infection (155).
The cyst form of D. fragilis is markedly smaller than the trophozoite stage in iron-hematoxylin-stained smears and also stains darkly in comparison (62). As discussed above, D. fragilis cysts are also incredibly rare in human stool specimens compared to trophozoites (62). Given their rarity and small size, it is not unreasonable that D. fragilis cysts were overlooked previously. However, the rarity of these forms in human clinical specimens suggests that human-to-human transmission probably does not rely solely on these cysts, unless an incredibly low infectious dose of cysts is required to establish a D. fragilis infection. Regardless, the identification of D. fragilis cysts has epidemiological implications, as they may represent a risk to drinking and bathing water. Waterborne transmission of Giardia occurs due to the long-term survival of the cysts in bathing and drinking water (162, 163). Subsequently, experiments to assess the infectivity of purified D. fragilis cysts following long-term storage in water would be worthwhile.
The exact role of cysts and pinworm in the transmission of D. fragilis has not been sufficiently explored to allow any solid conclusions to be drawn. However, there are a number of new leads to follow and information that should be considered. First, while associations between pinworm and D. fragilis have been identified, these associations should be viewed with caution. Polyparasitism is common for enteric parasites (26), particularly in young children (102), and can represents little more than a shared route of transmission (i.e., the fecal-oral route). Subsequently, reports by J. Ogren et al. (154) and D. Roser et al. (155) provide better support for the role of pinworm ova in D. fragilis transmission but do not confirm that D. fragilis parasites are viable in these ova. The animal experiments carried out by Munasinghe and colleagues (8) indicate that pinworm is not an absolute requirement for the transmission of D. fragilis. Similarly, the fact that D. fragilis cultures that contained only trophozoites (according to microscopic examination) were infectious to mice indicates that cysts are also not an absolute requirement for transmission (8). In order to elucidate the true role of pinworm ova and D. fragilis cysts in the transmission of this organism, further research is required. Ideally, these structures should be purified and fed to animals to confirm that they are capable of inducing a D. fragilis infection (72).
An alternative emerging theme of relevance is that some trichomonads produce pseudocysts as part of their normal life cycle (164, 165). Literature on trichomonads such as Tritrichomonas foetus and free-living Monocercomonas species (166–168), all of which are parabasalids that share an ancestor with D. fragilis (169), shows that these species produce pseudocysts under unfavorable environmental conditions. Pseudocysts are nonmotile, multinucleated, typically spherical forms without a true cyst wall. Pseudocysts of T. foetus are infectious and can be found in in vitro cultures, especially under conditions of stress, such as by cooling or changes in pH (167). It is unknown whether pseudocyst forms are present in the life cycle of D. fragilis, although given that they are thought to facilitate the survival of trichomonads under stress conditions, this is worthy of investigation (69). Similarly, it is possible that precystic forms also play a role in D. fragilis transmission between humans. These forms are more common in clinical specimens than are cysts (62), and it is therefore plausible that precystic forms of D. fragilis represent an additional mode of transmission, via the fecal-oral route (Fig. 7).
FIG 7.
Life cycle of D. fragilis showing current hypotheses on transmission. D. fragilis is ingested from the external environment by a host species in one or more possible forms (1). The preferred transmissible form is yet to be determined. Humans are thought to be the preferred host of D. fragilis, although gorillas, pigs, and rodents are also considered natural hosts (2). Once ingested, D. fragilis travels to the large intestine, where it multiplies by binary fission (3). D. fragilis trophozoites (A), precysts (B), and cysts (C) are passed into the environment in the feces (4), where they contaminate food and/or water sources. D. fragilis parasites are then ingested by a new host, completing the cycle. While D. fragilis trophozoites are infectious to laboratory mice, they are noninfectious to larger mammals. The infectivity of precysts and purified cysts is yet to be demonstrated. It has been suggested that D. fragilis may be transmitted in the ova of the human pinworm, Enterobius vermicularis (5). Recent reports have confirmed the presence of D. fragilis DNA within E. vermicularis ova, although it is unknown whether viable and/or transmissible D. fragilis is present in these ova.
DIENTAMOEBA CARRIAGE IN ANIMALS
A large number of animal species have been investigated as potential hosts of D. fragilis (129), although only a small number of animals have been confirmed as natural hosts. Pioneering investigators reported the presence of D. fragilis in stools of various primates, including wild monkeys from the Philippines (170), captive macaques (171), baboons (172), and Ecuadorian howler monkeys (173). One early investigation reports the finding of D. fragilis in sheep (174), and a recent Nigerian study describes D. fragilis from a wild rat (175). Early pioneering studies relied on microscopic examination of stained fecal smears, which is a robust diagnostic technique in the hands of a skilled microscopist (49). However, in some of these studies, the staining technique employed to detect D. fragilis was not disclosed, and photographs (or drawings) were not provided.
Recent studies have confirmed the natural occurrence of D. fragilis in two animal species (pigs and gorillas) by using microscopy and PCR (38, 176). A study by Stark et al. (176) described the first report of gorillas as a natural host of D. fragilis and also examined stools of various other captive animals and birds, including several species of monkey, some domesticated ruminants, and pigs. Nonetheless, D. fragilis was not detected in these other species (176). In support of the findings reported by Stark et al. (176), Lankester and colleagues (177) also found D. fragilis in the stool of a naturally infected captive gorilla. Interestingly, Lankester et al. (177) also described the presence of irritable bowel syndrome-like symptoms in this gorilla. These symptoms resolved upon treatment with metronidazole, which coincided with the eradication of D. fragilis.
Crotti et al. (161) were the first to identify D. fragilis in the stools of farmed pigs using Giemsa-stained fecal smears. The role of pigs as a natural host of D. fragilis was further substantiated in a later study by Caccio et al. (38), who used PCR and microscopy to confirm the presence of D. fragilis in farmed pigs. By capillary sequencing of PCR products derived from the D. fragilis rRNA genes, Caccio and colleagues (38) demonstrated that the D. fragilis rRNA and ITS genotypes infecting swine were also of the SSU subtype found in humans, suggesting that D. fragilis is a zoonosis.
Early attempts to establish an animal model were unsuccessful. Dobell was unable to establish a D. fragilis infection in himself or macaques using cultures given orally or in macaques inoculated rectally. He also inoculated six chicks rectally with cultures of D. fragilis. One of the chicks developed a cecal infection, which spontaneously cleared after 1 week. Cultures from this infection failed to infect three other chicks (2). In 1945, Knoll and Howell inoculated D. fragilis cultures orally and rectally into kittens; however, no infection or symptoms were demonstrated, and no amoebae were recovered at autopsy (111).
It was not until 2013 that a study by Munasinghe et al. (8) described the successful establishment of a rodent model of D. fragilis infection, and 1 year later, wild rats were described as a natural host of D. fragilis (175). Munasinghe and colleagues (8) were not the first investigators to attempt experimental infections in rodents. Wenrich (12) was unsuccessful at infecting laboratory rats. Kean and Malloch (112) successfully infected laboratory rats with D. fragilis but described these experiments in only minor detail. In most cases, experimental infections of animals (and humans) with cultured D. fragilis trophozoites failed, suggesting that D. fragilis trophozoites cannot survive under the acidic conditions of the stomach.
According to Munasinghe et al. (8), cultured trophozoites of D. fragilis were highly infectious to BALB/c mice but could not be used to establish infections in Sprague-Dawley rats. The rats became infected only after ingesting cysts present in the stools of BALB/c mice (8). According to data reported in the literature, no previous attempts had been made to infect mice with D. fragilis trophozoites. It is difficult to speculate on why BALB/c mice might be receptive to infection with D. fragilis trophozoites while other mammals are not. One contributing factor might be the pH of the rodent stomach (pH 3.0 to 4.0) (178), which is higher than that of the stomachs of other large mammals (around pH 2.0) and may be more conducive to trophozoite survival. Furthermore, gastric transit times are longer in larger animals than in small mammals (179), which may also be a contributing factor.
PATHOGENICITY
Some 100 years have passed since the discovery of D. fragilis, yet the debate about its pathogenicity is still ongoing. There is still little acceptance of the pathogenic potential of D. fragilis despite the vast evidence that has emerged from clinical studies. While there is certainly overwhelming circumstantial evidence incriminating D. fragilis as a pathogen, this is based primarily on case reports or prospective and retrospective studies describing symptomatic patients whose symptoms were resolved following therapeutic intervention and parasite eradication. Other studies have highlighted higher rates of carriage of D. fragilis in control groups or cohorts of patients who are asymptomatic (145). These conflicting data have led to uncertainty about the role that this parasite plays in GI disease.
The lack of a suitable animal model hampered the study of the pathogenicity of this organism for many years. However, the recently developed rodent model is promising and should lead to further research in this area (8). Three criteria of Koch's postulates were fulfilled by using this animal model. All mice inoculated with D. fragilis became infected, in contrast to the negative controls, which remained uninfected. In these experiments, infected rodent groups produced unformed stools and experienced statistically significant weight loss. Histopathology of infected tissue demonstrated a mild inflammatory response compared to that of the control group. Fecal calprotectin levels were also more than two times higher in the infected group than in uninfected controls. Additionally, polymorphonuclear white blood cells were microscopically detected in the feces of infected rodents. Finally, cysts recovered from the feces of infected mice could establish a new infection in naive mice and rats when administered orally (8). Infectious doses were as low as ∼600 trophozoites per mouse. All of these data would certainly indicate not only the highly infectious nature of the organism but also that D. fragilis is pathogenic in a rodent animal model. Chronic infections were also documented in these animal studies, with some infections lasting for 6 months, until the completion of the experiment.
With the recent publication of the D. fragilis transcriptome, several potential markers of pathogenicity were described, including amoebapore-like proteins and putative immunomodulatory proteins. However, the most abundant virulence factor transcripts detected were members of the cathepsin L-like cysteine protease family. Many of the cysteine protease transcripts identified in D. fragilis closely resembled cytotoxic cysteine proteases previously identified as virulence factors in T. vaginalis (41, 45). The role of cysteine proteases in E. histolytica virulence has been clearly demonstrated in overexpression studies (180). Transfection of E. histolytica with vectors that induced the overexpression of certain cysteine proteases increased their capacity to produce amoebic liver abscesses in laboratory animals (181). Similarly, E. histolytica isolates initially incapable of producing liver abscesses in laboratory animals were able to do so when overexpression of certain cysteine protease genes was induced (181).
Infections with E. histolytica have various clinical outcomes, and certain isolates are less likely to cause disease than others (182). Transcriptome studies of virulent and avirulent E. histolytica strains have identified differences in expression levels of certain genes between isolates, including cysteine proteases, lectins, and calmodulin (182). Transcripts encoding these proteins were identified in D. fragilis (41). While the identification of potential virulence factor transcripts does not confirm pathogenicity, these sequences provide a lead for future study. Asymptomatic carriage of D. fragilis has been reported in multiple studies (183), and it would be valuable to obtain laboratory isolates of these putative avirulent strains for direct comparison to those associated with illness. Comparing the transcriptomes of each isolate could determine whether the expression levels of key virulence proteins differ between these isolates. Similarly, studies that induce the overexpression of proteases and other key virulence proteins in D. fragilis could shed light on the pathogenic potential of this organism and identify likely mechanisms of pathogenicity.
PATHOLOGY
Due to limited research on the pathology of D. fragilis infections, information regarding the pathogenesis and pathology resulting from D. fragilis infection is minimal. The first pathological findings associated with D. fragilis infection were reported in 1954 from four patients who presented with appendicitis (184). They reported histopathological changes including lymphoid hyperplasia, organized and acute periappendicitis, catarrhal appendicitis, fibrosis of the appendix, and numerous D. fragilis trophozoites. It was postulated by these researchers that “D. fragilis elaborates a low-grade irritation that induces an inflammatory response” that causes fibrosis (184). These findings have not been substantiated by other researchers. The authors of this report also note the presence of red blood cells in trophozoites of D. fragilis within the appendix (184). Dobell was actually the first to note the erythrophagocytic nature of D. fragilis (2), a phenomenon also observed in later years in other studies (185). The only other additional study to look for D. fragilis within the appendix found that 4.8% of 414 appendices examined histopathologically were infected with D. fragilis (n = 20) trophozoites (186). That study failed to demonstrate any characteristic histopathology, and no fibrosis was found in any of the cases (186).
As discussed above, D. fragilis has also been associated with three cases of colitis. The most recent of these was a case of eosinophilic colitis in a female 4-year-old child who had a history of chronic diarrhea (104). Another case report described a Burmese woman who presented with ulcerative colitis (129). The third case of ulcerative colitis was documented in Canada in a 9-year-old boy (130). In contrast to these findings, a recent Scandinavian study found no association between active colitis and D. fragilis in 100 patients with IBD (148).
Kean and Malloch undertook experiments in 1966 to produce a D. fragilis infection in the cecum of rats. From preliminary studies, they reported that “edema of the mucosa was evident.” They also demonstrated that Dientamoeba trophozoites attach to the cecal mucosa and in doing so damage the underlying cells. However, no direct invasion of cells or ulceration was evident. Interestingly, no further details were given in that report (112). It was not until 47 years later that researchers would try replicating the rodent model of D. fragilis infection (8). Dientamoeba-infected mice were euthanized and underwent histopathological examination, which revealed minor pathological changes, including slight inflammation of the submucosa of the large intestine (Fig. 8). These inflammatory changes were confirmed by measuring levels of fecal calprotectin, a marker of inflammatory and neoplastic disease of the lower GI tract, with average readings taken over 28 days showing levels over twice those of uninfected controls (8). Infected mice and rats also exhibited statistically significant weight loss compared to controls. Stool samples from each infected group were unformed, and white cells were detected via microscopy, which were absent in the uninfected control groups (8). Chronic infections were also noted in these mice, with continued shedding of parasites being noted for 6 months, when the study was concluded. This evidence certainly suggests that D. fragilis is a pathogen of rodents, although the long-term effect that chronic D. fragilis infections are likely to have on the gut mucosa is yet to be determined.
FIG 8.
Intact, healthy submucosa of the large intestine of negative-control mice (A) compared to mice infected with D. fragilis (B). Infected mice exhibit mild inflammation and damage to the mucosal layer.
The lack of laboratory animal models has severely hampered past attempts to study the clinical manifestations of D. fragilis infection. Various animal species, including macaques, chickens, and kittens, have all been used as potential animal models of dientamoebiasis, with little or no success (2, 111, 112). The recent establishment of a rodent model of dientamoebiasis represents a great success for the field and will facilitate future studies on the pathogenesis of dientamoebiasis.
THERAPY
Numerous studies have demonstrated that antimicrobial treatment that leads to Dientamoeba eradication usually relieves clinical symptoms (187). As such, treatment of symptomatic patients for whom all other causes of gastrointestinal disease are ruled out is warranted. Despite the abundance of reports in the scientific literature highlighting the high incidence of D. fragilis carriage in humans, very little research has been conducted on the efficacy of various suitable antimicrobial compounds for the treatment of D. fragilis infections (Table 5). Several compounds, including carbarsone, clioquinol, diphetarsone, doxycycline, erythromycin, iodoquinol, metronidazole, tinidazole, ornidazole, oxytetracycline, paromomycin, secnidazole, and tetracycline, have been reported as effective treatments for dientamoebiasis (187). It should be noted, however, that no randomized controlled trials on the efficacy of these compounds using a statistically significant sample size have been undertaken. Furthermore, most of these reports are case studies, include only a small number of subjects, and fail to utilize adequate control groups. To adequately address this issue, large-scale, randomized, double-blind, controlled trials using all currently registered antimicrobial agents used in the treatment of Dientamoeba infections are needed. Until such time as these studies are undertaken, it is difficult to comment as to what are the most effective therapeutic agents for the treatment of dientamoebiasis. It should also be noted that since infections by other intestinal protozoa (including Blastocystis spp., Giardia, and E. histolytica) are also treated using many of the same drugs as those used for D. fragilis infection, many reported studies do not clarify which diagnostic tests were used to identify D. fragilis before and after therapy. Thus, some of the data may be questionable.
TABLE 5.
Treatment studies incorporating study sizes of >10 patients, treatment efficacy, and dosage summaries
| Antiparasitic agent | Treatment efficacy(ies) (%) (no. of patients) | Dosage(s)a | Reference |
|---|---|---|---|
| Clioquinol | 81.5 (27) | 40 mg/kg of body wt/day for 10–21 days | 190 |
| 83 (12) | 250 mg t.i.d. for 7 days | 193 | |
| Iodoquinol | 83.3 (12) | 650 mg p.o. t.i.d. for 20 days | 138 |
| Paromomycin | 98 (61) | 500 mg t.i.d. for 7 days | 193 |
| 80 for parasite clearance and 87 for clinical improvement (15) | 25–35 mg/kg daily for 4–5 days | 192 | |
| Metronidazole | 70 (91) | 30 mg/kg/day p.o. for 10 days | 103 |
| 69.6 for parasite eradication and 76.8 for clinical improvement (56) | 20 mg/kg for children and 1.5 g for adults daily | 194 | |
| 80 (35) | 400–750 mg p.o. every 8 h or daily for 3–10 days | 106 | |
| 66.7 (15) | 500–750 mg p.o. t.i.d. for 10 days | 123 | |
| Secnidazole | 97 for parasite eradication and 100 for clinical improvement (35) | 30 mg/kg for child stat dose,b 2 g for adult stat dose | 121 |
| Ornidazole | 92.9 for parasite eradication and 96.4 for clinical improvement (56) | 30 mg/kg for child stat dose, 2 g for adult stat dose | 194 |
t.i.d., three times daily; p.o., orally.
In medical terminology, a stat dose is a dose that must be administered immediately.
Arsenic-Based Compounds
Early case reports demonstrated that antiamoeba compounds commonly used in the early 20th century, including emetine-bismuth-iodine and the arsenic compound carbarsone, were effective for the treatment of D. fragilis infections (105). Hakansson was one of the first parasitologists to advocate the use of antimicrobials for the treatment of D. fragilis. He successfully treated himself and 12 patients with the arsenic compound carbarsone (56, 57). Knoll and Howell (111) administered carbarsone to three children and three adults with acute and chronic D. fragilis infections. In all patients, the clinical symptoms improved quickly after treatment. Despite its efficacy in eradicating D. fragilis, carbarsone is no longer available for human use. The newer arsenic compound diphetarsone showed 100% efficacy (188). Despite being a widely used first-line treatment for intestinal amoebiasis, side effects associated with the use of arsenicals, such as encephalopathy, polyneuritis, visual disturbances, and dermatitis, led to the drug being removed from clinical practice and not licensed for human use (188).
Tetracycline and Erythromycin
Tetracycline is one antimicrobial currently recommended by the Centers for Disease Control and Prevention for the treatment of dientamoebiasis. However, this recommendation is based on only three case reports. Therefore, the use of tetracycline for treatment has a very weak basis. Dardick (189) recommended tetracycline for D. fragilis treatment based on a single case report of a patient who was successfully treated with this drug. Two other studies, one comprising a single patient (143) and another that compared oxytetracycline and doxycycline treatments in 13 patients without the use of an adequate control group (117), also recommended the use of these agents. No large-scale studies have examined the efficacy of tetracycline for eradicating D. fragilis, and until such evaluations are undertaken, one cannot recommend the use of tetracycline as a treatment option with much confidence. Only one small-scale study comprising 6 pediatric patients looked at the use of erythromycin for Dientamoeba treatment. Preiss et al. (103) reported resolution of clinical symptoms and eradication of the parasite in 50% (3/6) of patients treated with this compound. As such, no conclusions can be made regarding the efficacy of erythromycin treatment with data from a single case report. Both agents have worldwide availability.
Iodoquinol
Iodoquinol is commonly used to treat D. fragilis infections, particularly in the United States (187). It is commonly used as a luminal amoebicide, but its exact mode of action is unknown. Spencer et al. were the first to report the use of iodoquinol for treatment of dientamoebiasis (113). Spencer and colleagues concluded that therapy with iodoquinol or metronidazole was effective based on a study involving 18 pediatric patients, although the therapeutic efficacy of the drugs used individually was not confirmed. Millet et al. (138) treated 12 patients with D. fragilis infection with iodoquinol, and elimination of the parasite was achieved in 10 of the 12 patients. Cuffari et al. (104) treated five patients with iodoquinol, with clinical improvement being shown for four patients. In contrast to these reports, Preiss et al. (103) reported treating five children with iodoquinol, with only 1/5 responding to treatment. It should be noted that a low dosage and a short duration of therapy were used in this study compared to previous studies. Clioquinol, a compound closely related to iodoquinol, is reportedly an effective treatment for D. fragilis infection. Bosman et al. (190) reported that this agent was successful in treating 27/33 pediatric patients with D. fragilis infection. Iodoquinol is readily available throughout North and South America; however, it has limited availability in the United Kingdom, Europe, and Australasia.
Paromomycin
The aminoglycoside paromomycin was first reported as an agent for the treatment of dientamoebiasis in 1967, with 21/21 cases responding to treatment (191). A more recent study reported that four pediatric patients were clinically and parasitologically cured after treatment with paromomycin (123). A larger study involving 15 pediatric patients with dientamoebiasis who were treated with paromomycin with follow-up 1 month later noted parasite elimination and complete resolution of symptoms, leading the authors of this study to state that paromomycin was a viable treatment option (192). An additional small case series report involving five symptomatic patients treated with paromomycin showed that treatment led to parasite clearance and clinical improvement in all patients (106). The only large study comprised a cohort of 93 patients who were retrospectively analyzed. Paromomycin was given to 61 patients, and eradication rates of 98% were reported (193). Paromomycin has worldwide availability.
Metronidazole
Of all the treatment options available for D. fragilis infections, studies reporting the use of metronidazole are the most discordant. While metronidazole was effective in some studies, several others describe it as being suboptimal for the treatment of dientamoebiasis (187). To complicate the issue further, most studies reporting on the use of metronidazole are small-scale case reports, and information on treatment duration and dosage is often incomplete. This makes the results difficult to interpret. In a study from New Zealand (125), three patients infected with Dientamoeba were treated with metronidazole. Success was achieved in 2/3 patients; however, 1 patient needed a second course of metronidazole in combination with oxytetracycline to achieve parasite clearance and resolution of symptoms (125). The largest cohort of patients treated with metronidazole was from Sweden (120), with 32 patients infected with D. fragilis being enrolled in the study. It was reported that only four patients responded to the treatment (120); however, no details were given as to the exact dosages or duration of treatment, so it is difficult to comment on the clinical effect of metronidazole in these circumstances. Vandenberg et al. (123) reported that 12/15 patients responded to metronidazole treatment with both parasitological and clinical cure; however, no specific information on dosage and duration was provided in this study.
Preiss et al. (117) were the first to report on the use of metronidazole in a large cohort of 123 pediatric patients infected with D. fragilis. These investigators reported a treatment efficacy of 70% with a single dose, while 30% of patients required up to three additional doses to eliminate D. fragilis and the accompanying abdominal complaints. These investigators subsequently recommended a 10-day course of metronidazole for D. fragilis infections. An Australian study showed that metronidazole was effective in 80% of patients, with 20% showing treatment failure/relapses (106). Metronidazole is commonly available throughout the world.
Secnidazole and Ornidazole
Recently, newer 5-nitroimidazole derivatives have become available. These compounds have the advantage of requiring only a single oral stat dose and having fewer side effects than metronidazole. A Turkish study evaluated the use of secnidazole in 35 patients with D. fragilis infection (121). Eradication of the parasite occurred in 34 patients with a stat dose; the remaining patient required a second dose, whose infection was subsequently resolved. This suggests that secnidazole may be effective in treating dientamoebiasis (121).
Ornidazole, another newer 5-nitroimidazole, was used in a randomized double-blind study comparing the efficacies of metronidazole and ornidazole in 112 patients with D. fragilis infection (194). Ornidazole outperformed metronidazole by achieving both clinical cure (96.4%) and parasite eradication (92.9%). Only 6 patients recorded minor side effects in the ornidazole treatment group, as opposed to 18 in the metronidazole group. However, sourcing these newer 5-nitroimidazole derivatives may be difficult in some areas.
In Vitro Susceptibility Testing
The parasitologist W. Balamuth was the first to perform antimicrobial studies on D. fragilis in 1953 (195), using six ameobicidal compounds available at the time: emetine-bismuth-iodine, clioquinol, carbarsone, prodigiosin, chlortetracycline (Aureomycin), and a chemical derivative of carbarsone. The arsenical compounds showed good activity against D. fragilis in a monophasic medium (195). However, none of these drugs are licensed for human use today.
Susceptibility testing of D. fragilis ATCC 30948 was performed with modern drug options, including iodoquinol, paromomycin, tetracycline, and metronidazole, in a dixenic culture system (93). The MICs were as follows: 128 μg/ml for iodoquinol, 16 μg/ml for paromomycin, 32 μg/ml for tetracycline, and 32 μg/ml for metronidazole. It is difficult to correlate these MICs to clinical responses, as this study was undertaken using a dixenic culture system containing Klebsiella pneumoniae and Bacteroides vulgatus. It should be noted that the ATCC strain belongs to SSU ribosomal DNA (rDNA) genotype 2, while the majority of clinical isolates belong to the genotype 1 subpopulation (31, 127).
Nagata et al. (196) performed susceptibility testing on four clinical isolates (SSU rDNA genotype 1) of D. fragilis from Sydney, Australia. Diloxanide furoate, furazolidone, iodoquinol, metronidazole, nitazoxanide, ornidazole, paromomycin, secnidazole, ronidazole, tetracycline, and tinidazole were tested. The 5-nitroimidazole derivatives were the most active compounds against D. fragilis in vitro, with minimum lethal concentrations as follows: 8 to 16 μg/ml for ornidazole and ronidazole, 31 μg/ml for metronidazole and tinidazole, 31 to 63 μg/ml for secnidazole, 63 μg/ml for nitazoxanide, 250 μg/ml for tetracycline, 250 to 500 μg/ml for furazolidone, 500 μg/ml for iodoquinol, 500 μg/ml for paromomycin, and >500 μg/ml for diloxanide furoate. Once again, these studies were undertaken using xenic culture systems containing bacterial flora derived from patients' stool specimens. The absence of an axenic culture system makes interpretation of in vitro susceptibility results difficult. The antimicrobial action of these compounds against the bacteria in the cultures could have an indirect negative impact on D. fragilis growth, as the parasites use these bacteria as a source of nutrition (196).
Barratt et al. (197) evaluated the in vitro antiprotozoal activities of various dry plant extracts commonly used as alternate therapies against parasitic infections. They found that pomegranate peel extract, garlic extract, goldenseal extract, black walnut extract, wormwood extract, wormseed extract, pumpkin seed extract, grapefruit seed extract, and ginger root extract all had no effect on D. fragilis.
The activity of several benzimidazoles against D. fragilis was tested in vitro using four clinical isolates (198). Susceptibility testing showed that albendazole, flubendazole, mebendazole, nocodazole, triclabendazole, and thiabendazole had no effect against Dientamoeba. Two beta-tubulin transcripts were sequenced from a clinical isolate of D. fragilis genotype 1, and the amino acid sequences of each beta-tubulin predicted susceptibility to benzimidazoles. This suggests that beta-tubulin sequences cannot be used as reliable markers for prediction of resistance to benzimidazoles in trichomonads (198). It should be noted that while Trichomonas and Giardia are susceptible to benzimidazoles, H. meleagridis is resistant, although the exact mechanism of resistance remains unknown (198).
CONCLUSIONS
Numerous clinical and epidemiological studies have confirmed that D. fragilis is the most prevalent trichomonad parasite that infects humans. It is therefore inexcusable that so little research has been undertaken on this peculiar organism. As debate still surrounds the pathogenic potential of D. fragilis, more research is needed. Arguably, of all the potentially pathogenic protozoa that infect humans, the one that we know the least about is Dientamoeba. Its life cycle and mode of transmission are poorly defined. The pathogenesis of dientamoebiasis and its exact mode of action are unknown. No axenic culture systems are available for this organism, which impedes future research. The diagnostic tests available for D. fragilis are limited compared to those for other protozoa, and the tests that are available are in limited use. Molecular epidemiological studies on D. fragilis are warranted. At least two distinct 18S rDNA subtypes of D. fragilis exist, and differences in clinical outcome reported by various investigators suggest that there is still greater genetic diversity to be found. Further animal studies would facilitate an improved understanding of not only the life cycle and transmission of D. fragilis but also the pathogenesis of dientamoebiasis. While recent progress has been made on these aspects, there is still a great deal of work to be done.
Clifford Dobell, one of the parasitologists to first describe D. fragilis, dedicated most of his working life to the research of this peculiar organism. He wrote the following statement in reference to D. fragilis in 1940 (2):
To the protozoologist—if not to the physician—Dientamoeba fragilis is now, perhaps, the most interesting of all the intestinal amoebae of Man: for we know less about it than about any of the others, and its life-history and activities are still mysterious.
Ever since I first saw this curious organism, in 1917, I have been intrigued by its peculiarities, and have taken every opportunity of studying it further: yet after more than 20 years' work and cogitation I am still baffled….
Over 75 years have passed since these words were written, and remarkably, very little has changed. Our group has been researching this parasite for over 12 years now, and we too remain baffled, as with every discovery made, more questions arise. Subsequently, we remain immensely curious about this parasite and hope to continue our work on Dientamoeba in order to address these questions, such that our understanding of this organism continues to improve.
ACKNOWLEDGMENTS
This work was supported jointly by St Vincent's Hospital, Sydney, and the University of Technology, Sydney.
We dedicate the manuscript to Cecil Clifford Dobell (1886 to 1949), a parasitologist who dedicated most of his working life toward Dientamoeba. Much of what we still know today about this peculiar parasite is directly attributed to this man, and for that we thank him.
Cecil Clifford Dobell, to whom this paper is dedicated.
Biographies

Damien Stark is a Senior Hospital Scientist in the Microbiology department at St. Vincent's Hospital in Sydney, Australia. His qualifications include a B.Sc., a M.Sc., and a Ph.D. He is also a Fellow of the Australian Society of Microbiology, Australian College of Tropical Medicine, and a Fellow of the Faculty of Science of the Royal College of Pathologists Australasia. He has over 20 years of experience in clinical microbiology laboratories. His research interests include clinical parasitology with a particular interest in Dientamoeba fragilis.

Joel Barratt was appointed as a Chancellor's Postdoctoral Fellow at the University of Technology, Sydney (UTS), in 2014 and works in close collaboration with Damien Stark at St. Vincent's Hospital Sydney and under the supervision of Professor John Ellis. He completed his honors degree at the UTS under the supervision of Professor John Ellis on the protozoan parasite Neospora caninum. He completed his Ph.D. under the supervision of Professor John Ellis and Dr. Damien Stark on various aspects of Dientamoeba fragilis. As a Chancellor's Postdoctoral Fellow, he is involved in several projects in the areas of clinical and veterinary parasitology, including projects on the parasites Neospora caninum and Dientamoeba fragilis and the trypanosomatid parasites.

Douglas Chan is currently a Ph.D. student in the School of Life Sciences at the University of Technology, Sydney. In 2014, he graduated with a bachelor of Science (Honors) in Bioscience at the University of Technology, Sydney, for his research on the rat lungworm, Angiostrongylus cantonensis. He is currently completing a Ph.D. under the supervision of Professor John Ellis, Dr. Damien Stark, and Dr. Joel Barratt on a project which aims to study the transmission pathways employed by Dientamoeba fragilis.

John T. Ellis completed a Ph.D. on leishmaniasis with Professor J. M. Crampton at the Liverpool School of Tropical Medicine in 1986 and subsequently did postdoctoral research on Eimeria vaccines at Houghton Poultry Research Station and parasite phylogeny at the Flinders University of South Australia. He was appointed to the academic staff of the UTS in 1992 as a Lecturer in Microbiology. Over the last 24 years, he has continued to study parasitic protozoa of veterinary and medical importance. He is currently Professor of Molecular Biology, and his present research interests include the development of vaccines and diagnostics for protozoal diseases of economic importance. He was awarded the degree of D.Sc. by Liverpool University in 2006 for his pioneering research on cyst-forming coccidia. He is a Fellow of the Royal Society for Tropical Medicine and Hygiene and is an editor for the journal Parasitology and the Journal of Medical Microbiology.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CMR.00076-15.
REFERENCES
- 1.Jepps MW, Dobell C. 1918. Dientamoeba fragilis n.g., n. sp.: a new intestinal amoeba from man. Parasitology 10:352–367. [Google Scholar]
- 2.Dobell C. 1940. Researches on intestinal protozoa in monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments and speculations. Parasitology 32:417–461. [Google Scholar]
- 3.Hess M, Liebhart D, Bilic I, Ganas P. 2015. Histomonas meleagridis—new insights into an old pathogen. Vet Parasitol 208:67–76. doi: 10.1016/j.vetpar.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Ockert G, Schneider W. 1974. Electron microscopic findings in Dientamoeba fragilis. Z Gesamte Hyg 20:555–557. (In German.). [PubMed] [Google Scholar]
- 5.Silard R, Burghelea B, Panaitescu D, Burcos V. 1984. Ultrastructure of Dientamoeba fragilis: a study of the mononucleated stage. Arch Roum Pathol Exp Microbiol 43:87–101. [PubMed] [Google Scholar]
- 6.Gerbod D, Edgcomb VP, Noel C, Zenner L, Wintjens R, Delgado-Viscogliosi P, Holder ME, Sogin ML, Viscogliosi E. 2001. Phylogenetic position of the trichomonad parasite of turkeys, Histomonas meleagridis (Smith) Tyzzer, inferred from small subunit rRNA sequence. J Eukaryot Microbiol 48:498–504. doi: 10.1111/j.1550-7408.2001.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 7.Banik GR, Birch D, Stark D, Ellis JT. 2012. A microscopic description and ultrastructural characterisation of Dientamoeba fragilis: an emerging cause of human enteric disease. Int J Parasitol 42:139–153. doi: 10.1016/j.ijpara.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Munasinghe VS, Vella NG, Ellis JT, Windsor PA, Stark D. 2013. Cyst formation and faecal-oral transmission of Dientamoeba fragilis—the missing link in the life cycle of an emerging pathogen. Int J Parasitol 43:879–883. doi: 10.1016/j.ijpara.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Kofoid CA, Kornhauser SI, Plate JT. 1919. Intestinal parasites in overseas and home service troops of the U.S. Army. JAMA 72:1721–1724. [Google Scholar]
- 10.Haughwout FG, Horrilleno FS. 1920. The intestinal animal parasites found in one hundred sick Filipino children. Philipp J Sci 16:1–73. [Google Scholar]
- 11.Robertson A. 1923. Specimens from a human case of infection with Dientamoeba fragilis, Jepps and Dobell, 1917. Proc R Soc Med 16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenrich DH. 1944. Studies on Dientamoeba fragilis (Protozoa). IV. Further observations, with an outline of present days knowledge of this species. J Parasitol 30:322–338. [Google Scholar]
- 13.Gasse PP. 1953. Famille des Dientamoebidae Grasse, nov. (E. Chatton, principal author: ordre des amoebiens nus ou Amoebaea), p 50–54. In Grasse PP. (ed), Traite de zoologie. Masson et Cie, Paris, France. [Google Scholar]
- 14.Bird RG, Sargeaunt P, Upton CP. 1970. Uni- and binucleate trophozoites of Dientamoeba fragilis. Trans R Soc Trop Med Hyg 64:18. [PubMed] [Google Scholar]
- 15.Dwyer DM, Honigberg BM. 1972. Immunologic analysis by quantitative fluorescent antibody methods of effects of prolonged cultivation on Histomonas meleagridis (Smith). Z Parasitenkd 39:39–52. doi: 10.1007/BF00329219. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer DM. 1972. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba, and Entamoeba. I Quantitative fluorescent antibody methods. J Protozool 19:316–325. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer DM. 1972. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba, and Entamoeba. II. Gel diffusion methods. J Protozool 19:326–332. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer DM. 1974. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba and Entamoeba. III. Immunoelectrophoresis technics. J Protozool 21:139–145. doi: 10.1111/j.1550-7408.1974.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 19.Camp RR, Mattern CF, Honigberg BM. 1974. Study of Dientamoeba fragilis Jepps & Dobell. I. Electronmicroscopic observations of the binucleate stages. II. Taxonomic position and revision of the genus. J Protozool 21:69–82. [DOI] [PubMed] [Google Scholar]
- 20.Levine ND, Corliss JO, Cox FE, Deroux G, Grain J, Honigberg BM, Leedale GF, Loeblich AR III, Lom J, Lynn D, Merinfeld EG, Page FC, Poljansky G, Sprague V, Vavra J, Wallace FG. 1980. A newly revised classification of the protozoa. J Protozool 27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- 21.Silberman JD, Clark CG, Sogin ML. 1996. Dientamoeba fragilis shares a recent common evolutionary history with the trichomonads. Mol Biochem Parasitol 76:311–314. doi: 10.1016/0166-6851(95)02516-2. [DOI] [PubMed] [Google Scholar]
- 22.Delgado-Viscogliosi P, Viscogliosi E, Gerbod D, Kulda J, Sogin ML, Edgcomb VP. 2000. Molecular phylogeny of parabasalids based on small subunit rRNA sequences, with emphasis on the Trichomonadinae subfamily. J Eukaryot Microbiol 47:70–75. doi: 10.1111/j.1550-7408.2000.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 23.Malik SB, Brochu CD, Bilic I, Yuan J, Hess M, Logsdon JM Jr, Carlton JM. 2011. Phylogeny of parasitic parabasalia and free-living relatives inferred from conventional markers vs. Rpb1, a single-copy gene. PLoS One 6:e20774. doi: 10.1371/journal.pone.0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda S, Mantini C, Meloni D, Inoue J, Kitade O, Viscogliosi E, Ohkuma M. 2012. Molecular phylogeny and evolution of parabasalia with improved taxon sampling and new protein markers of actin and elongation factor-1alpha. PLoS One 7:e29938. doi: 10.1371/journal.pone.0029938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cepicka I, Hampl V, Kulda J. 2010. Critical taxonomic revision of parabasalids with description of one new genus and three new species. Protist 161:400–433. doi: 10.1016/j.protis.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2:3–12. doi: 10.4161/gmic.2.1.14755. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EH, Windsor JJ, Clark CG. 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin Microbiol Rev 17:553–570. doi: 10.1128/CMR.17.3.553-570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windsor JJ, Clark CG, Macfarlane L. 2004. Molecular typing of Dientamoeba fragilis. Br J Biomed Sci 61:153–155. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JA, Clark CG. 2000. Cryptic genetic diversity in Dientamoeba fragilis. J Clin Microbiol 38:4653–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int J Parasitol 35:57–62. doi: 10.1016/j.ijpara.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Peek R, Reedeker FR, van Gool T. 2004. Direct amplification and genotyping of Dientamoeba fragilis from human stool specimens. J Clin Microbiol 42:631–635. doi: 10.1128/JCM.42.2.631-635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stensvold CR, Clark CG, Roser D. 2013. Limited intra-genetic diversity in Dientamoeba fragilis housekeeping genes. Infect Genet Evol 18:284–286. doi: 10.1016/j.meegid.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Barratt JL, Banik GR, Harkness J, Marriott D, Ellis JT, Stark D. 2010. Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137:1867–1878. doi: 10.1017/S0031182010000764. [DOI] [PubMed] [Google Scholar]
- 34.Windsor JJ, Macfarlane L, Clark CG. 2006. Internal transcribed spacer dimorphism and diversity in Dientamoeba fragilis. J Eukaryot Microbiol 53:188–192. doi: 10.1111/j.1550-7408.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 35.Bart A, van der Heijden HM, Greve S, Speijer D, Landman WJ, van Gool T. 2008. Intragenomic variation in the internal transcribed spacer 1 region of Dientamoeba fragilis as a molecular epidemiological marker. J Clin Microbiol 46:3270–3275. doi: 10.1128/JCM.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stark D, Barratt J, Ellis J, Harkness J, Marriott D. 2009. Repeated Dientamoeba fragilis infections: a case report of two families from Sydney, Australia. Infect Dis Rep 1:e4. doi: 10.4081/idr.2009.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussein EM, Al-Mohammed HI, Hussein AM. 2009. Genetic diversity of Dientamoeba fragilis isolates of irritable bowel syndrome patients by high-resolution melting-curve (HRM) analysis. Parasitol Res 105:1053–1060. doi: 10.1007/s00436-009-1515-9. [DOI] [PubMed] [Google Scholar]
- 38.Caccio SM, Sannella AR, Manuali E, Tosini F, Sensi M, Crotti D, Pozio E. 2012. Pigs as natural hosts of Dientamoeba fragilis genotypes found in humans. Emerg Infect Dis 18:838–841. doi: 10.3201/eid1805.111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willhoeft U, Hamann L, Tannich E. 1999. A DNA sequence corresponding to the gene encoding cysteine proteinase 5 in Entamoeba histolytica is present and positionally conserved but highly degenerated in Entamoeba dispar. Infect Immun 67:5925–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond LS, Clark CG. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol 40:340–344. doi: 10.1111/j.1550-7408.1993.tb04926.x. [DOI] [PubMed] [Google Scholar]
- 41.Barratt JL, Cao M, Stark DJ, Ellis JT. 2015. The transcriptome sequence of Dientamoeba fragilis offers new biological insights on its metabolism, kinome, degradome and potential mechanisms of pathogenicity. Protist 166:389–408. doi: 10.1016/j.protis.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM Jr, Henze K, Gupta A, et al. 2007. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusdian G, Woehle C, Martin WF, Gould SB. 2013. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell Microbiol 15:1707–1721. doi: 10.1111/cmi.12144. [DOI] [PubMed] [Google Scholar]
- 44.Noel CJ, Diaz N, Sicheritz-Ponten T, Safarikova L, Tachezy J, Tang P, Fiori PL, Hirt RP. 2010. Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics 11:99. doi: 10.1186/1471-2164-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barratt J, Stark D, Ellis J. 2012. Cytotoxic and proteolytic molecules of the human parasite Dientamoeba fragilis, identified by RNA seq, provide support for its pathogenic capacity. Toxicon 60:163–164. doi: 10.1016/j.toxicon.2012.04.135. [DOI] [Google Scholar]
- 46.Šlapeta J, Müller N, Stack CM, Walker G, Lew-Tabor A, Tachezy J, Frey CF. 2012. Comparative analysis of Tritrichomonas foetus (Riedmüller, 1928) cat genotype, T. foetus (Riedmüller, 1928) cattle genotype and Tritrichomonas suis (Davaine, 1875) at 10 DNA loci. Int J Parasitol 42:1143–1149. doi: 10.1016/j.ijpara.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Stark DJ, Beebe N, Marriott D, Ellis JT, Harkness J. 2006. Dientamoebiasis: clinical importance and recent advances. Trends Parasitol 22:92–96. doi: 10.1016/j.pt.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Bruijnesteijn van Coppenraet LE, Wallinga JA, Ruijs GJ, Bruins MJ, Verweij JJ. 2009. Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin Microbiol Infect 15:869–874. doi: 10.1111/j.1469-0691.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 49.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. Comparison of microscopy, two xenic culture techniques, conventional and real-time PCR for the detection of Dientamoeba fragilis in clinical stool samples. Eur J Clin Microbiol Infect Dis 29:411–416. doi: 10.1007/s10096-010-0876-4. [DOI] [PubMed] [Google Scholar]
- 50.Stark D, Al-Qassab SE, Barratt JL, Stanley K, Roberts T, Marriott D, Harkness J, Ellis JT. 2011. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J Clin Microbiol 49:257–262. doi: 10.1128/JCM.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verweij JJ, Mulder B, Poell B, van Middelkoop D, Brienen EAT, van Lieshout L. 2007. Real-time PCR for the detection of Dientamoeba fragilis in fecal samples. Mol Cell Probes 21:400–404. doi: 10.1016/j.mcp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Brug SL. 1938. Observations on Dientamoeba fragilis. Ann Trop Med Parasitol 30:441–452. [Google Scholar]
- 53.Wenrich DH. 1937. Studies on Dientamoneba fragilis (protozoa). II. Report of unusual morphology in one case with suggestions as to pathogenicity. J Parasitol 23:183–196. [Google Scholar]
- 54.Robinson GL, Ng PH. 1968. The size of Dientamoeba fragilis in culture. Trans R Soc Trop Med Hyg 62:156–158. doi: 10.1016/0035-9203(68)90076-X. [DOI] [PubMed] [Google Scholar]
- 55.Wenrich DH, Stabler RM, Arnett JH. 1935. Entamoeba histolytica and other intestinal protozoa in 1060 college freshmen. Am J Trop Med 15:331–345. [Google Scholar]
- 56.Hakansson EG. 1936. Dientamoeba fragilis, a cause of illness: report of a case. Am J Trop Med Hyg 1936:175–178. [Google Scholar]
- 57.Hakansson EG. 1937. Dientamoeba fragilis: some further observations. Am J Trop Med 17:349–362. [Google Scholar]
- 58.Sawangjaroen N, Luke R, Prociv P. 1993. Diagnosis by faecal culture of Dientamoeba fragilis infections in Australian patients with diarrhoea. Trans R Soc Trop Med Hyg 87:163–165. doi: 10.1016/0035-9203(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 59.Borges FP, Wiltuschnig RC, Tasca T, De Carli GA. 2004. Scanning electron microscopy study of Tritrichomonas augusta. Parasitol Res 94:158–161. doi: 10.1007/s00436-004-1189-2. [DOI] [PubMed] [Google Scholar]
- 60.Benchimol M, Monteiro S, Chang TH, Alderete JF. 2002. Virus in Trichomonas—an ultrastructural study. Parasitol Int 51:293–298. doi: 10.1016/S1383-5769(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 61.Wendel KA, Rompalo AM, Erbelding EJ, Chang TH, Alderete JF. 2002. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J Infect Dis 186:558–561. doi: 10.1086/341832. [DOI] [PubMed] [Google Scholar]
- 62.Stark D, Garcia LS, Barratt JL, Phillips O, Roberts T, Marriott D, Harkness J, Ellis JT. 2014. Description of Dientamoeba fragilis cyst and precystic forms from human samples. J Clin Microbiol 52:2680–2683. doi: 10.1128/JCM.00813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kofoid CA. 1923. Amoeba and man. University of California Press, Berkeley, CA. [Google Scholar]
- 64.Kudo R. 1926. Observations on Dientamoeba fragilis. Am J Trop Med Hyg 6:299–305. [Google Scholar]
- 65.Wenrich D. 1936. Studies on Dientamoeba fragilis (Protozoa). I. Observations with special reference to nuclear structure. J Parasitol 22:76–83. [Google Scholar]
- 66.Munsch M, Lotfi A, Hafez HM, Al-Quraishy S, Mehlhorn H. 2009. Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitol Res 104:683–689. doi: 10.1007/s00436-008-1246-3. [DOI] [PubMed] [Google Scholar]
- 67.Zaragatzki E, Hess M, Grabensteiner E, Abdel-Ghaffar F, Al-Rasheid KAS, Mehlhorn H. 2010. Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitol Res 106:977–983. doi: 10.1007/s00436-010-1777-2. [DOI] [PubMed] [Google Scholar]
- 68.Zaragatzki E, Mehlhorn H, Abdel-Ghaffar F, Rasheid KAS, Grabensteiner E, Hess M. 2010. Experiments to produce cysts in cultures of Histomonas meleagridis—the agent of histomonosis in poultry. Parasitol Res 106:1005–1007. doi: 10.1007/s00436-010-1776-3. [DOI] [PubMed] [Google Scholar]
- 69.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission. Parasitology 138:557–572. doi: 10.1017/S0031182010001733. [DOI] [PubMed] [Google Scholar]
- 70.Greenway D. 1928. Dientamoeba fragilis en la Argentina. Arch Argent Enferm Apar Dig 3:897. [Google Scholar]
- 71.Piekarski G. 1948. Zur Frage der Cystenbildung bei Dientamoeba fragilis. Z Hyg Infektionskr 127:496–500. [PubMed] [Google Scholar]
- 72.Clark CG, Roser D, Stensvold CR. 31 January 2014. Transmission of Dientamoeba fragilis: pinworm or cysts? Trends Parasitol doi: 10.1016/j.pt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Windsor JJ, Rafay AM. 1997. Laboratory detection of Dientamoeba fragilis. Br J Biomed Sci 54:223–224. [PubMed] [Google Scholar]
- 74.Yang J, Scholten T. 1977. Dientamoeba fragilis: a review with notes on its epidemiology, pathogenicity, mode of transmission, and diagnosis. Am J Trop Med Hyg 26:16–22. [DOI] [PubMed] [Google Scholar]
- 75.Taliaferro WH, Becker ER. 1924. A note on the human intestinal amoeba, Dientamoeba fragilis. Am J Hyg 4:71–74. [Google Scholar]
- 76.Burrows RB. 1967. A new fixative and technics for the diagnosis of intestinal parasites. Tech Bull Regist Med Technol 37:208–212. [PubMed] [Google Scholar]
- 77.Ockert G, Schulz U. 1972. Pathogenetic role of Dientamoeba fragilis. Dtsch Gesundheitsw 27:1156–1158. (In German.). [PubMed] [Google Scholar]
- 78.Walker JC, Bahr G, Ehl AS. 1985. Gastrointestinal parasites in Sydney. Med J Aust 143:480. [DOI] [PubMed] [Google Scholar]
- 79.Windsor JJ, Johnson EH. 1999. Dientamoeba fragilis: the unflagellated human flagellate. Br J Biomed Sci 56:293–306. [PubMed] [Google Scholar]
- 80.van Gool T, Weijts R, Lommerse E, Mank TG. 2003. Triple faeces test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur J Clin Microbiol Infect Dis 22:284–290. [DOI] [PubMed] [Google Scholar]
- 81.Ragavan AD, Govind SK. 2015. Modified Fields' stain: ideal to differentiate Dientamoeba fragilis and Blastocystis sp. Parasitol Res 114:1163–1166. doi: 10.1007/s00436-014-4296-8. [DOI] [PubMed] [Google Scholar]
- 82.Hiatt RA, Markell EK, Ng E. 1995. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am J Trop Med Hyg 53:36–39. [PubMed] [Google Scholar]
- 83.Boeck WC, Drbohlav J. 1925. The cultivation of Endamoeba histolytica. Proc Natl Acad Sci U S A 11:235–238. doi: 10.1073/pnas.11.5.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson GL. 1968. Laboratory cultivation of some human parasitic amoebae. J Gen Microbiol 53:69–79. doi: 10.1099/00221287-53-1-69. [DOI] [PubMed] [Google Scholar]
- 85.Dobell C, Laidlaw PP. 1926. On the cultivation of Entamoeba histolytica and some other entozoic amoeba. Parasitology 18:283–318. doi: 10.1017/S0031182000005278. [DOI] [Google Scholar]
- 86.Cleveland LR, Collier J. 1930. Various improvements in cultivation of Endamoeba histolytica. Am J Hyg 12:606–613. [Google Scholar]
- 87.Balamuth W. 1946. Improved egg yolk medium for cultivation of Entamoeba histolytica and other intestinal protozoa. Am J Hyg 16:380–384. [DOI] [PubMed] [Google Scholar]
- 88.Diamond LS. 1982. A new liquid medium for xenic cultivation of Entamoeba histolytica and other lumen-dwelling protozoa. J Parasitol 68:958–959. doi: 10.2307/3281016. [DOI] [PubMed] [Google Scholar]
- 89.Munasinghe VS, Stark D, Ellis JT. 2012. New advances in the in-vitro culture of Dientamoeba fragilis. Parasitology 139:864–869. doi: 10.1017/S0031182012000145. [DOI] [PubMed] [Google Scholar]
- 90.Windsor JJ, Macfarlane L, Hughes-Thapa G, Jones SK, Whiteside TM. 2003. Detection of Dientamoeba fragilis by culture. Br J Biomed Sci 60:79–83. [DOI] [PubMed] [Google Scholar]
- 91.Clark CG, Diamond LS. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan FT, Guan MX, Mackenzie AM. 1993. Application of indirect immunofluorescence to detection of Dientamoeba fragilis trophozoites in fecal specimens. J Clin Microbiol 31:1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan FT, Guan MX, Mackenzie AM, Diaz-Mitoma F. 1994. Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole. Antimicrob Agents Chemother 38:1157–1160. doi: 10.1128/AAC.38.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. 2014. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol 52:712–720. doi: 10.1128/JCM.02877-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2006. Evaluation of three diagnostic methods, including real-time PCR, for detection of Dientamoeba fragilis in stool specimens. J Clin Microbiol 44:232–235. doi: 10.1128/JCM.44.1.232-235.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Canale E, Biasolo MA, Tessari A, Bettanello S, Besutti V, Mengoli C. 2009. Real time PCR for Dientamoeba fragilis: a comparison between molecular and microscopical approach. Microbiol Med 24:133–138. [Google Scholar]
- 97.David E, Guimaraes S, de Oliveira A, Goulart de Oliveira-Sequeira T, Nogueira Bittencourt G, Moraes Nardi A, Martins Ribolla P, Bueno Franco R, Branco N, Tosini F, Bella A, Pozio E, Caccio S. 2015. Molecular characterization of intestinal protozoa in two poor communities in the State of Sao Paulo, Brazil. Parasit Vectors 8:103. doi: 10.1186/s13071-015-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarafraz S, Farajnia S, Jamali J, Khodabakhsh F, Khanipour F. 2013. Detection of Dientamoeba fragilis among diarrheal patients referred to Tabriz health care centers by nested PCR. Trop Biomed 30:113–118. [PubMed] [Google Scholar]
- 99.Julio C, Furtado C, Rocha R, Escobar C, Brito MJ, Oleastro M. 21 July 2015. Detection of Dientamoeba fragilis in Portuguese children with acute gastroenteritis between 2011 and 2013. Parasitology doi: 10.1017/S0031182015000906. [DOI] [PubMed] [Google Scholar]
- 100.Stark D, Roberts T, Ellis JT, Marriott D, Harkness J. 2014. Evaluation of the EasyScreen enteric parasite detection kit for the detection of Blastocystis spp., Cryptosporidium spp, Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagn Microbiol Infect Dis 78:149–152. doi: 10.1016/j.diagmicrobio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 101.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2013. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur J Clin Microbiol Infect Dis 32:1303–1310. doi: 10.1007/s10096-013-1880-2. [DOI] [PubMed] [Google Scholar]
- 102.Barry MA, Weatherhead JE, Hotez PJ, Woc-Colburn L. 2013. Childhood parasitic infections endemic to the United States. Pediatr Clin North Am 60:471–485. doi: 10.1016/j.pcl.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Preiss U, Ockert G, Broemme S, Otto A. 1991. On the clinical importance of Dientamoeba fragilis infections in childhood. J Hyg Epidemiol Microbiol Immunol 35:27–34. [PubMed] [Google Scholar]
- 104.Cuffari C, Oligny L, Seidman EG. 1998. Dientamoeba fragilis masquerading as allergic colitis. J Pediatr Gastroenterol Nutr 26:16–20. doi: 10.1097/00005176-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 105.Gittings JC, Waltz AD. 1927. Dientamoeba fragilis. Am J Dis Child 34:542–546. [Google Scholar]
- 106.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. A review of the clinical presentation of dientamoebiasis. Am J Trop Med Hyg 82:614–619. doi: 10.4269/ajtmh.2010.09-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borody TJ, Warren EF, Wettstein A, Robertson G, Recabarren P, Fontella A, Herdnman K, Surace R. 2002. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. J Gastroenterol Hepatol 17(Suppl):A103. [Google Scholar]
- 108.Krogsgaard LR, Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. 2015. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin Gastroenterol Hepatol 13:507–513. doi: 10.1016/j.cgh.2014.07.065. [DOI] [PubMed] [Google Scholar]
- 109.Sapero JJ, Johnson CM. 1939. Entamoeba histolytica and other intestinal parasites: incidence in variously exposed groups of the Navy. U S Nav Med Bull 37:297–301. [Google Scholar]
- 110.Hood M. 1940. Diarrhea caused by D fragilis. J Lab Clin Med 25:914–918. [Google Scholar]
- 111.Knoll EW, Howell KM. 1945. Studies on Dientamoeba fragilis; its incidence and possible pathogenicity. Am J Clin Pathol 15:178–183. doi: 10.1093/ajcp/15.5.178. [DOI] [PubMed] [Google Scholar]
- 112.Kean BH, Malloch CL. 1966. The neglected ameba: Dientamoeba fragilis. A report of 100 “pure” infections. Am J Dig Dis 11:735–746. [DOI] [PubMed] [Google Scholar]
- 113.Spencer MJ, Garcia LS, Chapin MR. 1979. Dientamoeba fragilis. An intestinal pathogen in children? Am J Dis Child 133:390–393. [PubMed] [Google Scholar]
- 114.Spencer MJ, Chapin MR, Garcia LS. 1982. Dientamoeba fragilis: a gastrointestinal protozoan infection in adults. Am J Gastroenterol 77:565–569. [PubMed] [Google Scholar]
- 115.Spencer MJ, Millet VE, Garcia LS, Rhee L, Masterson L. 1983. Parasitic infections in a pediatric population. Pediatr Infect Dis 2:110–113. doi: 10.1097/00006454-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Turner JA. 1985. Giardiasis and infections with Dientamoeba fragilis. Pediatr Clin North Am 32:865–880. [DOI] [PubMed] [Google Scholar]
- 117.Preiss U, Ockert G, Bromme S, Otto A. 1990. Dientamoeba fragilis infection, a cause of gastrointestinal symptoms in childhood. Klin Padiatr 202:120–123. doi: 10.1055/s-2007-1025503. [DOI] [PubMed] [Google Scholar]
- 118.Grendon JH, DiGiacomo RF, Frost FJ. 1995. Descriptive features of Dientamoeba fragilis infections. J Trop Med Hyg 98:309–315. [PubMed] [Google Scholar]
- 119.Windsor JJ, Rafay AM, Shenoy AK, Johnson EH. 1998. Incidence of Dientamoeba fragilis in faecal samples submitted for routine microbiological analysis. Br J Biomed Sci 55:172–175. [PubMed] [Google Scholar]
- 120.Norberg A, Nord CE, Evengard B. 2003. Dientamoeba fragilis—a protozoal infection which may cause severe bowel distress. Clin Microbiol Infect 9:65–68. doi: 10.1046/j.1469-0691.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- 121.Girginkardesler N, Coskun S, Cuneyt Balcioglu I, Ertan P, Ok UZ. 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin Microbiol Infect 9:110–113. doi: 10.1046/j.1469-0691.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 122.Crotti D, D'Annibale ML, Fonzo G, Lalle M, Caccio SM, Pozio E. 2005. Dientamoeba fragilis is more prevalent than Giardia duodenalis in children and adults attending a day care centre in central Italy. Parasite 12:165–170. doi: 10.1051/parasite/2005122165. [DOI] [PubMed] [Google Scholar]
- 123.Vandenberg O, Peek R, Souayah H, Dediste A, Buset M, Scheen R, Retore P, Zissis G, van Gool T. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int J Infect Dis 10:255–261. doi: 10.1016/j.ijid.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 124.Ogren J, Dienus O, Lofgren S, Einemo IM, Iveroth P, Matussek A. 15 July 2015. Dientamoeba fragilis prevalence coincides with gastrointestinal symptoms in children less than 11 years old in Sweden. Eur J Clin Microbiol Infect Dis doi: 10.1007/s10096-015-2442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oxner RB, Paltridge GP, Chapman BA, Cook HB, Sheppard PF. 1987. Dientamoeba fragilis: a bowel pathogen? N Z Med J 100:64–65. [PubMed] [Google Scholar]
- 126.Welch JS, Stuart JE. 1976. A longitudinal study of parasite infections in 120 Queensland Aboriginal children. Med J Aust 44:14–16. [DOI] [PubMed] [Google Scholar]
- 127.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. J Clin Microbiol 43:2718–2723. doi: 10.1128/JCM.43.6.2718-2723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Talis B, Stein B, Lengy J. 1971. Dientamoeba fragilis in human feces and bile. Isr J Med Sci 7:1063–1069. [PubMed] [Google Scholar]
- 129.Shein R, Gelb A. 1983. Colitis due to Dientamoeba fragilis. Am J Gastroenterol 78:634–636. [PubMed] [Google Scholar]
- 130.Ring FA, Hershfield NB, Machin GA, Scott RB. 1984. Sulfasalazine-induced colitis complicating idiopathic ulcerative colitis. Can Med Assoc J 131:43–45. [PMC free article] [PubMed] [Google Scholar]
- 131.Markell EK, Havens RF, Kuritsubo RA, Wingerd J. 1984. Intestinal protozoa in homosexual men of the San Francisco Bay area: prevalence and correlates of infection. Am J Trop Med Hyg 33:239–245. [DOI] [PubMed] [Google Scholar]
- 132.Ortega HB, Borchardt KA, Hamilton R, Ortega P, Mahood J. 1984. Enteric pathogenic protozoa in homosexual men from San Francisco. Sex Transm Dis 11:59–63. doi: 10.1097/00007435-198404000-00001. [DOI] [PubMed] [Google Scholar]
- 133.Peters CS, Sable R, Janda WM, Chittom AL, Kocka FE. 1986. Prevalence of enteric parasites in homosexual patients attending an outpatient clinic. J Clin Microbiol 24:684–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mendez OC, Szmulewicz G, Menghi C, Torres S, Gonzalez G, Gatta C. 1994. Comparison of intestinal parasite infestation indexes among HIV positive and negative populations. Medicina (B Aires) 54:307–310. (In Spanish.). [PubMed] [Google Scholar]
- 135.Chan F, Stewart N, Guan M, Robb I, Fuite L, Chan I, Diaz-Mitoma F, King J, MacDonald N, Mackenzie A. 1996. Prevalence of Dientamoeba fragilis antibodies in children and recognition of a 39 kDa immunodominant protein antigen of the organism. Eur J Clin Microbiol Infect Dis 15:950–954. doi: 10.1007/BF01690516. [DOI] [PubMed] [Google Scholar]
- 136.Naiman HL, Sekla L, Albritton WL. 1980. Giardiasis and other intestinal parasitic infections in a Manitoba residential school for the mentally retarded. Can Med Assoc J 122:185–188. [PMC free article] [PubMed] [Google Scholar]
- 137.Melvin DM, Brooke MM. 1962. Parasitologic surveys on Indian reservations in Montana, South Dakota, New Mexico, Arizona and Wisconsin. Am J Trop Med Hyg 11:765–772. [DOI] [PubMed] [Google Scholar]
- 138.Millet V, Spencer MJ, Chapin M, Stewart M, Yatabe JA, Brewer T, Garcia LS. 1983. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig Dis Sci 28:335–339. doi: 10.1007/BF01324950. [DOI] [PubMed] [Google Scholar]
- 139.Mollari M, Anzulovic JV. 1938. Cultivation and pathogencity of Dientamoeba fragilis, with a case report. J Trop Med Hyg 41:246–247. [Google Scholar]
- 140.Yoeli M. 1955. A report on intestinal disorders accompanied by large numbers of Dientamoeba fragilis. J Trop Med Hyg 58:38–41. [PubMed] [Google Scholar]
- 141.Steinitz H, Talis B, Stein B. 1970. Entamoeba histolytica and Dientamoeba fragilis and the syndrome of chronic recurrent intestinal amoebiasis in Israel. Digestion 3:146–153. doi: 10.1159/000197025. [DOI] [PubMed] [Google Scholar]
- 142.Chang SL. 1973. Parasitization of the parasite. JAMA 223:1510. doi: 10.1001/jama.1973.03220130054020. [DOI] [PubMed] [Google Scholar]
- 143.Butler WP. 1996. Dientamoeba fragilis. An unusual intestinal pathogen. Dig Dis Sci 41:1811–1813. [DOI] [PubMed] [Google Scholar]
- 144.de Wit MA, Koopmans MP, Kortbeek LM, van Leeuwen NJ, Vinjé J, van Duynhoven YT. 2001. Etiology of gastroenteritis in sentinel general practices in the Netherlands. Clin Infect Dis 33:280–288. doi: 10.1086/321875. [DOI] [PubMed] [Google Scholar]
- 145.Bruijnesteijn van Coppenraet LE, Dullaert-de Boer M, Ruijs GJ, van der Reijden WA, van der Zanden AG, Weel JF, Schuurs TA. 2015. Case-control comparison of bacterial and protozoan microorganisms associated with gastroenteritis: application of molecular detection. Clin Microbiol Infect 21:592.e9–592.e19. doi: 10.1016/j.cmi.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 146.de Jong MJ, Korterink JJ, Benninga MA, Hilbink M, Widdershoven J, Deckers-Kocken JM. 2014. Dientamoeba fragilis and chronic abdominal pain in children: a case-control study. Arch Dis Child 99:1109–1113. doi: 10.1136/archdischild-2014-305942. [DOI] [PubMed] [Google Scholar]
- 147.Engsbro AL, Stensvold CR, Vedel Nielsen H, Bytzer P. 2014. Prevalence, incidence, and risk factors of intestinal parasites in Danish primary care patients with irritable bowel syndrome. Scand J Infect Dis 46:204–209. doi: 10.3109/00365548.2013.861609. [DOI] [PubMed] [Google Scholar]
- 148.Petersen AM, Stensvold CR, Mirsepasi H, Engberg J, Friis-Moller A, Porsbo LJ, Hammerum AM, Nordgaard-Lassen I, Nielsen HV, Krogfelt KA. 2013. Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand J Gastroenterol 48:638–639. doi: 10.3109/00365521.2013.780094. [DOI] [PubMed] [Google Scholar]
- 149.Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. 17 December 2013. Prevalence, incidence, and risk factors of intestinal parasites in Danish primary care patients with irritable bowel syndrome. Scand J Infect Dis doi: 10.3109/00365548.2013.861609. [DOI] [PubMed] [Google Scholar]
- 150.Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. 2012. Treatment of Dientamoeba fragilis in patients with irritable bowel syndrome. Am J Trop Med Hyg 87:1046–1052. doi: 10.4269/ajtmh.2012.11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stensvold CR, Arendrup MC, Molbak K, Nielsen HV. 2007. The prevalence of Dientamoeba fragilis in patients with suspected enteroparasitic disease in a metropolitan area in Denmark. Clin Microbiol Infect 13:839–842. doi: 10.1111/j.1469-0691.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 152.Crotti D, D'Annibale ML. 2007. Intestinal infections caused by Dientamoeba fragilis and Giardia duodenalis in our experience. Recenti Prog Med 98:361–366. (In Italian.). [PubMed] [Google Scholar]
- 153.Girginkardesler N, Kurt O, Kilimcioglu AA, Ok UZ. 2008. Transmission of Dientamoeba fragilis: evaluation of the role of Enterobius vermicularis. Parasitol Int 57:72–75. doi: 10.1016/j.parint.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Ogren J, Dienus O, Lofgren S, Iveroth P, Matussek A. 2013. Dientamoeba fragilis DNA detection in Enterobius vermicularis eggs. Pathog Dis 69:157–158. doi: 10.1111/2049-632X.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roser D, Nejsum P, Carlsgart AJ, Nielsen HV, Stensvold CR. 2013. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp Parasitol 133:57–61. doi: 10.1016/j.exppara.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 156.Calderaro A, Gorrini C, Montecchini S, Peruzzi S, Piccolo G, Rossi S, Gargiulo F, Manca N, Dettori G, Chezzi C. 2010. Evaluation of a real-time polymerase chain reaction assay for the detection of Dientamoeba fragilis. Diagn Microbiol Infect Dis 67:239–245. doi: 10.1016/j.diagmicrobio.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 157.Ockert G. 1975. Epidemiology of Dientamoeba fragilis Jepps and Dobell, 1918. 3. Further studies on Enterobius transmission through eggs. J Hyg Epidemiol Microbiol Immunol 19:17–21. (In German.). [PubMed] [Google Scholar]
- 158.Ockert G. 1972. Epidemiology of Dientamoeba fragilis Jepps and Dobell, 1918. 2. Attempt at species transfer with Enterobius eggs. J Hyg Epidemiol Microbiol Immunol 16:213–221. (In German.). [PubMed] [Google Scholar]
- 159.Ockert G. 1972. Epidemiology of Dientamoeba fragilis Jepps and Dobell 1918. 1. Spread of the species in child collectives. J Hyg Epidemiol Microbiol Immunol 16:213–221. (In German.). [PubMed] [Google Scholar]
- 160.Menghi CI, Makiya R, Gatta CL, Mendez OC. 2005. Dientamoeba fragilis: molecular biology techniques for the elucidation of its mode of transmission. Parasitol Latinoam 60:25–31. [Google Scholar]
- 161.Crotti D, Sensi M, Crotti S, Grelloni V, Manuali E. 2007. Dientamoeba fragilis in swine population: a preliminary investigation. Vet Parasitol 145:349–351. doi: 10.1016/j.vetpar.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 162.Bertrand I, Gantzer C, Chesnot T, Schwartzbrod J. 2004. Improved specificity for Giardia lamblia cyst quantification in wastewater by development of a real-time PCR method. J Microbiol Methods 57:41–53. doi: 10.1016/j.mimet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 163.Wright MS, Collins PA. 1997. Waterborne transmission of Cryptosporidium, Cyclospora and Giardia. Clin Lab Sci 10:287–290. [PubMed] [Google Scholar]
- 164.Mattern CF, Daniel WA. 1980. Tritrichomonas muris in the hamster: pseudocysts and the infection of newborn. J Protozool 27:435–439. doi: 10.1111/j.1550-7408.1980.tb05392.x. [DOI] [PubMed] [Google Scholar]
- 165.Friedhoff KT, Kuhnigk C, Muller I. 1991. Experimental infections in chickens with Chilomastix gallinarum, Tetratrichomonas gallinarum, and Tritrichomonas eberthi. Parasitol Res 77:329–334. doi: 10.1007/BF00930910. [DOI] [PubMed] [Google Scholar]
- 166.Borges FP, Gottardi B, Stuepp C, Larre AB, de Brum Vieira P, Tasca T, De Carli GA. 2007. Morphological aspects of Monocercomonas sp. and investigation on probable pseudocysts occurrence. Parasitol Res 101:1503–1509. doi: 10.1007/s00436-007-0667-8. [DOI] [PubMed] [Google Scholar]
- 167.Pereira-Neves A, Ribeiro KC, Benchimol M. 2003. Pseudocysts in trichomonads—new insights. Protist 154:313–329. doi: 10.1078/143446103322454095. [DOI] [PubMed] [Google Scholar]
- 168.Benchimol M. 2004. Trichomonads under microscopy. Microsc Microanal 10:528–550. doi: 10.1017/S1431927604040905. [DOI] [PubMed] [Google Scholar]
- 169.Hauck R, Hafez HM. 2009. Partial sequence of the beta-tubulin of Histomonas meleagridis and the activity of benzimidazoles against H. meleagridis in vitro. Parasitol Res 104:1183–1189. doi: 10.1007/s00436-008-1309-5. [DOI] [PubMed] [Google Scholar]
- 170.Hegner R, Chu HJ. 1930. A comparative study of the intestinal protozoa of wild monkeys and man. Am J Epidemiol 12:62–108. [Google Scholar]
- 171.Knowles R, DasGupta BM. 1936. Some observations on the intestinal protozoa of macaques. Indian J Med Res 24:547–556. [Google Scholar]
- 172.Myers BJ, Kuntz RE. 1968. Intestinal protozoa of the baboon Papio doguera Pucheran, 1856. J Protozool 15:363–365. doi: 10.1111/j.1550-7408.1968.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 173.Helenbrook WD, Wade SE, Shields WM, Stehman SV, Whipps CM. 2015. Gastrointestinal parasites of Ecuadorian mantled howler monkeys (Alouatta palliata aequatorialis) based on fecal analysis. J Parasitol 101:341–350. doi: 10.1645/13-356.1. [DOI] [PubMed] [Google Scholar]
- 174.Noble GA, Noble ER. 1952. Entamoeba in farm animals. J Parasitol 38:571–595. doi: 10.2307/3273985. [DOI] [PubMed] [Google Scholar]
- 175.Ogunniyi T, Balogun H, Shasanya B. 2014. Ectoparasites and endoparasites of peridomestic house-rats in Ile-Ife, Nigeria and implication on human health. Iran J Parasitol 9:134–140. [PMC free article] [PubMed] [Google Scholar]
- 176.Stark D, Phillips O, Peckett D, Munro U, Marriott D, Harkness J, Ellis J. 2008. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet Parasitol 151:21–26. doi: 10.1016/j.vetpar.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 177.Lankester F, Kiyang JA, Bailey W, Unwin S. 2010. Dientamoeba fragilis: initial evidence of pathogenicity in the western lowland gorilla (Gorilla gorilla gorilla). J Zoo Wildl Med 41:350–352. doi: 10.1638/2009-0190.1. [DOI] [PubMed] [Google Scholar]
- 178.McConnell EL, Basit AW, Murdan S. 2008. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol 60:63–70. doi: 10.1211/jpp.60.1.0008. [DOI] [PubMed] [Google Scholar]
- 179.Kararli TT. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 180.Tillack M, Nowak N, Lotter H, Bracha R, Mirelman D, Tannich E, Bruchhaus I. 2006. Increased expression of the major cysteine proteinases by stable episomal transfection underlines the important role of EhCP5 for the pathogenicity of Entamoeba histolytica. Mol Biochem Parasitol 149:58–64. doi: 10.1016/j.molbiopara.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 181.Matthiesen J, Bar AK, Bartels AK, Marien D, Ofori S, Biller L, Tannich E, Lotter H, Bruchhaus I. 2013. Overexpression of specific cysteine peptidases confers pathogenicity to a nonpathogenic Entamoeba histolytica clone. mBio 4:e00072-13. doi: 10.1128/mBio.00072-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Davis PH, Schulze J, Stanley SL Jr. 2007. Transcriptomic comparison of two Entamoeba histolytica strains with defined virulence phenotypes identifies new virulence factor candidates and key differences in the expression patterns of cysteine proteases, lectin light chains, and calmodulin. Mol Biochem Parasitol 151:118–128. doi: 10.1016/j.molbiopara.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 183.Lukes J, Stensvold CR, Jirku-Pomajbikova K, Wegener Parfrey L. 2015. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog 11:e1005039. doi: 10.1371/journal.ppat.1005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burrows RB, Swerdlow MA, Frost JK, Leeper CK. 1954. Pathology of Dientamoeba fragilis infections of the appendix. Am J Trop Med Hyg 3:1033–1039. [PubMed] [Google Scholar]
- 185.Swerdlow MA, Burrows RB. 1955. Dientamoeba fragilis, an intestinal pathogen. JAMA 158:176–178. doi: 10.1001/jama.1955.02960030026008. [DOI] [PubMed] [Google Scholar]
- 186.Cerva L, Schrottenbaum M, Kliment V. 1991. Intestinal parasites: a study of human appendices. Folia Parasitol (Praha) 38:5–9. [PubMed] [Google Scholar]
- 187.Nagata N, Marriott D, Harkness J, Ellis JT, Stark D. 2012. Current treatment options for Dientamoeba fragilis infections. Int J Parasitol Drugs Drug Resist 2:204–215. doi: 10.1016/j.ijpddr.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Keystone JS, Proctor E, Glenn C, McIntyre L. 1983. Safety and efficacy of diphetarsone in the treatment of amoebiasis, non-pathogenic amoebiasis and trichuriasis. Trans R Soc Trop Med Hyg 77:84–86. doi: 10.1016/0035-9203(83)90022-6. [DOI] [PubMed] [Google Scholar]
- 189.Dardick KR. 1983. Tetracycline treatment of Dientamoeba fragilis. Conn Med 47:69–70. [PubMed] [Google Scholar]
- 190.Bosman DK, Benninga MA, van de Berg P, Kooijman GC, van Gool T. 2004. Dientamoeba fragilis: possibly an important cause of persistent abdominal pain in children. Ned Tijdschr Geneeskd 148:575–579. (In Dutch.). [PubMed] [Google Scholar]
- 191.Simon M, Shookhoff HB, Terner H, Weingarten B, Parker JG. 1967. Paromomycin in the treatment of intestinal amebiasis; a short course of therapy. Am J Gastroenterol 48:504–511. [PubMed] [Google Scholar]
- 192.Vandenberg O, Souayah H, Mouchet F, Dediste A, van Gool T. 2007. Treatment of Dientamoeba fragilis infection with paromomycin. Pediatr Infect Dis J 26:88–90. doi: 10.1097/01.inf.0000247139.89191.91. [DOI] [PubMed] [Google Scholar]
- 193.van Hellemond JJ, Molhoek N, Koelewijn R, Wismans PJ, van Genderen PJ. 2012. Is paromomycin the drug of choice for eradication of Dientamoeba fragilis in adults? Int J Parasitol Drugs Drug Resist 2:162–165. doi: 10.1016/j.ijpddr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kurt O, Girginkardesler N, Balcioglu IC, Ozbilgin A, Ok UZ. 2008. A comparison of metronidazole and single-dose ornidazole for the treatment of dientamoebiasis. Clin Microbiol Infect 14:601–604. doi: 10.1111/j.1469-0691.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- 195.Balamuth W. 1953. Comparative action of selected amebicidal agents and antibiotics against several species of human intestinal amebae. Am J Trop Med Hyg 2:191–205. [DOI] [PubMed] [Google Scholar]
- 196.Nagata N, Marriott D, Harkness J, Ellis JT, Stark D. 2012. In vitro susceptibility testing of Dientamoeba fragilis. Antimicrob Agents Chemother 56:487–494. doi: 10.1128/AAC.05125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barratt J, Ellis J, Harkness J, Marriott D, Stark D. 2013. Evaluation of the in vitro antiprotozoal activity of various dry plant extracts against Dientamoeba fragilis. J Infect Dis Ther 1:111. [Google Scholar]
- 198.Stark D, Barratt JL, Roberts T, Marriott D, Harkness JT, Ellis J. 2014. Activity of benzimidazoles against Dientamoeba fragilis (Trichomonadida, Monocercomonadidae) in vitro and correlation of beta-tubulin sequences as an indicator of resistance. Parasite 21:41. doi: 10.1051/parasite/2014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. [DOI] [PubMed] [Google Scholar]
- 200.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sukanahaketu S. 1977. The presence of Dientamoeba fragilis in the Ascaris lumbricoides ova: the first report from Thailand. J Med Assoc Thai 60:256–258. [PubMed] [Google Scholar]
- 202.Sargeaunt PG, Williams JE, Kumate J, Jimenez E. 1980. The epidemiology of Entamoeba histolytica in Mexico City. A pilot survey I. Trans R Soc Trop Med Hyg 74:653–656. doi: 10.1016/0035-9203(80)90158-3. [DOI] [PubMed] [Google Scholar]
- 203.Sargeaunt PG, Williams JE, Jackson TF, Simjee AE. 1982. A zymodeme study of Entamoeba histolytica in a group of South African schoolchildren. Trans R Soc Trop Med Hyg 76:401–402. doi: 10.1016/0035-9203(82)90200-0. [DOI] [PubMed] [Google Scholar]
- 204.Keystone JS, Yang J, Grisdale D, Harrington M, Pillon L, Andreychuk R. 1984. Intestinal parasites in metropolitan Toronto day-care centres. Can Med Assoc J 131:733–735. [PMC free article] [PubMed] [Google Scholar]
- 205.Muller R, Lillywhite J, Bending JJ, Catford JC. 1987. Human cysticercosis and intestinal parasitism amongst the Ekari people of Irian Jaya. J Trop Med Hyg 90:291–296. [PubMed] [Google Scholar]
- 206.Kaminsky RG. 1991. Parasitism and diarrhoea in children from two rural communities and marginal barrio in Honduras. Trans R Soc Trop Med Hyg 85:70–73. doi: 10.1016/0035-9203(91)90162-R. [DOI] [PubMed] [Google Scholar]
- 207.Tavarez LA, Pena F, Placencia F, Mendoza HR, Polanco D. 1991. Prevalence of protozoans in children with acute diarrheal disease. Arch Domin Pediatr 27:43–47. (In Spanish.). [PubMed] [Google Scholar]
- 208.Meropol SB. 1995. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med 149:887–892. doi: 10.1001/archpedi.1995.02170210061011. [DOI] [PubMed] [Google Scholar]
- 209.van Gool T, Dankert J. 1996. 3 emerging protozoal infections in the Netherlands: Cyclospora, Dientamoeba, and Microspora infections. Ned Tijdschr Geneeskd 140:155–160. (In Dutch.). [PubMed] [Google Scholar]
- 210.Lindo JF, Dubon JM, Ager AL, De Gourville EM, Solo-Gabriele H, Klaskala WI, Baum MK, Palmer CJ. 1998. Intestinal parasitic infections in human immunodeficiency virus (HIV)-positive and HIV-negative individuals in San Pedro Sula, Honduras. Am J Trop Med Hyg 58:431–435. [DOI] [PubMed] [Google Scholar]
- 211.Ayadi A, Bahri I. 1999. Dientamoeba fragilis: pathogenic flagellate? Bull Soc Pathol Exot 92:299–301. (In French.). [PubMed] [Google Scholar]
- 212.Lainson R, da Silva BA. 1999. Intestinal parasites of some diarrhoeic HIV-seropositive individuals in North Brazil, with particular reference to Isospora belli Wenyon, 1923 and Dientamoeba fragilis Jepps & Dobell, 1918. Mem Inst Oswaldo Cruz 94:611–613. doi: 10.1590/S0074-02761999000500008. [DOI] [PubMed] [Google Scholar]
- 213.Crotti D, D'Annibale ML. 2001. Dientamoeba fragilis and dientamoebiasis: aspects of clinical parasitology and laboratory diagnosis. Parassitologia 43:135–138. (In Italian.). [PubMed] [Google Scholar]
- 214.Karaman U, Atambay M, Aycan O, Yologlu S, Daldal N. 2006. Incidence of intestinal parasites in municipal sanitary workers in Malatya. Turkiye Parazitol Derg 30:181–183. (In Turkish.). [PubMed] [Google Scholar]
- 215.Crotti D, D'Annibale ML. 2007. Human intestinal parasitosis: role of Dientamoeba fragilis in human infections. Ann Ig 19:27–34. (In Italian.) [PubMed] [Google Scholar]
- 216.Crotti D, D'Annibale ML. 2007. Role of Dientamoeba fragilis in human bowel infections. Infez Med 15:30–39. (In Italian.) [PubMed] [Google Scholar]
- 217.Kasssem HH, Zaed HA, Sadaga GA. 2007. Intestinal parasitic infection among children and neonatus admitted to Ibn-Sina Hospital, Sirt, Libya. J Egypt Soc Parasitol 37:371–380. [PubMed] [Google Scholar]
- 218.Menghi CI, Iuvaro FR, Dellacasa MA, Gatta CL. 2007. Survey of intestinal parasites among an aboriginal community in Salta. Medicina (B Aires) 67:705–708. (In Spanish.) [PubMed] [Google Scholar]
- 219.Ozcakir O, Gureser S, Erguven S, Yilmaz YA, Topaloglu R, Hascelik G. 2007. Characteristics of Blastocystis hominis infection in a Turkish university hospital. Turkiye Parazitol Derg 31:277–282. [PubMed] [Google Scholar]
- 220.Rayan HZ, Ismail OA, El Gayar EK. 2007. Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. J Egypt Soc Parasitol 37:599–608. [PubMed] [Google Scholar]
- 221.Stark D, Fotedar R, van Hal S, Beebe N, Marriott D, Ellis JT, Harkness J. 2007. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney, Australia. Am J Trop Med Hyg 76:549–552. [PubMed] [Google Scholar]
- 222.Cengiz ZT, Akbayram S, Cicek M, Yilmaz H. 2009. Intestinal parasitoses detected in primary schoolchildren in the Van Province. Turkiye Parazitol Derg 33:289–293. (In Turkish.) [PubMed] [Google Scholar]
- 223.Schuster H, Jackson RS. 2009. Prevalence of Dientamoeba fragilis among patients consulting complementary medicine practitioners in the British Isles. J Clin Pathol 62:182–184. doi: 10.1136/jcp.2008.059659. [DOI] [PubMed] [Google Scholar]
- 224.Gonzalez-Moreno O, Domingo L, Teixidor J, Gracenea M. 2011. Prevalence and associated factors of intestinal parasitisation: a cross-sectional study among outpatients with gastrointestinal symptoms in Catalonia, Spain. Parasitol Res 108:87–93. doi: 10.1007/s00436-010-2044-2. [DOI] [PubMed] [Google Scholar]
- 225.Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R. 2010. Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107:679–684. doi: 10.1007/s00436-010-1918-7. [DOI] [PubMed] [Google Scholar]
- 226.Church C, Neill A, Schotthoefer AM. 2010. Intestinal infections in humans in the Rocky Mountain region, United States. J Parasitol 96:194–196. doi: 10.1645/GE-2229. [DOI] [PubMed] [Google Scholar]
- 227.Guidetti C, Ricci L, Vecchia L. 2010. Prevalence of intestinal parasitosis in Reggio Emilia (Italy) during 2009. Infez Med 18:154–161. (In Italian.) [PubMed] [Google Scholar]
- 228.Banik GR, Barratt JL, Marriott D, Harkness J, Ellis JT, Stark D. 27 April 2011. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology doi: 10.1017/S0031182011000448. [DOI] [PubMed] [Google Scholar]
- 229.Calik S, Karaman U, Colak C. 2011. Prevalence of microsporidium and other intestinal parasites in children from Malatya, Turkey. Indian J Microbiol 51:345–349. doi: 10.1007/s12088-011-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gijsbers CF, Benninga M, Buller H. 2011. Clinical and laboratory findings in 220 children with recurrent abdominal pain. Acta Paediatr 100:1028–1032. doi: 10.1111/j.1651-2227.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 231.Karaman U, Turan A, Depecik F, Gecit I, Ozer A, Karci E, Karadan M. 2011. Frequency of intestinal parasites among administrators and workers in sanitary and non-sanitary institutions. Turkiye Parazitol Derg 35:30–33. (In Turkish.) doi: 10.5152/tpd.2011.08. [DOI] [PubMed] [Google Scholar]
- 232.Siala E, Guidara R, Ben Abdallah R, Ben Ayed S, Ben Alaya N, Zallaga N, Bouratbine A, Aoun K. 2011. The intestinal parasites in the food handlers of Tunis area: study of 8502 stool samples (1998-2008). Arch Inst Pasteur Tunis 88:77–84. (In French.). [PubMed] [Google Scholar]
- 233.Staat MA, Rice M, Donauer S, Mukkada S, Holloway M, Cassedy A, Kelley J, Salisbury S. 2011. Intestinal parasite screening in internationally adopted children: importance of multiple stool specimens. Pediatrics 128:e613–e622. doi: 10.1542/peds.2010-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dogan N, Oz Y, Kocman NU, Nursal AF. 2012. Comparison of individual differences in the direct microscopic examination in the diagnosis of intestinal parasites. Turkiye Parazitol Derg 36:211–214. (In Turkish.) doi: 10.5152/tpd.2012.51. [DOI] [PubMed] [Google Scholar]
- 235.Garcia JA, Cimerman S. 2012. Detection of Dientamoeba fragilis in patients with HIV/AIDS by using a simplified iron hematoxylin technique. Rev Soc Bras Med Trop 45:156–158. doi: 10.1590/S0037-86822012000200003. [DOI] [PubMed] [Google Scholar]
- 236.Jimenez-Gonzalez DE, Martinez-Flores WA, Reyes-Gordillo J, Ramirez-Miranda ME, Arroyo-Escalante S, Romero-Valdovinos M, Stark D, Souza-Saldivar V, Martinez-Hernandez F, Flisser A, Olivo-Diaz A, Maravilla P. 2012. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res 110:1269–1275. doi: 10.1007/s00436-011-2626-7. [DOI] [PubMed] [Google Scholar]
- 237.Al-Hindi AI, Shammala BM. 2013. Dientamoeba fragilis in Gaza Strip: a neglected protozoan parasite. Iran J Parasitol 8:249–255. [PMC free article] [PubMed] [Google Scholar]
- 238.Gulmez D, Saribas Z, Akyon Y, Erguven S. 2013. The results of Hacettepe University Faculty of Medicine Parasitology Laboratory in 2003-2012: evaluation of 10 years. Turkiye Parazitol Derg 37:97–101. (In Turkish.) doi: 10.5152/tpd.2013.23. [DOI] [PubMed] [Google Scholar]
- 239.Maas L, Dorigo-Zetsma JW, de Groot CJ, Bouter S, Plotz FB, van Ewijk BE. 17 October 2013. Detection of intestinal protozoa in paediatric patients with gastrointestinal symptoms by multiplex real-time PCR. Clin Microbiol Infect doi: 10.1111/1469-0691.12386. [DOI] [PubMed] [Google Scholar]
- 240.Mumcuoglu I, Coskun FA, Aksu N, Purnak T, Gungor C. 2013. Role of Dientamoeba fragilis and Blastocystis spp. in irritable bowel syndrome. Turkiye Parazitol Derg 37:73–77. (In Turkish.) doi: 10.5152/tpd.2013.19. [DOI] [PubMed] [Google Scholar]