Abstract
Hippocampal pathology is central to Alzheimer’s disease (AD) and other forms of dementia such as frontotemporal lobar degeneration (FTLD). Autopsy studies have shown that certain hippocampal subfields are more vulnerable than others to AD and FTLD pathology, in particular the subiculum and cornu ammonis 1 (CA1).
We conducted shape analysis of hippocampi segmented from structural T1 MRI images on clinically diagnosed dementia patients and controls. The subjects included 19 AD and 35 FTLD patients (13 frontotemporal dementia [FTD], 13 semantic dementia [SD] and 9 progressive nonfluent aphasia [PNFA]) and 21 controls.
Compared to controls, SD displayed severe atrophy of the whole left hippocampus. PNFA and FTD also displayed atrophy on the left side, restricted to the hippocampal head in FTD. AD finally displayed most atrophy in left hippocampal body with relative sparing of the hippocampal head. Consistent with pathological studies most deformation was found in CA1 and subiculum areas in FTLD and AD.
1. Introduction
The hippocampal formation is a key site of pathology in Alzheimer’s disease (AD). However, pathology of the hippocampus is also common in several other forms of dementia, including frontotemporal lobar degeneration (FTLD).
The hippocampus can be divided anatomically in different ways. In gross anatomical terms, three regions may be discerned: the hippocampal head (HH), which is the most anterior part, the body (HB) and the tail (HT) (Duvernoy, 2005). On basis of its cytoarchitecture, the hippocampus can be divided into the cornu ammonis subfields (CA1-4), the dentate gyrus (DG) and the subiculum (Duvernoy, 2005).
A three-part subdivision is also valid from a functional or molecular genomic perspective, according to which the structure can be divided into anterior, intermediate and posterior sections (Fanselow and Dong, 2010). The anterior hippocampus is associated with regulation of affect, stress and emotions, and the posterior part with cognitive functions such as memory and spatial learning (Moser and Moser, 1998). An intermediate part has also been identified but its specific functions are less well understood. Each subpart of hippocampus also has a unique connectivity pattern (Fanselow and Dong, 2010). The anterior hippocampus is thus connected to the amygdala and medial prefrontal cortex, regions associated with generation and regulation of emotional response (Fanselow and Dong, 2010; Moser and Moser, 1998). The posterior hippocampus has connections with several subcortical nuclei associated with spatial learning. In contrast to the gross anatomical division, however, this functional subdivision has no sharp boundaries; instead connectivity changes gradually moving from anterior to posterior through the structure (Fanselow and Dong, 2010).
Due to of its central role in the consolidation of memory and emotional processing and its vulnerability in different kinds of dementia, the hippocampal formation has been extensively studied with structural magnetic resonance imaging (MRI) (Apostolova, et al., 2010a; Apostolova, et al., 2006b; Barnes, et al., 2006; Frisoni, et al., 2008; Frisoni, et al., 1999; Probst, et al., 2007; van de Pol, et al., 2006). As hippocampal pathology is viewed as a central landmark in AD, it has also been used as a diagnostic biomarker in clinical practice (Wahlund, et al., 1999). While earlier volumetric MRI studies investigated pathology by measuring the total volume loss of hippocampus (van de Pol, et al., 2006), recent studies attempt to identify regional pathology within the structure. This can be undertaken by analyzing the shape deformation of a particular sub-region, a method used in several studies of AD (Apostolova, et al., 2006a; Apostolova, et al., 2010b; Chupin, et al., 2009; Frisoni, et al., 2008; Frisoni, et al., 2006; Gerardin, et al., 2009; Morra, et al., 2009) and rarely in other forms of dementia (Apostolova, et al., 2010a; Sabattoli, et al., 2008).
It has generally been found that certain hippocampal subfields seem more sensitive than others to neuropathology. For example, in AD most studies have shown reduced volume in the subiculum and CA1 (Frisoni, et al., 2008; Gerardin, et al., 2009; Miller, et al., 2009; Scher, et al., 2007; Wang, et al., 2009). This concurs with the results of several neuropathological studies on AD patients (Scher, et al., 2007).
FTLD is, from a neuropathological point of view, an umbrella term encompassing several different neuropathological processes that lead to common clinical phenotypes (Cairns, et al., 2007). The different neuropathological variants may, however, share some sites of hippocampal pathology, namely, the CA1, subiculum and DG (Amador-Ortiz, et al., 2007; Cairns, et al., 2007; Graham, et al., 2005; Kersaitis, et al., 2004; Piguet, et al., 2011).
The clinical international consensus criteria for FTLD include three syndromes: frontotemporal dementia (FTD), progressive nonfluent aphasia (PNFA), and semantic dementia (SD) (Neary, et al., 1998). FTD is diagnosed primarily on the basis of early decline in interpersonal conduct, early emotional blunting, and an early loss of insight or concern about such changes. This transformation of character should be the dominant feature at onset and throughout the course. The diagnosis of PNFA is made in patients who insidiously develop nonfluent speech with agrammatism (a pattern of simplified sentence structure), phonological paraphasias, or anomia. Apraxia of speech is a common feature whereas word comprehension and comportment are preserved initially. SD, in contrast, is defined by fluent but “empty” spontaneous speech, anomia combined with impaired word comprehension and associative agnosia or prosopagnosia. Surface alexia and surface agraphia may occur in patients who use alphabetic scripts. For the diagnosis of AD the American Psychiatric Association ([DSM-IV-TR], 2000) requires memory deficits as a core diagnostic feature, in addition to at least one of the following symptoms: aphasia, apraxia, agnosia or deficits in executive functioning.
To our knowledge hippocampal shape analysis has not yet been performed on clinically diagnosed FTLD patients. Two studies divided manually outlined anatomical sections traced in coronal plane into subdivisions, moving antero-posteriorly along the long axis of the hippocampal formation in AD and FTLD patients. In one study, the volumes of these subdivisions were then plotted as an antero-posterior volumetric “profile”, revealing that FTD patients showed only anterior atrophy while AD patients had reduced volume in all hippocampal subdivisions (Laakso, et al., 2000). In another study, the anterior-posterior “profile” was divided into 20 subparts, which contained the combined area (in mm3) of the hippocampus and amygdale (Barnes, et al., 2006). In this study SD, FTD and AD patients were included. Both FTLD subtypes had proportionally more anterior than posterior atrophy; while AD again displayed a more evenly distributed atrophy throughout hippocampus.
In the present study we aimed to investigate the atrophic deformation of the hippocampus in the three classical clinical subtypes of FTLD, that is FTD, SD and PNFA, as well asin AD.
Our aims were to investigate the following questions:
Is the regional atrophic deformation of hippocampus in vivo in both the FTLD subtypes and AD consistent with the relative neuropathological vulnerability of the subiculum and the CA11?
Is there more pronounced deformation of the anterior parts of hippocampus in the subtypes of FTLD than in AD?
Does each subtype of FTLD have a characteristic pattern of hippocampal atrophy?
2. Methods
2.1 Participants
The participants included in this investigation have been studied before in relation to cortical and striatal structure (Lindberg, et al., 2009; Looi, et al., 2008; Looi, et al., 2009; Looi, et al., 2011; Looi, et al., 2010).
Participants were recruited retrospectively from the Memory Clinic at the Karolinska University Hospital Huddinge, Stockholm, Sweden. All participants went through the standard investigation procedure at the memory clinic (Andersson, 2007). Laboratory investigations for all subjects were done on blood, CSF and urine (including vitamin B12, folic acid levels and thyroid function). Clinical diagnoses were determined at a multidisciplinary consensus conference with physicians, neuropsychologists, speech-language pathologists and nurses. FTLD syndromes were diagnosed following international consensus criteria (Neary, et al., 1998). Patients with FTLD and AD at different stages of the disease were included. Diagnoses of AD were based on criteria of the ICD-10 International Classification of Diseases, Tenth Revision (WHO, 1992). The control group (CTL) comprised individuals referred to the memory clinic because of mild subjective forgetfulness in everyday life. Objective cognitive impairment was ruled out through comprehensive neuropsychological assessment (impairment was defined as performance 1.5 SD unit below the age-normal mean on any cognitive test). To further minimize the risk of including participants with neurodegenerative diseases in very early stages, we included only those participants whose performance did not deteriorate over a minimum of 2-years follow-up.
Volumetric data was obtained for 13 FTD, 9 PNFA, 13 SD and 19 AD patients, as well as 21 CTL subjects. Nine SD patients had more temporal atrophy on the left and 4 more atrophy on the right side. For analysis of degree of asymmetry in temporal atrophy, the SD group was thus divided into SDL (SD with predominantly left side atrophy) and SDR (SD with predominantly right side atrophy). SD was also divided into SD4, with severe left temporal atrophy, and SD03, with milder left temporal atrophy (see below under visual rating). Background data included age at scanning, Mini-Mental State Examination (MMSE) score (Folstein, et al., 1975) and intracranial volume. Illness duration was also investigated by calculating the number of months/years between first indications of symptoms (in medical records) and the MRI investigation. A non-parametric Kruskal-Wallis ANOVA with a Mann-Whitney U test as post hoc was used for investigating differences in illness duration, age at scanning and MMSE scores. The study was approved by the Regional Ethical Review Board in Stockholm, Sweden. Background data are presented in Table 1. The dementia groups did not differ in age, but all dementia groups had, as expected, significantly lower MMSE scores than the CTL group.
Table 1.
Background data of the included patients
| Group | Age at scan | Illness duration | MMSE | N | M/F | ICV |
|---|---|---|---|---|---|---|
| CTL | 62.9(53.2-78) | NA | 28.9(25-30) | 21 | 7/14 | 1408(1116-1673) |
| AD | 63.7(50.3-74.9) | 2.49 (0.6-5.1) | 22.3(7-29)* | 19 | 8/12 | 1373(1138-1574) |
| PNFA | 65(57.2-78.3) | 3.6 (0.1-8.13) | 16.9(0-28* | 9 | 3/6 | 1373(1296-1542) |
| SD | 64(51.6-76.9) | 3.9(1.31–7.73) | 22.9(5-29)* | 13 | 5/8 | 1440(1204-1682) |
| FTD | 59.8(41.9-72.1) | 1.7 (0.25-3.4) | 20.83(10-30)* | 13 | 4/9 | 1373(1296-1542) |
| SDL | 63.3(51.6-76.9) | 4.1 (I.3-7.7) | 22.4(5-29)* | 9 | 2/7 | 1449(1237-1682) |
| SDR | 65(57.5-73.8) | 3.45 (2.3-5.3) | 24.3(20-27) | 4 | 3/1 | 1420(1204-1610) |
| SD03 | 62.2(51.6-73.8) | 3.47(1.31-5.85) | 25.4(24-27) | 8 | 3/5 | 1339(1237-1610) |
| SD4 | 66.2(56.3-76.9) | 4.59(2.4-7.73)+ | 19.4(5-29) | 5 | 2/3 | 1507(1204-1682) |
CTL=controls. Second column: mean(max-min) age at scan, Third column= mean (max-min) illness duration. Fourth column= mean(max-min) scores on minimal mental state exame. N= number of subjects. M/F= the number of males/females included in the study. ICV= intracranial volume mean(max-min).
= significant difference from FTD in Kruskal-Wallis Anova with a Mann-Whitney U-test post-hoc.
= significantly different from controls in Kruskal-Wallis Anova with a Mann-Whitney U-test post-hoc.
2.2 Image Acquisition
T1-weighted MR images were acquired on a 1.5T Magnetom Vision Plus scanner (Siemens Medical Systems, Erlangen, Germany). A 3D magnetization-prepared rapid gradient echo pulse sequence (TR, 11.4 ms; TE, 4.4 ms; TI, 300 ms; flip angle, 10°; NEX, 1) was used to obtain 72 contiguous coronal 2.5-mm sections with a 512 × 144 matrix and a 230-mm FOV. Original images were subsequently up-interpolated to 1×1×1 mm image resolution before the computation of morphometric analysis.
2.3 Image analysis
2.3.1 Hippocampus
Volumetric analysis was performed using HERMES BMAP Morphy Display (Nuclear Diagnostics AB, Stockholm, Sweden). Hippocampus was measured following rules proposed by Malykhin et al. (2007). This protocol divides the hippocampus into hippocampal head (HH), body (HB) and tail (HT). The most posterior part of the HH was defined as the first slice in which the uncal apex was clearly visible (Malykhin, et al., 2007). The superior border was the alveus, the uncal recess and the inferior horn of the lateral ventricle. The medial border was the inferior horn of the lateral ventricle or the WM of the parahippocampal gyrus. More anteriorly, the superior border of the HH was the amygdala. The fimbria was included as the superomedial border of the HB. The white matter of the parahippocampal gyrus was used to separate the subiculum from the entorhinal cortex by an imaginary line along the HB to the quadrigeminal cistern. The lateral border was the inferior horn of the lateral ventricle or the adjacent white matter. The superior medial border was the quadrigeminal cistern. Anterior border was the last slice before the appearance of the uncus.
The anterior border of the HT was the slice in which the fornix was seen in full profile, or was separated from the wall of the ventricle. As in the HB, the fimbria was included but not the fornix. The white matter of the fornix defined the superior and lateral border boundaries, and the white matter of the parahippocampal gyrus the inferior border. The medial border was the CSF of the quadrigeminal cistern (Malykhin, et al., 2007). OL was trained by NM for the hippocampus measurements. An interrater intraclass correlation coefficient (ICC) was calculated for volumetric data on 20 hippocampi. The ICC was 0.95 for the total volume in comparison with NM. Intrarater ICC was calculated on two occasions on 10 hippocampi and was on both occasions above 0.96. An anatomical atlas of the hippocampus (Duvernoy, 2005) was used to interpret hippocampal anatomy. We used the gross-anatomical division of the region into HH, HB and HT, as specified by above, in the interpretation of the results. On basis of the regional deformation of the structure, we also estimated which cytoarchitectonic subfields that displayed atrophy in the dementia groups.
2.3.2 Intracranial volume
Intracranial volume was measured by stereology point-counting technique following borders proposed by Eritaia et al. (Eritaia, et al., 2000). Measurements were performed by BZ.
2.3.3 Visual rating
Medial temporal lobe (MTL) pathology was also rated in dementia patients. Rating was performed in accordance with the Scheltens scale by an experienced radiologist (LC). The MTL scale ranges from 0 (no atrophy) to 4 (severe atrophy) (Scheltens, et al., 1992). The visual rating scale was used to investigate the progression of atrophy in SD. In a sub analysis the SD patients were divided into two groups SD4 (with a left side MTL rating of 4) and SD03 (with a left side visual rating below 4) (table 1). The whole SD group had significant atrophy all over hippocampus. In order to find early sites of pathology a separate shape analysis of left hippocampus in the SD03 group was performed.
2.3.4 Statistical analysis
Statistical analysis was performed with Statistica 10 (StatSoft, Inc., 2011). Volumetric data were analyzed by one-way-analysis of variance with Tukey HSD post-hoc test. All volumetric data were normalized by intracranial volume (ICV) by the formula volume of region/intracranial volume.
2.3.5 Shape analysis
Shape analysis was undertaken in an automated fashion using the University of North Carolina shape analysis toolkit (http://www.nitrc.org/projects/spharm-pdm); a detailed description of the methodology is available in Styner et al. (2006). Segmented 3D label maps are initially processed to fill interior holes, ensure spherical topology and perform minimal smoothing. These are then mapped into spherical harmonic shape description (SPHARM-PDM), whereby boundary surfaces of each shape are mapped under area-preservation onto a spherical parametrization, followed by describing the original surface locations via sets of coefficients weighting spherical harmonic basis functions (Brechbuhler, et al., 1995). The correspondence between surfaces is established by parameter-based rotation, itself based on first-order expansion of the spherical harmonics, and is then uniformly sampled into a set of 1002 surface points. This surface is then aligned to a study-averaged template for each structure (left and right hippocampus) using rigid-body Procrustes alignment (Bookstein, 1997), with normalization for head size using an ICV based scaling factor, fi, where fi=(Mean(ICV)/ICVi)1/3. We then compute local non-parametric statistical tests that compares the local surface coordinates for group mean differences at the 1002 surface locations to compare shapes between groups (Styner et al., 2006; Levitt et al., 2009). The group difference metric between groups of surface coordinates is derived from the Hotelling T2 two-sample metric. As the shape analysis involves computing 1002 hypothesis tests, one per surface location, a correction for multiple testing is necessary, as an uncorrected analysis would be overly optimistic. The shape analysis uses permutation tests for the computation of raw uncorrected p-values. A false discovery rate (FDR) correction (Genovese et al., 2002) is applied instead of a Bonferroni based multiple comparisons, resulting in a less pessimistic control for type 1 errors and less conservative estimate of false negatives (Pantazis et al., 2004; Styner et al., 2004). Shape statistical analysis significance maps showing local statistical p-values, raw and corrected for FDR, are generated. In results only FDR corrected data are shown. A global shape difference is computed, summarizing average group differences across the surface. Shape statistical analysis also provides visualizations of group test local effect size via mean difference magnitude displacement, which display the magnitude of deflation (mm) between the same point on the mean surface of group 1 and the mean surface of group 2, in a manner analogous to a vector map.
Given the expected effect size in our study, the results of SPHARM-PDM shape statistics can be considered robust despite the relative low number of samples (Walterfang, et al., 2011).
3. Results
3.1 Volumetric analysis
Compared to controls the normalized left hippocampal volume was significantly smaller in all dementia groups. The right-sided volume was significantly smaller in FTD and SD (Table 2) compared to controls. No significant difference was found between dementia groups on either the left or right side volumes. The mean hippocampal volume was larger on the right side in all dementia groups while controls displayed a non-significant larger mean left volume. In SDL and PNFA there was a significant difference showing smaller left than right hippocampal volume. SDR had smaller right than left side volume; however this difference was not significant.
Table 2.
Volumetric data of hippocampus
| HC L | HC R | HC L | HC R | |
|---|---|---|---|---|
| CTL | NA | NA | 3.26 (2.99-3.54) | 3.21 (2.98-3.44) |
| AD | 0.005 | 0.166 | 2.66* (2.42-2.9) | 2.75 (2.48-3.02) |
| PNFA | 0.001 | 0.92 | 2.50* (2.06-2.94) | 2.94 (2.62-3.25)¤ |
| SD | 0.0002 | 0.01 | 2.41* (2.20-2.61) | 2.61* (2.33-2.89) |
| SDL | 0.0001 | 0.16 | 2.29* (1.95-2.63) | 2.79 (2.46-3.12)¤ |
| SDR | 0.09 | 0.003 | 2.67* (2.04-3.30) | 2.21* (1.47-2.95) |
| FTD | 0.0002 | 0.012 | 2.61* (2.32-2.9) | 2.72* (2.38-3.06) |
HC L= Left hippocampus, HC R=Right hippocampus. Column 1&2 denotes P-values in one-way-ANOVA withTukey post-hoc test. Column 3&4 denotes the raw mean with a 95% confidence interval volume of hippocampus in investigated groups.
=significant different compared to controls.
= significant difference between right and left hippocampal volume in T-test (p < or = 0,01).
AD: Alzheimer’s disease, FTD: Frontotemporal dementia, PNFA: Progressive nonfluent aphasia, SD: Semantic dementia, SDL: SD with predominantly left side atrophy, SDR: SD with predominantly right side atrophy
3.2 Hippocampal deformation in the dementia subtypes
3.2.1 AD compared with controls
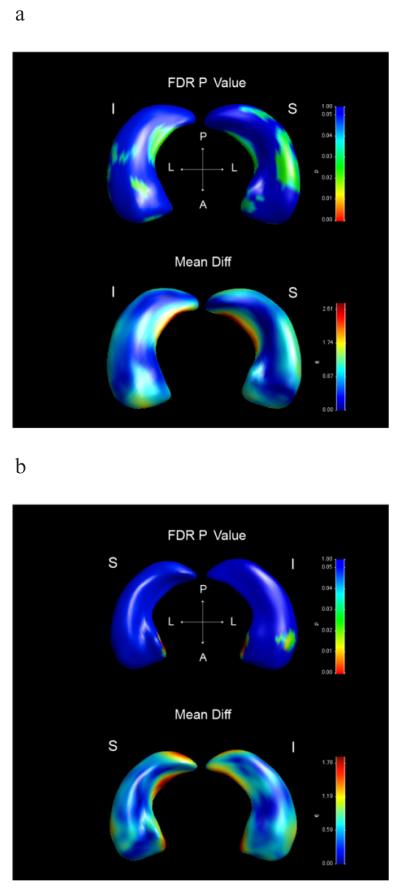
Left hippocampus differed significantly in global shape between AD and controls (p=0.0018). Inferior and superior views also revealed localized shape differences. Atrophy was found in the medial part of the HB in the subiculum area and the lateral part of the HB in the CA1 area. Furthermore, a small part of the anterior hippocampal pole in the CA2-3 area and inferior part of the HH in the subiculum area displayed atrophy (Figure 2a).
Figure 2. Shape statistics and displacements maps.
This figure contains Shape statistics maps (above) and displacements maps below. The statistical shape maps display the significance of local deformations. P-values color significance scale is identical for all images, and warmer colors refer to smaller P-values (less than 0.05). Blue color corresponds to P-values above 0.05. False discovery rate (FDR) corrected P-values are displayed. In the displacements maps color scale is unique for each image, and corresponds to the millimeters of atrophy of the surface in that region. Warmer color corresponds to greater deflation and colors such as green and blue corresponds to lesser deflation. I=inferior view, S=superior view, A=anterior, P=posterior, L=lateral.
2a) Left hippocampus in AD compared to controls.
2b) Right hippocampus in AD compared to controls.
2c) Left hippocampus in FTD compared to controls.
2d) Left hippocampus in PNFA compared to controls.
2e) Left hippocampus in SD compared to controls.
2f) Left hippocampus in SD with visual rating below 4 compared to controls.
The right hippocampus did not display a global shape difference between AD and controls. Significant localized atrophy was however found in the most medial part of the HH corresponding to the subiculum area and in a small spot of the inferior lateral part of the HH in the CA1 and subiculum area (Figure 2b).
3.2.2 FTD compared with controls
There was a significant global shape difference in the left hippocampus between FTD and controls (p=0.0087). Significant atrophy was also found in the anterior pole of the HH in the subiculum area (Figure 2c).
The right hippocampus did not display a global shape or regional difference between FTD and controls.
3.2.3 PNFA compared with controls
The left hippocampus displayed a significant global shape difference between PNFA and controls (p=0.0011). Viewing the hippocampus from above indicated that the most anterior part of the HH and posterior lateral parts of the HB and HT were atrophic, involving CA1-3 and the subiculum. A small part at the medial border of the body in the subiculum area was also affected. The inferior view indicates that a large part of the body was affected involving the CA1-2 area (Figure 2d). The right hippocampus did not display any global shape or regional significant deformation in PNFA.
3.2.4 SD compared with controls
Severe localized atrophy was found in most parts of the left hippocampus in SD. The global shape difference was thus highly significant when comparing SD with controls (p=0.0002). (Figure 2e). There was also a global shape difference in the right hippocampus between SD and controls (p=0.0098), however, no regional significant difference remained after FDR correction.
3.2.5 SD with visual MTL rating of 0-3
The superior view of the left hippocampus shows atrophy of the HH in the anterior pole in the CA1 and subiculum area, in the lateral part of the HH in the CA1 area, and also in the superior medial part of the HH in the CA2-3 area. A small part in the CA2-3 area in the junction between the HB and the HT was also affected. An inferior view indicated that subiculum and the CA1 displayed atrophy in the HH, while a small part of the medial subicular area of the HB as well as a small part the subiculum and CA1 area of the HT (Figure 2f). No global or regional shape difference was found on the right side in SD with left visual MTL rating between 0-3.
4. Discussion
We have investigated global volume loss and regional shape deformation of the hippocampus in three clinical FTLD subtypes and AD. The variance in morphology of the hippocampus across the groups will be discussed in terms of volumetry and different levels of neuroanatomy.
4.1 Volumetry
Global volumetric data revealed hippocampal atrophy in all dementia types on the left side, while only two FTLD subtypes (SD & FTD) were atrophic on the right side. This is consistent with previous findings (Shi, et al., 2009; van de Pol, et al., 2009).
4.2 Morphology: gross anatomy
From gross anatomical viewpoint, the FTD patients only displayed atrophy of left anterior HH. SD patients showed marked atrophy deformation in the whole left hippocampus, while PNFA had deformation in the left HH and HB. AD finally displayed more pronounced deformation of the left HB and some small spots in the HH.
4.2.1 SD
A previous study on SD showed limited atrophy particularly in the HT (Barnes, et al., 2006). As we found more posterior pathology in SD, including the left HT, we hypothesized that this may be an effect of disease progression. To test this we excluded the 5 SD patients that had a visual left medial temporal lobe rating score of 4 (severe pathology). A subset shape analysis was then performed comparing the remaining 8 patients with the same control group (Table 1; SD03). We indeed found more limited posterior pathology in these milder cases of SD (Figure 2f.).
4.2.2 PNFA
Similarity in hippocampal morphology between the clinical FTLD subtypes and AD may potentially be related to the presence of AD pathology in these subtypes (Alladi, et al., 2007). PNFA was the FTLD subtype that displayed most similarity of hippocampal morphology with AD. Both AD and PNFA show deformation of the medial part of the HH and the lateral part of the HB. In a study of clinically diagnosed FTLD patients, around 44% of the PNFA cases had pathology consistent with AD post mortem, while this number was much smaller in SD (10%) and FTD (7%) (Alladi, et al., 2007). Perhaps some of the PNFA patients included in the study were atypical AD patients, contributing to the resemblance between these two groups.
4.2.3 AD
In AD, the left hippocampus displays more pronounced pathology in the body, while the right hippocampus displays atrophy in the medial part of the hippocampal head. Pathological studies of AD suggest that atrophy starts in the entorhinal area (Braak and Braak, 1991) which is adjacent to the medial hippocampal head (Pruessner, et al., 2002). Thus it is very plausible that the atrophy we found in the right HH is more an effect of initial entorhinal rather than hippocampal atrophy. One could argue that if such were the case, then more medial atrophy would be seen in the left HH as well. The medial edge of the HH may however be too small a region to be reliably interpreted in a small group like our AD group (as anatomical variability may be a confounding factor). The interpretation of entorhinal pathology is strengthened in the context of a previous study by Gerardin et al. (2009). This study found medial atrophy of the HH on both side in both AD and in mild cognitive impairment (Gerardin, et al., 2009). An interesting contrast between AD and SD is this. In SD most of the left hippocampus is markedly atrophic; however the medial part of the L HH is relatively spared (Figure 2e). It has been suggested that atrophy in SD starts in the lateral temporal areas and the temporal pole and progresses later to the hippocampus (Graham, et al., 1999a; Graham, et al., 1999b; Graham, et al., 2000; Maguire, et al., 2000). Thus one possible interpretation of this could thus be that atrophy has progressed from medial entorhinal areas in AD but from lateral temporal areas in SD.
4.3 Comparison with previous studies on this dementia-cohort
The patients included in this investigation have been studied before. We have previously shown that the FTLD patients display different degrees of frontal lobe pathology: FTD>PNFA>SD>AD (Lindberg, et al., 2009). The same pattern has also been found in the caudate and in the putamen (Looi, et al., 2008; Looi, et al., 2009). The overall significance of shape deformation of left hippocampus in this study reveals the following pattern: SD>PNFA>AD>FTD while only SD displays a significant deformation on the right side. We have further shown that frontal versus temporal cortical pathology could be used to discriminate SD from FTD and PNFA patients, with the most temporal changes seen in SD and the least in FTD and PNFA (Lindberg, et al., 2009). Maybe a characteristic sign of AD-pathology is manifested in proportionally more atrophy of the HB compared to the HH.
All dementia types in this investigation displayed more atrophy on the left hemisphere. This has previously been found in AD, PNFA and SD (Shi, et al., 2009; van de Pol, et al., 2006) but seems more unexpected in FTD, particularly since we previously showed that this group of FTD patients displayed more atrophy of the right frontal lobe (Lindberg, et al., 2009). Perhaps a lateralized difference has been emphasized by the characteristics of the controls group that displayed a non-significant larger mean volume of the left hippocampus, while the FTD group displayed the opposite pattern.
4.4 Morphology: functional anatomy
In the introduction we also discussed a functional division. As this study did not involve any formal behavioral test, comment about the relevance of the distribution of hippocampal atrophy for emotional behavior is admittedly speculative, yet does indicate possibilities for further research. It can be noted that particularly FTD and SD displayed proportionally more anterior atrophy than PNFA and AD. As discussed in the introduction, the anterior parts of hippocampus are associated with emotional processing (Fanselow and Dong, 2010). It is interesting to note that the international consensus criteria for FTLD describe alteration in emotional behavior both in SD (“loss of sympathy and empathy”) and FTD (“decline in social interpersonal conduct”), while PNFA is defined by having “early preservation of social skills” (Neary, et al., 1998). Furthermore, the American Psychiatric Association ([DSM-IV-TR], 2000) does not include emotional or social dysfunction as diagnostic criteria for AD. Thus the two dementia types that displayed most anterior hippocampal atrophy in this study have been described to develop emotional and social dysfunction. Whether anterior hippocampus by itself may be relevant for this changes can, however, not be investigated in our data. Atrophy of this region could for example also be an indirect indicator of more general atrophy of the anterior temporal lobe (including amygdala and temporal pole).
4.5 Morphology: cytoarchitecture
On basis of the shape analysis, we infer that the subiculum and the CA1 are the two most atrophic cytoarchitectonic subfields in both AD and FTLD. Some involvement was however found also in CA2-3, particularly in the left HH (in AD, SD & PNFA) and in some parts of the body (in SD & PNFA).
4.6 Limitations
A limitation of this study is the difference in illness duration between different patients of the same dementia group and/or between different subgroups of dementia. However, comparative disease duration in clinically defined disease is itself a problematic concept due to probable differences in pathophysiology resulting in disparate clinical features, each with different temporal progressions. This makes it impossible to differentiate between primary sites of pathology and later spreading from primary sites, except by longitudinal study, which was not attempted here. Another limitation is that we did not have pathological confirmation about the underlying cause of the disease in the dementia groups. It has to be emphasized, however, that the relationship between atrophy and pathologically confirmed cases is a quite different approach than used in this study; particularly since the different pathologies may result in similar clinical symptoms (Rabinovici and Miller, 2010).
A third limitation is that we can only study the subfields of hippocampus indirectly (by the location of the deformation) which may yield different information from the actual volume of these subfields (Malykhin, et al., 2010). To measure the subfields volume would, however, require high resolution images that were not available in these patients. The use of a subjective memory complaint, but no cognitive impairment, group as controls was validated by the long term follow-up showing no decline in cognition in the controls.
5. Conclusions
This study demonstrates that both the FTLD subtypes and AD are most vulnerable to atrophy in CA1 and the subiculum. In contrast to FTLD, AD displayed proportionally more atrophy of the HB than the HH. FTD only displayed atrophy in the HH, while SD displayed atrophy of the whole hippocampus. There was a strong resemblance in the pattern of hippocampal atrophy between AD and PNFA. This may potentially reflect the fact that patients with the clinical diagnosis of PNFA more often display AD neuropathology than other FTLD subtypes.
Figure 1. Raw volume of hippocampus in included patients groups.
Figure 1 displays the raw (in cm3) volume of hippocampus in included groups. X-axis denotes included groups, Y-axis denotes the volume of hippocampus. HC=hippocampus. L=left, R=right. AD: Alzheimer’s disease, FTD: Frontotemporal dementia, PNFA: Progressive nonfluent aphasia, SD: Semantic dementia, SDL: SD with predominantly left side atrophy, SDR: SD with predominantly right side atrophy
Acknowledgments
This work was supported by Swedish Brain power. M Styner was supported by NIH grants P40 HD 03110 (IDDRC) and U54 EB005149-01 (NA-MIC),
Stiftelsen gamla tjänarinnor.
Stiftelsen Ragnhild och Einar Lundströms Minne.
JCL Looi self-funded travel costs to assist in conduct of this research.
This research has made use of the SMILE medical imaging laboratory at Karolinska University Hospital, Stockholm, Sweden
Footnotes
The DG is located within the hippocampus and is therefore beyond reach for a shape analysis of the total hippocampus.
None of the coauthors have any disclosures of conflicts of interest.
References
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(Pt 10):2636–45. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007;113(3):245–52. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C. Predictors of cognitive decline in memory clinic patients. Karolinska institut. 2007.
- Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM. Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov Disord. 2010a;25(6):687–8. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006a;129(Pt 11):2867–73. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006b;63(5):693–9. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, Mistur R, Tsui WH, de Leon MJ. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010b;31(7):1077–88. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol. 2006;63(10):1434–9. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brechbuhler C, Gerig G, Kubler O. Parametrization of closed surfaces for 3-D shape description. Computer Vision, Graphics, Image Processing. 1995.
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupin M, Gerardin E, Cuingnet R, Boutet C, Lemieux L, Lehericy S, Benali H, Garnero L, Colliot O. Fully automatic hippocampus segmentation and classification in Alzheimer's disease and mild cognitive impairment applied on data from ADNI. Hippocampus. 2009;19(6):579–87. doi: 10.1002/hipo.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus : functional anatomy, vascularization, and serial sections with MRI. 3rd ed Springer; Berlin ; New York: 2005. [Google Scholar]
- Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, Velakoulis D, Pantelis C. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med. 2000;44(6):973–7. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Ganzola R, Canu E, Rub U, Pizzini FB, Alessandrini F, Zoccatelli G, Beltramello A, Caltagirone C, Thompson PM. Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain. 2008;131(Pt 12):3266–76. doi: 10.1093/brain/awn280. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Laakso MP, Beltramello A, Geroldi C, Bianchetti A, Soininen H, Trabucchi M. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer's disease. Neurology. 1999;52(1):91–100. doi: 10.1212/wnl.52.1.91. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage. 2006;32(1):104–10. doi: 10.1016/j.neuroimage.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Chetelat G, Chupin M, Cuingnet R, Desgranges B, Kim HS, Niethammer M, Dubois B, Lehericy S, Garnero L, Eustache F, Colliot O. Multidimensional classification of hippocampal shape features discriminates Alzheimer's disease and mild cognitive impairment from normal aging. Neuroimage. 2009;47(4):1476–86. doi: 10.1016/j.neuroimage.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Davies R, Xuereb J, Halliday G, Kril J, Creasey H, Graham K, Hodges J. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain. 2005;128(Pt 3):597–605. doi: 10.1093/brain/awh348. [DOI] [PubMed] [Google Scholar]
- Graham KS, Murre JM, Hodges JR. Episodic memory in semantic dementia: a computational approach based on the TraceLink model. Prog Brain Res. 1999a;121:47–65. doi: 10.1016/s0079-6123(08)63066-6. [DOI] [PubMed] [Google Scholar]
- Graham KS, Patterson K, Hodges JR. Episodic memory: new insights from the study of semantic dementia. Curr Opin Neurobiol. 1999b;9(2):245–50. doi: 10.1016/s0959-4388(99)80035-x. [DOI] [PubMed] [Google Scholar]
- Graham KS, Simons JS, Pratt KH, Patterson K, Hodges JR. Insights from semantic dementia on the relationship between episodic and semantic memory. Neuropsychologia. 2000;38(3):313–24. doi: 10.1016/s0028-3932(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Kersaitis C, Halliday GM, Kril JJ. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 2004;108(6):515–23. doi: 10.1007/s00401-004-0917-0. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Frisoni GB, Kononen M, Mikkonen M, Beltramello A, Geroldi C, Bianchetti A, Trabucchi M, Soininen H, Aronen HJ. Hippocampus and entorhinal cortex in frontotemporal dementia and Alzheimer's disease: a morphometric MRI study. Biol Psychiatry. 2000;47(12):1056–63. doi: 10.1016/s0006-3223(99)00306-6. [DOI] [PubMed] [Google Scholar]
- Lindberg O, Ostberg P, Zandbelt BB, Oberg J, Zhang Y, Andersen C, Looi JC, Bogdanovic N, Wahlund LO. Cortical morphometric subclassification of frontotemporal lobar degeneration. AJNR Am J Neuroradiol. 2009;30(6):1233–9. doi: 10.3174/ajnr.A1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, Botes L, Svensson L, Wahlund LO. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR Am J Neuroradiol. 2008;29(8):1537–43. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Svensson L, Lindberg O, Zandbelt BB, Ostberg P, Orndahl E, Wahlund LO. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: differential volumes in dementia subtypes and controls. AJNR Am J Neuroradiol. 2009;30(8):1552–60. doi: 10.3174/ajnr.A1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Walterfang M, Styner M, Niethammer M, Svensson LA, Lindberg O, Ostberg P, Botes L, Orndahl E, Chua P, Velakoulis D, Wahlund LO. Shape analysis of the neostriatum in subtypes of frontotemporal lobar degeneration: Neuroanatomically significant regional morphologic change. Psychiatry Res. 2011 doi: 10.1016/j.pscychresns.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Looi JC, Walterfang M, Styner M, Svensson L, Lindberg O, Ostberg P, Botes L, Orndahl E, Chua P, Kumar R, Velakoulis D, Wahlund LO. Shape analysis of the neostriatum in frontotemporal lobar degeneration, Alzheimer's disease, and controls. Neuroimage. 2010;51(3):970–86. doi: 10.1016/j.neuroimage.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10(4):475–82. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Ogilvie CJ, Coupland NJ, Seres P, Camicioli R. Three-dimensional volumetric analysis and reconstruction of amygdala and hippocampal head, body and tail. Psychiatry Res. 2007;155(2):155–65. doi: 10.1016/j.pscychresns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Lebel RM, Coupland NJ, Wilman AH, Carter R. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage. 2010;49(2):1224–30. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Miller MI, Priebe CE, Qiu A, Fischl B, Kolasny A, Brown T, Park Y, Ratnanather JT, Busa E, Jovicich J, Yu P, Dickerson BC, Buckner RL. Collaborative computational anatomy: an MRI morphometry study of the human brain via diffeomorphic metric mapping. Hum Brain Mapp. 2009;30(7):2132–41. doi: 10.1002/hbm.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR, Jr., Schuff N, Weiner MW, Thompson PM. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp. 2009;30(9):2766–88. doi: 10.1002/hbm.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Piguet O, Halliday GM, Reid WG, Casey B, Carman R, Huang Y, Xuereb JH, Hodges JR, Kril JJ. Clinical phenotypes in autopsy-confirmed Pick disease. Neurology. 2011;76(3):253–9. doi: 10.1212/WNL.0b013e318207b1ce. [DOI] [PubMed] [Google Scholar]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol. 2007;114(4):335–45. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12(12):1342–53. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24(5):375–98. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabattoli F, Boccardi M, Galluzzi S, Treves A, Thompson PM, Frisoni GB. Hippocampal shape differences in dementia with Lewy bodies. Neuroimage. 2008;41(3):699–705. doi: 10.1016/j.neuroimage.2008.02.060. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55(10):967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher AI, Xu Y, Korf ES, White LR, Scheltens P, Toga AW, Thompson PM, Hartley SW, Witter MP, Valentino DJ, Launer LJ. Hippocampal shape analysis in Alzheimer's disease: a population-based study. Neuroimage. 2007;36(1):8–18. doi: 10.1016/j.neuroimage.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–64. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Julin P, Lindqvist J, Scheltens P. Visual assessment of medical temporal lobe atrophy in demented and healthy control subjects: correlation with volumetry. Psychiatry Res. 1999;90(3):193–9. doi: 10.1016/s0925-4927(99)00016-5. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Looi JC, Styner M, Walker RH, Danek A, Niethammer M, Evans A, Kotschet K, Rodrigues GR, Hughes A, Velakoulis D. Shape alterations in the striatum in chorea-acanthocytosis. Psychiatry Res. 2011;192(1):29–36. doi: 10.1016/j.pscychresns.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, Gertz HJ, Scheltens P. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(4):439–42. doi: 10.1136/jnnp.2005.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol LA, Verhey F, Frisoni GB, Tsolaki M, Papapostolou P, Nobili F, Wahlund LO, Minthon L, Frolich L, Hampel H, Soininen H, Knol DL, Barkhof F, Scheltens P, Visser PJ. White matter hyperintensities and medial temporal lobe atrophy in clinical subtypes of mild cognitive impairment: the DESCRIPA study. J Neurol Neurosurg Psychiatry. 2009;80(10):1069–74. doi: 10.1136/jnnp.2008.158881. [DOI] [PubMed] [Google Scholar]
- Wang L, Khan A, Csernansky JG, Fischl B, Miller MI, Morris JC, Beg MF. Fully-automated, multi-stage hippocampus mapping in very mild Alzheimer disease. Hippocampus. 2009;19(6):541–8. doi: 10.1002/hipo.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva: 1992. [Google Scholar]