Abstract
Pregnancy weight gain may lead to long-term increases in maternal BMI for some women. The objective of this study was to examine maternal body weight change 1y–2y postpartum, and to compare classifications of 2y weight retention with and without accounting for 1y–2y weight gain. Early pregnancy body weight (EPW, first trimester) was measured or imputed, and follow-up measures obtained before delivery, 1 year postpartum (1y) and 2 years postpartum (2y) in an observational cohort study of women seeking prenatal care in several counties in upstate New York (n = 413). Baseline height was measured; demographic and behavioral data were obtained from questionnaires and medical records. Associations of 1y–2y weight change (kg) and 1y–2y weight gain (≥2.25 kg) with anthropometric, socioeconomic, and behavioral variables were evaluated using linear and logistic regressions. While mean ± SE 1y–2y weight change was 0.009 ± 4.6 kg, 1y–2y weight gain (≥2.25 kg) was common (n = 108, 26%). Odds of weight gain 1y–2y were higher for overweight (ORadj = 2.63, CI95% = 1.43–4.82) and obese (ORadj = 2.93, CI95% = 1.62–5.27) women than for women with BMI <25. Two year weight retention (2y–EPW ≥2.25 kg) was misclassified in 38% (n = 37) of women when 1y–2y weight gain was ignored. One year weight retention (1YWR) (1y–EPW) was negatively related to 1y–2y weight change (βadj ± SE = −0.28 ± 0.04, P < 0.001) and weight gain (≥2.25 kg) (ORadj = 0.91, CI95% = 0.87–0.95). Relations between 1y weight retention and 1y–2y weight change were attenuated for women with higher early pregnancy BMI. Weight change 1y–2y was predicted primarily by an inverse relation with 1y weight retention. The high frequency of weight gain has important implications for classification of postpartum weight retention.
INTRODUCTION
Pregnancy has been identified as a period of risk for excessive weight gain leading to long-term weight retention and maternal obesity (1–7). Several studies have reported increases in maternal body weight ranging from 1.5 to 3.0 kg above pre-pregnancy weight when postpartum weight is measured up to 18 months postpartum (8–11), and from 0.5 to 6.2 kg when measured 2.5y–15y postpartum (6,12–15). Gestational weight gain (GWG) in excess of the Institute of Medicine guidelines is of particular concern, given associations with an increased risk of postpartum weight retention (16–21) suggesting a potential for long-term influence on maternal BMI (7,12,13,22). In addition, some studies have shown that high BMI before pregnancy is related to excessive GWG (23), increased risk of postpartum weight retention (24) and postpartum weight gain (25). Both high prepregnancy BMI and excessive GWG are thought to be indicators of general susceptibility to weight gain, implying a subgroup of women with increased susceptibility to a weight gain trajectory leading to maternal obesity. Other factors associated with maternal weight gain and obesity in previous studies include maternal income (24,26), age (27), education and parity (28), as well as behavioral factors such as infant feeding and smoking (11).
Reviews of the literature on determinants of postpartum weight retention have indicated a need for further research regarding postpartum maternal weight trends through the initial 2 years postpartum (2y), noting a scarcity of studies after 18 months postpartum (29–31). In particular, the potential misclassification of postpartum weight gain as retention of GWG deserves examination (27,31). Postpartum weight retention is conventionally calculated as the difference between body weight at a given time postpartum and early or prepregnancy body weight (4). However, weight retention according to this definition is a misnomer if it includes weight gain that originates solely within the postpartum period (31). This may be problematic given previous research suggesting that weight gain may occur for some women after the first year postpartum (10,27,28,31,32).
The primary objectives of this research were to describe change in maternal body weight between 1y and 2y postpartum and to examine its associations with maternal anthropometric, behavioral and socioeconomic factors. We also examined the implications of late postpartum weight change for classification of 2y weight retention.
METHODS AND PROCEDURES
Study design and subjects
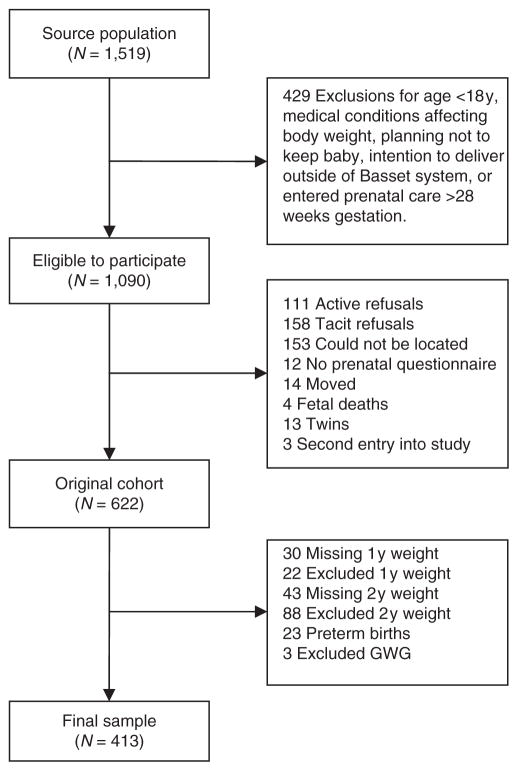
This was a prospective, observational cohort study, in which women seeking prenatal care from the Bassett Healthcare system, spanning 10 counties in upstate New York, were followed from early pregnancy until 2y postpartum. Details of the study design, recruitment, and data collection have been described elsewhere (24,33,34). The enrollment diagram is shown in Figure 1. Of the 622 women in the original cohort, 73 had missing body weight measurements at 1y (n = 30) or 2y (n = 43). Women were excluded from this analysis if the 1y body weight measurement was not taken within 9–19 months postpartum or if the 2y body weight measurement was not taken within 21–30 months postpartum (n = 110). These timeframes were in accordance with health provider scheduling practices for postpartum clinic visits. Additionally, women were excluded if they were more than 14 weeks pregnant at the time of either postpartum body weight measurements, or had a subsequent child born since enrollment before the 1y weight measurement or within 6 months of the 2y weight measurement. The 413 women included in this analysis were 1.4 ± 0.5 (SE) years older, had 0.5 ± 0.2 years more education, were more likely to be parous (62% vs. 52%), and were less likely to be low income (41% vs. 50%) than those excluded from this analysis (P < 0.05). There were no differences between included and excluded women according to early pregnancy BMI, GWG, or 1y weight retention. The sample was predominantly white (96%).
Figure 1.
Subject enrollment. 1y, 1 year postpartum; 2y, 2 years postpartum.
Data collection and measurements
Maternal height was measured at the first prenatal visit. Body weights were measured by health providers at prenatal and postpartum clinic visits. Early pregnancy weight (EPW, first trimester) was measured at the first prenatal visit or imputed, as described elsewhere (23). Briefly, for women whose first measured weights occurred in the second trimester (n = 75), EPW was imputed based on a regression model derived from the relation between measured EPW and later measured prenatal weight in the sample of women with measurements at both the first and second trimesters. Although self-reported prepregnancy weight was also obtained for all women, this measure was not used in the analysis due to the discrepancy between self-reported and measured weight that was especially marked in women with high BMI (23), as has been reported in the literature (29). A meta-analysis of studies examining the relation between GWG and postpartum weight retention showed that findings based on measured EPW fell within the range of those based on self-reported prepregnancy body weight (29). All analyses using categorical variables for GWG and early pregnancy BMI were replicated using continuous variables to assess potential misclassification; findings were not sensitive to the type of variable used, indicating that the potential bias on classification due to use of measured EPW was minimal.
Additional measurements of body weight were taken before delivery and at 1y and 2y postpartum. Last measured pregnancy weight was taken within 2 weeks of delivery for 92% of the sample (all women were measured within 6 weeks of delivery; results were similar when the sample was restricted to those whose last measured pregnancy weights were within 2 weeks of delivery).
Subject age at delivery (years), education (years), marital status (whether the mother was living and/or married to their partner), parity, and income (whether or not income was at or below 185% of the poverty level) were abstracted from medical records shortly after delivery. Data about smoking (smoker, nonsmoker, smoker who quit during pregnancy but resumed postpartum) and infant feeding (any breastfeeding at 1y, yes/no) were obtained from mailed questionnaires completed once during the prenatal period, and at 1y and 2y postpartum. This measure of breastfeeding was used in the analysis for consistency with previous studies using these data (24). Study procedures were approved by the institutional review boards of Cornell University and Bassett Healthcare.
Variables
Early pregnancy BMI (kg/m2) was calculated from the first measured body weight and height. A categorical variable was used to classify weight status as low, overweight, and obese (BMI <25.0, ≥25.0–<30.0, and ≥30.0, respectively). Eleven women with BMI <18.5 were included in the low BMI group because of insufficient numbers to allow for a separate category, and in order to avoid discarding data from these subjects who met all other inclusion criteria. Other categories are in accordance with Institute of Medicine cutpoints for BMI groups (4).
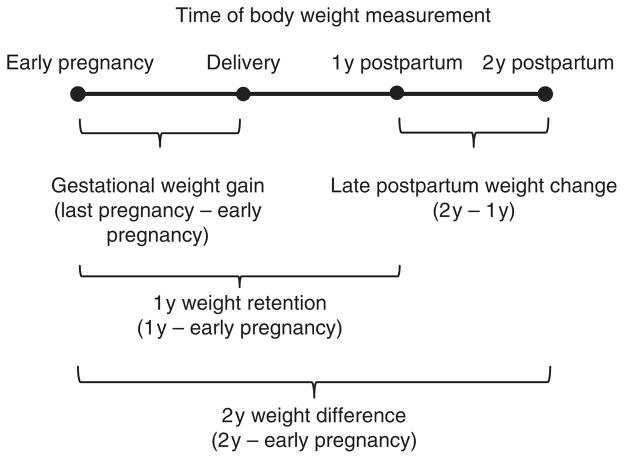
Figure 2 depicts a schematic representation of the definitions of the weight change measures. GWG was calculated as the difference between last measured pregnancy weight and EPW. A categorical variable was used to describe GWG according to the Institute of Medicine recommendations as less than, within, and exceeding guidelines (4). Guidelines stipulate recommended ranges of GWG according to maternal prepregnancy weight status (12.5–18 kg for underweight, 11.5–16 kg for normal weight, 7–11.5 kg for overweight, 5–9 kg for obese). One year weight retention (1YWR) was defined as the difference between body weight at 1y and EPW. Late postpartum weight change was defined as the difference between body weight at 2y and 1y. A dichotomous variable, late postpartum weight gain, was used to identify women with 1y–2y weight change ≥2.25 kg, in accordance with research indicating adverse health effects associated with this amount of weight gain (35). Although the difference between weight at 2y and EPW is conventionally called “weight retention,” we instead refer to this as the “2y weight difference” in consideration for potential postpartum weight gain before 2y postpartum. We also defined major late postpartum weight retention as having both 1y and 2y body weight measurements ≥4.55 kg above EPW in order to account for weight gain occurring after 1y. This classification thereby differentiates women who are at least 4.55 kg greater than EPW due partly to weight gain occurring between 1y and 2y from those with this amount of weight retention carried over from 1y. This cutpoint (4.55 kg) was used to enable comparability with previous research (24).
Figure 2.
Schematic representation of maternal body weight over time and body weight variable definitions for gestational weight gain, 1y weight retention, 2y weight difference, and late postpartum weight change. 1y, 1 year postpartum; 2y, 2 years postpartum.
Statistical analysis
Multiple linear and logistic regression models were used to examine relationships of 1y–2y weight change and weight gain (≥2.25 kg) with early pregnancy BMI and GWG. The inclusion of demographic and socioeconomic covariates in the final adjusted models was determined by Akaike Information Criterion, which compares both fit and number of parameters of competing models. Final models adjusted for time between weight measurements, breastfeeding, smoking, education, parity and income. 1YWR was included in the fully adjusted models. We tested for interactions between 1YWR with BMI, smoking, breastfeeding, parity and income using a multiplicative interaction term followed by stratified analyses where warranted. STATA version 11 (Stata, College Station, TX) was used for all statistical analyses.
RESULTS
Sixty-four percent (n = 265) of the sample was between ages 25–35 years at delivery. The sample was highly educated (38% with at least 15y of education), and was predominantly married or living with partner (92%) (Table 1). Mean ± SD early pregnancy BMI was 26.2 ± 6.0 kg/m2. More women had GWG above the Institute of Medicine guidelines (n = 193, 47%) than were within (n = 147, 36%) or below (n = 73, 18%) the guidelines, with a mean GWG of 13.5 ± 5.4 kg. Mean 1YWR was 1.3 ± 5.8 kg, with nearly one quarter (n = 101, 24%) of the sample having major retention (≥4.55 kg) at 1y. Late postpartum weight change was 0.009 ± 4.6 kg (interquartile range = −2.25, 2.25 kg), with approximately a quarter (n = 108, 26%) gaining at least 2.25 kg from 1y to 2y. Mean difference between 2y and EPW was 1.3 ± 6.2 kg.
Table 1.
Sample characteristics (n = 413)
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Demographic | |
| Age at delivery (years) | 29.3 ± 5.3 |
| Education (years) | 14.1 ± 2.3 |
| Marriage status | |
| Married or living with partner | 379 (92) |
| Not married or living with partner | 34 (8) |
| Income (n = 409) | |
| <185% PIR | 167 (41) |
| >185% PIR | 242 (59) |
| Parity | |
| Nulliparous | 156 (38) |
| Parous | 257 (62) |
| Behavioral | |
| Smoker | |
| Nonsmoker | 305 (75) |
| Quit during pregnancy | 38 (9) |
| Smoker | 66 (16) |
| Breastfeeding at 1 year (n = 401) | |
| Yes | 110 (27) |
| No | 291 (73) |
| Anthropometric | |
| Early pregnancy BMI (kg·m−2) | |
| <25 | 214 (52) |
| >25–<30 | 102 (25) |
| >30 | 97 (23) |
| Gestational weight gain | |
| <IOM guidelinesa | 73 (18) |
| Within IOM guidelines | 147 (36) |
| >IOM guidelines | 193 (47) |
| 1 year weight retention (1 year weight–EPW (kg)) | |
| ≥4.55 | 101 (24) |
| <4.55 | 312 (76) |
| Late postpartum weight change (2 year weight–1 year weight (kg)) | |
| >0 | 212 (51) |
| ≤0 | 201 (49) |
| Late postpartum weight gain (2 year weight–1 year weight (kg)) | |
| ≥2.25 | 108 (26) |
| <2.25 | 305 (74) |
EPW, early pregnancy weight; IOM, Institute of Medicine; PIR, poverty income ratio.
Variable indicating whether gestational weight gain falls within the IOM guidelines, which specify a range of recommended weight gain according to maternal prepregnancy BMI category.
Ninety-eight (24%) women were classified as having major 2y weight retention according to the conventional definition of 2y–EPW regardless of 1y weight (Table 2). However, only 61 (15%) of women were classified as having major 2y weight retention when defined as having both 1y and 2y weight ≥4.55 kg above EPW, indicating that 37 (38%) of the women with 2y weight ≥4.55 kg above EPW moved into that category due to weight gain between 1y and 2y postpartum (i.e., had 1y–EPW <4.55 kg and 2y–EPW ≥4.55 kg).
Table 2.
Classification of major 2 year postpartum weight retention (≥4.55 kg) with and without taking into account postpartum weight gain (n)
| 1 year weight–EPW (kg) | 2 year weight–EPW,(kg)
|
Total | |
|---|---|---|---|
| <4.55 | ≥4.55 | ||
|
|
|||
| <4.55 | 315 | 37 | 352 |
| ≥4.55 | 0 | 61 | 61b |
| Total | 315 | 98a | 413 |
EPW, early pregnancy weight.
Major 2 year weight retention conventionally defined as 2 year weight–EPW ≥4.55 kg without regard for 1 year weight.
Major 2 year weight retention defined as having both 1 and 2 year weight ≥4.55 kg above EPW. This classification excludes the 37 women with 2 year–EPW ≥4.55 kg due to weight gain occurring after 1 year postpartum.
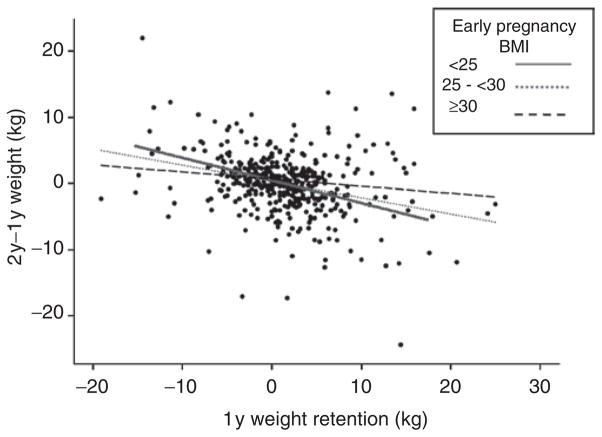
Maternal smoking was the only significant predictor of late postpartum weight change in the linear regression model unadjusted for 1YWR, with 1y–2y weight change being lower for women who quit smoking during pregnancy but resumed postpartum as compared with nonsmokers (Table 3). The fully adjusted model indicated an additional inverse relation between 1YWR and late postpartum weight change (β = −0.28 ± 0.04, P < 0.001). Inverse relationships of late postpartum weight change with breastfeeding at 1y postpartum and parity approached statistical significance in the fully adjusted model (P < 0.10). The relationship between 1YWR and late postpartum weight change was modified by early pregnancy BMI (Figure 3), such that it was attenuated for women who were obese in early pregnancy as compared to women with early pregnancy BMI <25. However, there remained a significant inverse relation between 1YWR and late postpartum weight change across all early pregnancy BMI categories.
Table 3.
Coefficient estimates ± SE from multiple linear regression models predicting weight change 1–2 years postpartum (kg)
| Independent variables | Model I β̂±SE |
P | Model II β̂±SE |
P |
|---|---|---|---|---|
| Early pregnancy BMI (<25 = ref) | ||||
| Overweight | 0.67 ± 0.59 | 0.26 | 0.26 ± 0.56 | 0.64 |
| Obese | 0.72 ± 0.60 | 0.23 | 0.65 ± 0.56 | 0.25 |
| Gestational weight gain (within IOM = ref) | ||||
| <IOM recommendations | −0.23 ± 0.68 | 0.73 | −0.43 ± 0.64 | 0.51 |
| >IOM recommendations | −0.48 ± 0.54 | 0.38 | 0.47 ± 0.53 | 0.37 |
| 1 year weight retention (kg) | n/a | −0.28 ± 0.04*** | <0.001 | |
| Smoker (no = ref) | ||||
| Quit during pregnancy | −2.21 ± 0.86 | 0.01 | −2.00 ± 0.81 | 0.01 |
| Smoker | −0.57 ± 0.66 | 0.39 | −0.79 ± 0.62 | 0.21 |
| Breastfeeding at 1 year (no = ref) | −0.75 ± 0.53 | 0.16 | −0.96 ± 0.50 | 0.06 |
| Education (years) | −0.17 ± 0.12 | 0.15 | −0.18 ± 0.11 | 0.12 |
| Parity (nulliparous = ref) | −0.53 ± 0.49 | 0.28 | −0.87 ± 0.46 | 0.06 |
| Income (≥185% poverty = ref) | −0.53 ± 0.53 | 0.32 | −0.17 ± 0.50 | 0.74 |
| Adjusted R2 | 0.009 | 0.12 | ||
IOM, Institute of Medicine.
Model I adjusted for between weight measurements and all covariates except 1 year weight retention. Model II additionally adjusted for 1 year weight retention.
Figure 3.
Late postpartum weight change (2y–1y weight (kg)) vs. 1y weight retention, by early pregnancy BMI. The relationship was significantly attenuated (P interaction = 0.01) for women who were obese in early pregnancy (βadj ± SE = −0.21 ± 0.10, P = 0.04) as compared with women with BMI <25 (βadj ± SE = −0.33 ± 0.04, P < 0.001). The relationship was not significantly different for women who were overweight in early pregnancy (βadj ± SE = −0.37 ± 0.08, P < 0.001) as compared to women with BMI <25. Adjusted for time between measurements, gestational weight gain, smoking, breastfeeding, education, parity, and income. 1y, 1 year postpartum; 2y, 2 years postpartum.
Early pregnancy BMI was positively related to late postpartum weight gain (≥2.25 kg) in multiple logistic regression models (Table 4). The ORadj of weight gain was 2.38 times (CI95% = 1.28, 4.42, P = 0.006) higher for overweight women and 2.98 times (1.63, 5.57, P < 0.001) higher for obese women as compared to women with low early pregnancy BMI in the fully adjusted model. The relationship between GWG and late postpartum weight gain was not significant in either model. The fully adjusted model showed a 9% (5%, 13%, P < 0.001) decreased odds of late postpartum weight gain associated with a 1 kg increase in 1YWR.
Table 4.
Odds ratios (CI95%) from multiple logistic regression models predicting late postpartum weight gain (2 year–1 year body weight ≥ 2.25 kg)
| Independent variables | Model I
|
P | Model II
|
P |
|---|---|---|---|---|
| ORadj (CI95%) | ORadj (CI95%) | |||
| Early pregnancy BMI (<25 = ref) | ||||
| Overweight | 2.63 (1.43, 4.82) | 0.002 | 2.38 (1.28, 4.42) | 0.006 |
| Obese | 2.93 (1.62, 5.27) | <0.001 | 2.98 (1.63, 5.57) | <0.001 |
| Gestational weight gain (within IOM = ref) | ||||
| <IOM recommendations | 0.98 (0.49, 1.98) | 0.73 | 0.91 (0.44, 1.88) | 0.8 |
| >IOM recommendations | 1.02 (0.58, 1.80) | 0.8 | 1.39 (0.77, 2.51) | 0.28 |
| 1 year weight retention (kg) | n/a | 0.91 (0.87, 0.95) | <0.001 | |
| Smoker (no = ref) | ||||
| Quit during pregnancy | 0.47 (0.18, 1.24) | 0.13 | 0.46 (0.17, 1.26) | 0.13 |
| Smoker | 0.94 (0.49, 1.80) | 0.85 | 0.85 (0.43, 1.66) | 0.63 |
| Breastfeeding at 1 year (no = ref) | 0.72 (0.41, 1.27) | 0.26 | 0.65 (0.36, 1.16) | 0.14 |
| Education (years) | 0.94 (0.83, 1.06) | 0.31 | 0.94 (0.83, 1.07) | 0.35 |
| Parity (nulliparous = ref) | 1.17 (0.71, 1.95) | 0.53 | 1.02 (0.61, 1.72) | 0.93 |
| Income (≥185% poverty level = ref) | 1.04 (0.61, 1.79) | 0.87 | 1.22 (0.70, 2.15) | 0.48 |
| Pseudo R2 | 0.07 | 0.11 | ||
CI, confidence interval; IOM, Institute of Medicine; OR, odds ratio.
Model I adjusted for between weight measurements and all covariates except 1 year weight retention. Model II additionally adjusted for 1 year weight retention.
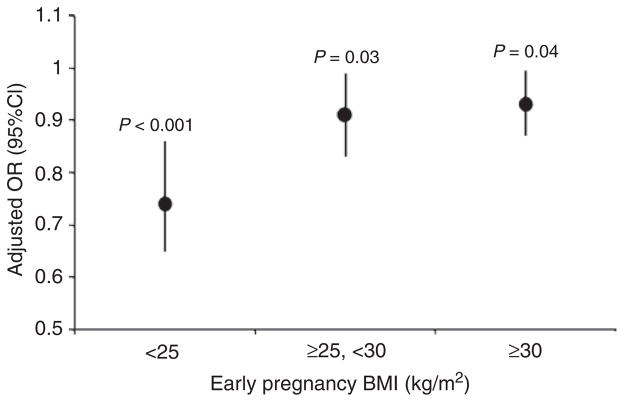
As in the linear model predicting late postpartum weight change, the relationship between late postpartum weight gain (≥2.25 kg) and 1YWR was modified by early pregnancy BMI, as shown in Figure 4. The ORadj of late postpartum weight gain associated with a 1 kg increase in 1YWR was 0.74 (0.65, 0.86, P < 0.001), 0.91 (0.83, 0.99, P = 0.03), and 0.93 (0.87, 0.995, P = 0.04) for women with low, overweight, and obese early pregnancy BMI, respectively. Relationships between 1YWR and late postpartum weight gain were significantly attenuated for overweight (P = 0.01) and obese women (P = 0.001) as compared to women with low early pregnancy BMI.
Figure 4.
Adjusted odds ratio (OR) and 95% confidence interval (95% CI) of late postpartum weight gain (2y–1y ≥2.25 kg) associated with a 1 kg increase in one year weight retention (1YWR), by early pregnancy BMI. Interaction terms for overweight (P = 0.01) and obese (P = 0.001) BMI are significantly different from zero. Adjusted for time between measurements, gestational weight gain, smoking, breastfeeding, education, parity, and income. 1y, 1 year postpartum; 2y, 2 years postpartum.
DISCUSSION
This study examined change in maternal body weight between 1y and 2y, and evaluated implications of postpartum weight gain for classification of postpartum weight retention. The mean difference between EPW and 2y body weight in this study is within the range reported in previous research with similar follow-up time (10,26,28,36). While mean weight change 1y–2y in this study was small (0.009 ± 4.6 kg), the high frequency of clinically significant weight gain ≥2.25 kg (26%) in this period suggests the difference between maternal body weight before and after pregnancy is likely due to both retention of GWG and weight gain external to pregnancy, and thus, may be more accurately referred to as “postpartum weight difference,” rather than “retention” of GWG. Postpartum weight gain after 1y has been suggested in other studies (10,28,32,37), though its implication for classification of postpartum weight retention has been overlooked. The results of the present study demonstrate that misclassification of postpartum weight retention occurred for a substantial proportion (38%) of the women with body weights ≥4.55 kg above EPW at 2y when late postpartum weight gain was ignored.
These data indicate that late postpartum weight change was unrelated to GWG, and was strongly and inversely related to 1YWR, contradicting the hypothesis that GWG and earlier weight retention, as indicators of general susceptibility to weight gain, lead to a perpetual weight gain trajectory. On average, between 1y and 2y, women lost 0.28 kg (±0.04, P < 0.001) for every 1 kg above EPW at 1y. Early pregnancy BMI was unrelated to mean late postpartum weight change, though odds of late postpartum weight gain (≥2.25 kg) was increased for overweight and obese women as compared with women with BMI <25, suggesting there is greater variability in late postpartum weight change for women with higher early pregnancy BMI. Results further indicate that the relationship between 1 YWR and late postpartum weight gain (≥2.25 kg) was attenuated for women with higher earlier pregnancy BMI. Reduced postpartum weight loss for obese mothers has been documented in previous research (25,37). Other studies of varying durations of follow-up have reported mixed results regarding the relation between prepregnancy body size and postpartum weight outcomes (5,6,8,11,15,16,18,20,38–40). These discrepancies may have resulted in part from the use of different outcome definitions, timing of measurements, and BMI cutpoints. The finding presented herein that overweight and obese women had an overall increased odds of late postpartum weight gain (≥2.25 kg) suggests that these women may also be more susceptible to misclassification as having major retention of GWG according to the conventional definition, leading to overestimation of the effect of GWG on postpartum BMI in this population.
We did not find statistically significant relationships between 1y and 2y postpartum weight gain (≥2.25 kg) and parity, maternal education, breastfeeding or smoking status in logistic regression models. However, we found significantly lower weight change 1y–2y for smokers who quit during pregnancy as compared with nonsmokers in linear models, supporting previous research (26). The associations of 1y–2y weight change with breastfeeding at 1y postpartum and parity approached significance in the linear model adjusted for 1YWR. Though breastfeeding is associated with weight change in the early postpartum period, the influence of breastfeeding on weight change after 6 months postpartum is considered to be small (10,37,41). Additionally, our data may not give precise enough estimates of the intensity of breastfeeding to detect a relationship with postpartum weight change. Previous research has been mixed regarding the relationship between postpartum weight and parity (13,15,26,27,37). Findings presented in this study demonstrating no statistically significant relationships with socioeconomic and behavioral covariates may suggest that late postpartum weight change is related to different factors than those associated with early pregnancy BMI, GWG, and 1YWR. One such factor may be that women who are further from EPW at 1y are relatively more conscientious of weight control between 1y and 2y in an attempt to return to prepregnancy weight as compared to women who have already reached this target weight by 1y. Late postpartum weight change may also reflect the complex influences of child-rearing that have been suggested to account for some of the observed association between parity and body weight in women (9,15,29).
Implications of these findings should be evaluated with respect to the relative strengths and limitations of the study. Due to the sample characteristics, the generalizability of these results to other racial/ethnic populations, to populations in other geographic regions, or to those outside the age range of those included in this study is limited. In addition, while the initial response rate of this study was high (>75%), there are differences between the final sample, the sample originally enrolled in the study, and the eligible population. However, these results show that adjusting for these variables did not substantially alter the estimates; estimates of relationships between late postpartum weight change and 1YWR were particularly robust to multiple alternative model specifications. Finally, a measure of body weight was not available for analysis between delivery and 1y for a majority of the sample, or between 1y and 2y; thus, the present study cannot distinguish 1YWR from weight gain that may have occurred between delivery and 1y, which may have lead to an overestimation of the absolute risk of major retention at 2y in this sample. Strengths of the current study include the prospective study design and the large sample size, which support the internal validity of the findings. The present analysis also addressed the possibility of confounding by adjusting for several potential behavioral and socioeconomic covariates. Additionally, our measure of GWG is strengthened by the use of measured EPW, which prevents bias associated with recalled prepregnancy weight that is likely to differ by maternal BMI.
In conclusion, these findings suggest that maternal weight gain is common after 1y, and may contribute to substantial misclassification of postpartum weight retention if this gain is ignored, leading in turn to biased estimates of the influence of GWG on maternal obesity. Our findings support previous assertions that the use of the term, “postpartum weight retention,” is likely to be appropriate only for a short duration after parturition (31). “Postpartum weight difference” is suggested as a more precise term that accounts for the potential for postpartum weight gain. Additionally, results indicate that misclassification of postpartum weight retention may be accentuated in women with high early pregnancy BMI due to an increased risk of weight gain after 1y. The significant inverse relationship between 1YWR and weight change after 1y across the range of early pregnancy BMI suggests that the difference between 1y and early pregnancy body weight is an important factor in the course of late postpartum weight change.
Acknowledgments
The authors thank Dr Kathleen Rasmussen, Dr Jeffery Sobal, Dr David Just and Dr Tonja Nansel for their helpful comments. This research was funded by NIH grant # HD29549, Cornell University and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
See the online ICMJE Conflict of Interest Forms for this article.
References
- 1.Bradley PJ. Conditions recalled to have been associated with weight gain in adulthood. Appetite. 1985;6:235–241. [PubMed] [Google Scholar]
- 2.Crerand CE, Wadden TA, Sarwer DB, et al. A comparison of weight histories in women with class III vs. class I–II obesity. Surg Obes Relat Dis. 2006;2:165–170. doi: 10.1016/j.soard.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.IOM (Institute of Medicine) Nutrition During Pregnancy. Report. National Academy Press; Washington, DC: 1990. [Google Scholar]
- 4.IOM (Institute of Medicine) Weight Gain During Pregnancy: Reexamining the Guidelines. Report. National Academy Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 5.Ohlin A, Rössner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 6.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 7.Rössner S. Pregnancy, weight cycling and weight gain in obesity. Int J Obes Relat Metab Disord. 1992;16:145–147. [PubMed] [Google Scholar]
- 8.Harris HE, Ellison GT, Holliday M, Lucassen E. The impact of pregnancy on the long-term weight gain of primiparous women in England. Int J Obes Relat Metab Disord. 1997;21:747–755. doi: 10.1038/sj.ijo.0800466. [DOI] [PubMed] [Google Scholar]
- 9.Harris HE, Ellison GT, Clement S. Relative importance of heritable characteristics and lifestyle in the development of maternal obesity. J Epidemiol Community Health. 1999;53:66–74. doi: 10.1136/jech.53.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janney CA, Zhang D, Sowers M. Lactation and weight retention. Am J Clin Nutr. 1997;66:1116–1124. doi: 10.1093/ajcn/66.5.1116. [DOI] [PubMed] [Google Scholar]
- 11.Linné Y, Rössner S. Interrelationships between weight development and weight retention in subsequent pregnancies: the SPAWN study. Acta Obstet Gynecol Scand. 2003;82:318–325. doi: 10.1080/j.1600-0412.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 12.Amorim AR, Rössner S, Neovius M, Lourenço PM, Linné Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15:1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 13.Smith DE, Lewis CE, Caveny JL, et al. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 14.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 15.Williamson DF, Madans J, Pamuk E, et al. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord. 1994;18:561–569. [PubMed] [Google Scholar]
- 16.Boardley DJ, Sargent RG, Coker AL, Hussey JR, Sharpe PA. The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstet Gynecol. 1995;86:834–838. doi: 10.1016/0029-7844(95)00283-W. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- 18.Kac G, Benício MH, Velásquez-Meléndez G, Valente JG, Struchiner CJ. Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of brazilian women. J Nutr. 2004;134:661–666. doi: 10.1093/jn/134.3.661. [DOI] [PubMed] [Google Scholar]
- 19.Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol. 2004;191:530–536. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Rössner S, Ohlin A. Pregnancy as a risk factor for obesity: lessons from the Stockholm Pregnancy and Weight Development Study. Obes Res. 1995;3(Suppl 2):267s–275s. doi: 10.1002/j.1550-8528.1995.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 21.Walker L, Freeland-Graves JH, Milani T, et al. Weight and behavioral and psychosocial factors among ethnically diverse, low-income women after childbirth: II. Trends and correlates. Women Health. 2004;40:19–34. doi: 10.1300/J013v40n02_02. [DOI] [PubMed] [Google Scholar]
- 22.Linné Y, Barkeling B, Rössner S. Long-term weight development after pregnancy. Obes Rev. 2002;3:75–83. doi: 10.1046/j.1467-789x.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- 23.Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103:48–54. doi: 10.1053/jada.2003.50001. [DOI] [PubMed] [Google Scholar]
- 24.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 25.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord. 2001;25:853–862. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen P, Baker JL, Henriksen TB, et al. Influence of psychosocial factors on postpartum weight retention. Obesity (Silver Spring) 2011;19:639–646. doi: 10.1038/oby.2010.175. [DOI] [PubMed] [Google Scholar]
- 27.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord. 2000;24:1660–1668. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- 28.Maddah M, Nikooyeh B. Weight retention from early pregnancy to three years postpartum: a study in Iranian women. Midwifery. 2009;25:731–737. doi: 10.1016/j.midw.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22:261–274. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 30.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–32. ix. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity - results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes (Lond) 2007;31:1642–1651. doi: 10.1038/sj.ijo.0803655. [DOI] [PubMed] [Google Scholar]
- 32.McKeown T, Record RG. The influence of reproduction on body weight in women. J Endocrinol. 1957;15:393–409. doi: 10.1677/joe.0.0150393. [DOI] [PubMed] [Google Scholar]
- 33.Hinton PS, Olson CM. Predictors of pregnancy-associated change in physical activity in a rural white population. Matern Child Health J. 2001;5:7–14. doi: 10.1023/a:1011315616694. [DOI] [PubMed] [Google Scholar]
- 34.Kendall A, Olson CM, Frongillo EA., Jr Evaluation of psychosocial measures for understanding weight-related behaviors in pregnant women. Ann Behav Med. 2001;23:50–58. doi: 10.1207/S15324796ABM2301_8. [DOI] [PubMed] [Google Scholar]
- 35.Truesdale KP, Stevens J, Lewis CE, et al. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes (Lond) 2006;30:1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorsdottir I, Birgisdottir BE. Different weight gain in women of normal weight before pregnancy: postpartum weight and birth weight. Obstet Gynecol. 1998;92:377–383. doi: 10.1016/s0029-7844(98)00187-2. [DOI] [PubMed] [Google Scholar]
- 37.Onyango AW, Nommsen-Rivers L, Siyam A, et al. WHO Multicentre Growth Reference Study Group. Post-partum weight change patterns in the WHO Multicentre Growth Reference Study. Matern Child Nutr. 2011;7:228–240. doi: 10.1111/j.1740-8709.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linné Y, Dye L, Barkeling B, Rössner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004;12:1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 39.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 40.Walker LO. Weight gain after childbirth: a women’s health concern? Ann Behav Med. 1995;17:132–141. doi: 10.1007/BF02895062. [DOI] [PubMed] [Google Scholar]
- 41.Baker JL, Gamborg M, Heitmann BL, et al. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88:1543–1551. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]