Abstract
Background and objectives
Acute-On-Chronic liver failure (ACLF) is an emerging entity. The present study was undertaken to analyze the clinical profile and natural course of ACLF patients.
Patients and methods
ACLF was defined as per Asia Pacific Association for the Study of Liver consensus criteria 2009. Patients fulfilling these criteria with some deviations were included and prospectively evaluated for clinical profile, etiologies of acute decompensation (AD) and underlying chronic liver disease, and short-term natural course [3 months].
Results
Out of 123 patients with ACLF (mean age: 45.83 ± 12.05 years; male:female 109:14), 45.53% cases had prior history of AD, and 54.47% presented for the first time as ACLF. Etiologies of cirrhosis were alcohol, cryptogenic, and chronic hepatitis B virus infection in 65.04%, 23.57%, and 11.38% cases, respectively. Recent history of alcohol intake (within 4 weeks) [42.27%] followed by bacterial infections [36.58%] were the common etiologic precipitants for AD. Only 87 (70.73%) out of 123 cases could be followed up for a duration of 3 months; 62 (71.26%) cases died by 3 months. Most deaths occurred in the alcoholics compared to nonalcoholics [(43/53) 81.13% vs. (19/34) 55.88%; P = 0.01]. No significant difference in mortality rate was observed between ACLF cases with history of prior AD compared to newly diagnosed ACLF cases [30/40 (75%) vs. 32/47 (68.09%); P = 0.477]. The prognostic markers [MELD, MELD-Na, CTP] were not significantly different between survivors and nonsurvivors.
Conclusion
ACLF patients in our population had high short-term mortality rates with majority of deaths in alcoholics. Alcohol intake and bacterial infections were mainly responsible for AD in our study.
Abbreviations: ACLF, acute-on-chronic liver failure; AD, acute decompensation; ALD, alcoholic liver disease; ALT, alanine transaminase; APASL, Asian Pacific Association for the Study of the Liver; CLD, chronic liver disease; CTP, Child-Turcotte-Pugh; EASL-AASLD, European Association for the Study of the Liver-American Association for the Study of Liver Diseases; HBV, hepatitis B virus; HE, hepatic encephalopathy; HEV, hepatitis E virus; HRS, hepatorenal syndrome; INR, International Normalized Ratio; MELD, Model for End-Stage Liver Disease; MELD-Na, Model for End-Stage Liver Disease Sodium; PT, prothrombin time; SD, standard deviation; SIRS, systemic inflammatory response syndrome
Keywords: ascites, encephalopathy, hepatic decompensation, renal failure, sepsis
Acute-on-chronic liver failure (ACLF) is an emerging entity denoting an acute deterioration of liver function in patients with chronic liver disease (CLD). However, there is lack of consensus on the definition of “ACLF” and its acute precipitants. This term was first used in 1995 to describe a condition in which two insults to liver are operating simultaneously, one of them being ongoing and chronic and the other one is acute.1 A characteristic feature of ACLF is rapid progression and high short-term mortality, varying from 50 to 90%.2 Currently, two consensus working definitions for this syndrome exist in the literature; the first one propounded by the Asia Pacific Association for the Study of the Liver (APASL) in 2009 defines ACLF as “Acute hepatic insult manifesting as jaundice (serum bilirubin ≥5 mg/dl) and coagulopathy [International Normalized Ratio (INR) Prothrombin Time (PT) ≥1.5], complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease.”3 The second, put forth at the European Association for Study of Liver–American Association for Study of Liver Disease (EASL-AASLD) single topic symposium in 2013, defined ACLF as “Acute deterioration of preexisting, chronic liver disease, usually related to a precipitating event and associated with increased mortality at 3 months due to multisystem organ failure”.2, 4, 5 Alcohol and drugs constitute the majority of acute insults in the West, whereas infectious etiologies predominate in the East. Among the infectious etiologies, reactivation of hepatitis B virus (HBV) infection is one of the major causes of ACLF in Asia.6, 7 In India, superadded infection with hepatitis E virus (HEV) acts as an important acute precipitant leading to ACLF.8, 9, 10 Among the noninfectious etiologies, active alcohol consumption is considered a major acute insult in the western countries11, 12; consumption of hepatotoxic drugs and herbal indigenous medications act as an important acute precipitants in the Asia Pacific region.13, 14 There is paucity of prospective studies on ACLF and its short-term (3 months) natural outcome in our region, which is a region constrained with no facility for liver transplantation. The study was performed with an aim to assess the clinical and biochemical profile, etiologies of underlying CLD, acute insults, and factors affecting the short-term natural outcome of ACLF patients in Coastal Eastern India.
Materials and Methods
Consecutive patients [total 123 patients] of ACLF fulfilling modified APASL Consensus criteria 2009 admitted to the Department of Gastroenterology, S.C.B. Medical College and Hospital, Cuttack between August 2012 and May 2014 were included and prospectively evaluated for 3 months, or till death, whichever occurred earlier. Patients with pregnancy, age <18 years, portal vein thrombosis, hepatocellular carcinoma, and unwillingness to participate in the study were excluded from the study protocol. The study was approved by the Institutional Ethics Committee. In the present study, cirrhosis was diagnosed on the basis of supportive history, clinical findings, biochemical abnormalities, ultrasonographic abnormalities [presence of heterogenous coarse shrunken liver with irregular margins and other signs of portal hypertension], and gastroscopic demonstration of varices. In the study, we also included cirrhotics with prior history of acute decompensation (AD), which is a departure from the APASL criteria that exclude patients with prior decompensation. Besides, we also included cases decompensating following insults like nonhepatotropic infections/sepsis and acute variceal bleeding. These are also deviations from the APASL 2009 criteria. AD was defined as rapid decompensation in the form of development of hepatic encephalopathy (HE) and/or ascites commensurate with the CANONIC study criteria.4 Although, the role of nonhepatic acute insults (nonhepatotropic infections/sepsis and variceal bleeding) in ACLF has not been unambiguously defined in the APASL recommendations, we included them as acute precipitants because of their frequent inclusion as precipitating events in different studies from the West,11, 15 and their frequent association in our cirrhotic population. All the cases were searched for possible causes of AD including intake of hepatotoxic medication, active alcohol drinking (alcohol consumption within 4 weeks of onset of jaundice), infections (urinary tract infection, respiratory tract infection, gastrointestinal infection, etc.), variceal bleeding, viral hepatitis [hepatitis A virus (HAV), HEV, HBV flare], recent history of major surgery, etc. In our study, HBV flare was diagnosed when there was abrupt rise of alanine transaminase (ALT) >5 times upper limit of normal on a background of chronic hepatitis B related cirrhosis. Whenever suspected, the cases were also investigated for autoimmune liver disease, Wilson disease, and Hemochromatosis. In some patients, more than one acute insult was deemed to be responsible for the precipitation of ACLF; in such cases, they were considered as multiple acute insults. All the cases were also analyzed for the varied modes of presentation (jaundice, ascites, HE, upper gastrointestinal bleeding, etc.) and various related complications including hepatorenal syndrome (HRS), cardio-respiratory failure, septicemia, etc. The cases were further evaluated using prognostic scorings such as Child-Turcotte-Pugh (CTP) score, Model for End-Stage Liver Disease (MELD) score, and Model for End-Stage Liver Disease Sodium (MELD-Na) score at baseline.
Statistical Analysis
All the results were expressed as mean ± standard deviation (SD) or frequency (in percent). Normally distributed quantitative and categorical variables were compared using student's t-test and Chi-square test, respectively. Nonparametric unpaired data were compared using Mann–Whitney U-test. All the analyses were performed using SPSS 17 software. A ‘P-value of <0.05’ was considered statistically significant.
Results
The mean age of presentation of ACLF patients in our study was 45.83 ± 12.05 years; majority were males (male:female ratio 109:14). All 123 ACLF cases had cirrhosis of liver as underlying CLD. The etiologies of cirrhosis were alcohol, cryptogenic, and chronic HBV infection in 80 (65.04%), 29 (23.57%), and 14 (11.38%) cases, respectively. Fifty-six (45.52%) patients had cirrhosis of liver with prior history of AD, whereas the rest 67 (54.47%) patients had no prior history of AD. Mean duration of development of ascites from the onset of jaundice was 13.25 ± 7.66 days. Eighty-eight (71.54%) cases had single acute predisposing event, whereas the rest 35 (28.45%) had multiple acute insults (≥2 acute insults). Of the 35 cases with multiple acute predispositions, 30 had 2 acute predispositions and remaining 5 cases had 3 acute predisposing events. Recent history of active alcohol drinking followed by bacterial infections was the leading causes of acute insults. Most cases who decompensated following hepatotoxic medications had ingested indigenous drugs provided by quacks, while 2 cases were attributed to use of antitubercular drugs (ATT). Majority of cases (97.56%) presented with ascites followed by HE (40.65%) cases. Forty-seven (38.21%) cases presented with both ascites and HE. Sixty-four (52%) cases had portal hypertensive gastropathy. Baseline biochemical parameters and prognostic markers of all the cases [survivors as well as nonsurvivors] at the time of enrollment are shown in Table 1. Baseline clinical presentations, biochemical parameters, and prognostic markers in the cases with and without prior history of AD and with single and multiple acute predispositions are shown in Table 2, Table 3, respectively. Sixty-two (50.4%) cases died within 3 months, whereas 25 (20.32%) cases survived, while 36 (29.26%) cases were lost to follow-up. Thus, out of the 87 who were followed up, 71.26% cases had high short-term [3 months] mortality and only 28.73% patients survived beyond 3 months. More deaths occurred in the alcoholics compared to nonalcoholics [(43/53) 81.13% vs. (19/34) 55.88%; P = 0.01]. Out of the 52 cases with active alcohol drink as acute insult, only 36 (69.23%) cases could be followed up and 34 (94.44%) cases died. Out of 71 cases with nonalcoholic acute predispositions, only 51 (71.83%) cases could be followed up; of these, 28 (54.9%) cases died. Short-term/early (3 months) mortality was significantly higher in cases with alcoholic precipitation compared to cases with nonalcoholic acute precipitations (P value <0.0001). Out of the 67 newly diagnosed ACLF cases, only 46 (68.65%) cases could be followed up; of them 32 (69.56%) died by 3 months. Out of 56 cases with history of prior AD, 41 (73.21%) cases could be followed up, and 30 (73.17%) died by 3 months. Short-term mortality in ACLF cases basing on history of decompensation, etiology of CLD, clinical presentations, and as per acute predisposing events are shown in Table 4, Table 5, respectively. The comparative short-term mortality in ACLF cases with reference to underlying cirrhosis is shown in Figure 1. Short-term mortality was not significantly different when cases with history of prior AD were compared to cases without history of prior AD (P value: 0.67). Short-term mortality was not significantly different between cases with multiple acute predispositions compared to cases with single acute insult (81.48% vs. 66.12%; P value >0.05). Conventional Prognostic markers (CTP, MELD, MELD-Na) were not statistically different between the survivors and nonsurvivors, between cases with and without history of prior AD, and between cases with single and multiple acute precipitants (P value >0.05).
Table 1.
Baseline Biochemical Parameters (Mean ± SD) and Prognostic Markers in All the Cases at the Time of Enrollment, and in Survived and Expired Cases.
| Biochemical parameters and prognostic markers | Total cases (N = 123) | Nonsurvivors (N = 62) | Survivors (N = 25) | ‘P’ value (Compared between survivors and nonsurvivors) |
|---|---|---|---|---|
| TLC/cmma | 9050 (3800–32,000) | 9000 (4000–32,000) | 10,000 (5000–20,000) | 0.57 |
| Urea (mg/dl)a | 28 (5.1–223) | 28.5 (5.1–152) | 43 (17–119) | 0.16 |
| Creatinine (mg/dl)a | 1.1 (0.5–8.1) | 1.03 (0.5–5.4) | 1.2 (0.6–8.1) | 0.07 |
| Sodium (mmol/L) | 131.82 ± 6.55 | 131.05 ± 6.86 | 131.92 ± 7.47 | 0.6 |
| Total bilirubin (mg/dl)a | 8.7 (5.1–40) | 9.4 (5.1–40) | 7.4 (5.1–19.2) | 0.21 |
| AST (IU/L)a | 98 (23–559) | 98 (23–509) | 97 (27–599) | 0.63 |
| ALT (IU/L)a | 64 (20–370) | 63 (20–370) | 62 (29–219) | 0.89 |
| ALP (IU/L)a | 260 (61–975) | 242 (61–975) | 312 (107–493) | 0.08 |
| Albumin (g/dl) | 2.74 ± 0.56 | 2.75 ± 0.6 | 2.78 ± 0.46 | 0.83 |
| INR (PT) | 2.46 ± 0.8 | 2.53 ± 0.81 | 2.49 ± 0.97 | 0.83 |
| CTP | 12.26 ± 1.27 | 12.24 ± 1.69 | 12.29 ± 1.18 | 0.87 |
| MELD | 27.47 ± 6.53 | 27.68 ± 6.16 | 27.76 ± 5.47 | 0.95 |
| MELD-Na | 29.74 ± 5.6 | 29.72 ± 5.21 | 30.19 ± 4.8 | 0.68 |
N: Number; TLC: total leukocyte count; AST: aspartate transaminase; ALT: alanine transaminase; ALP: alkaline phosphatase; INR (PT): International Normalized Ratio (Prothrombin Time); CTP: Child Turcotte Pugh; MELD: Model for End-Stage Liver Disease; MELD-Na: Model for End-Stage Liver Disease Sodium.
Median (range) value.
Table 2.
Baseline Clinical Presentations in Cases With and Without Prior History of (h/o) Acute Decompensation (AD) and With Single and Multiple Acute Predispositions.
| Clinical presentation | Cases without prior h/o AD, N (%) | Cases with prior h/o AD, N (%) | ‘P’ value | Cases with single acute insult, N (%) | Cases with multiple acute insults, N (%) | ‘P’ value |
|---|---|---|---|---|---|---|
| Hepatorenal syndrome (HRS) | 22 (32.83) | 10 (17.85) | 0.05 | 21 (23.86) | 11 (31.42) | 0.35 |
| Hepatic encephalopathy (HE) | 23 (34.32) | 27 (48.21) | 0.11 | 36 (40.9) | 14 (40) | 1 |
| Spontaneous bacterial peritonitis (SBP) | 5 (7.46) | 10 (17.85) | 0.08 | 6 (6.81) | 9 (25.71) | 0.002 |
| Upper gastrointestinal (UGI) bleed | 12 (17.91) | 7 (12.5) | 0.43 | 10 (11.36) | 9 (25.71) | 0.04 |
| Ascites | 64 (95.52) | 56 (100) | 0.08 | 88 (100) | 32 (91.42) | 0.004 |
| Ascites with HE | 20 (29.85) | 27 (48.21) | 0.03 | 32 (36.36) | 15 (42.85) | 0.53 |
| Hyponatremia | 40 (59.7) | 41 (73.21) | 0.1 | 59 (67.04) | 22 (62.85) | 0.59 |
Table 3.
Baseline Biochemical Parameters (Mean ± SD), and Prognostic Markers in Cases With and Without Prior History of Acute Decompensation and With Single and Multiple Acute Predispositions.
| Biochemical parameters and prognostic markers | Cases without prior H/O AD (N = 67) | Cases with prior H/O AD (N = 56) | ‘P’ value | Cases with single acute insult (N = 88) | Cases with multiple acute insult (N = 35) | ‘P’ value |
|---|---|---|---|---|---|---|
| TLC/cmma | 9050 (4400–32,000) | 9000 (3800–20,000) | 0.63 | 8600 (3800–20,000) | 10,400 (5200–32,000) | 0.02 |
| Urea (mg/dl)a | 27 (5.1–152) | 29.5 (14–223) | 0.95 | 26 (14–223) | 32 (5.1–152) | 0.18 |
| Creatinine (mg/dl)a | 1.2 (0.6–6.8) | 1 (0.5–8.1) | 0.01 | 1.08 (0.5–8.1) | 1.1 (0.5–5.4) | 0.38 |
| Sodium (mmol/L) | 132.10 ± 6.69 | 131.36 ±6.45 | 0.53 | 131.89 ±5.76 | 131.46 ± 8.35 | 0.74 |
| Total bilirubin (mg/dl)a | 9.2 (5.1–36) | 7.8 (5.2–40) | 0.19 | 9.1 (5.1–40) | 7.8 (5.1–36) | 0.67 |
| AST (IU/L)a | 110 (27–599) | 86 (23–509) | 0.054 | 98 (23–599) | 96 (27–492) | 0.51 |
| ALT (IU/L)a | 71 (20–330) | 55 (26–370) | 0.004 | 64 (20–370) | 63 (29–325) | 0.81 |
| ALP (IU/L)a | 263 (69–975) | 259 (61–920) | 0.59 | 266 (61–975) | 235 (97–653) | 0.16 |
| Albumin (g/dl) | 2.75 ± 0.54 | 2.71 ± 0.57 | 0.68 | 2.76 ± 0.53 | 2.65 ± 0.62 | 0.31 |
| INR (PT) | 2.38 ± 0.78 | 2.56 ± 0.83 | 0.24 | 2.42 ±0.84 | 2.56 ± 0.71 | 0.4 |
| CTP | 12.17 ± 1.21 | 12.37 ± 1.33 | 0.38 | 12.17 ±1.32 | 12.57 ± 1.01 | 0.1 |
| MELD | 28.21 ± 7.48 | 26.61 ± 5.15 | 0.17 | 27.25 ± 6.85 | 28.20 ± 5.54 | 0.46 |
| MELD-Na | 30.18 ± 6.45 | 29.23 ± 4.42 | 0.34 | 29.48 ± 5.89 | 30.63 ± 4.61 | 0.30 |
N: number; SD: standard deviation; H/O AD: history of acute decompensation; TLC: total leukocyte count; AST: aspartate transaminase; ALT: alanine transaminase; ALP: alkaline phosphatase; INR (PT): International Normalized Ratio (Prothrombin Time); CTP: Child Turcotte Pugh; MELD: Model for End-Stage Liver Disease; MELD-Na: Model for End-Stage Liver Disease Sodium.
Median (range) value.
Table 4.
Short-Term (3 Months) Mortality in Cases as per History of Decompensation, Etiology of Chronic Liver Disease, and Clinical Presentations.
| Cases with | Total cases, N (%) | Followed up, N (%) | Survived, N (%) | Expired, N (%) |
|---|---|---|---|---|
| First onset of AD | 67 (54.47) | 46 (68.65) | 14 (30.43) | 32 (69.56) |
| Prior history of AD | 56 (45.52) | 41 (73.21) | 11 (26.82) | 30 (73.17) |
| Alcoholic cirrhosis | 80 (65.04) | 53 (66.25) | 10 (18.86) | 43 (81.13) |
| HBV-related cirrhosis | 14 (11.38) | 11 (78.57) | 6 (54.54) | 5 (45.45) |
| Cryptogenic cirrhosis | 29 (23.57) | 23 (79.31) | 9 (39.13) | 14 (60.86) |
| Hepatorenal syndrome | 32 (26.01) | 26 (81.25) | 8 (30.76) | 18 (69.23) |
| Hepatic encephalopathy | 50 (40.65) | 40 (80) | 14 (35) | 26 (65) |
| Ascites with HE | 47 (38.21) | 39 (82.97) | 14 (35.89) | 25 (64.1) |
| Ascites | 120 (97.56) | 84 (70) | 24 (28.57) | 60 (71.42) |
| SBP | 15 (12.19) | 11 (73.33) | 5 (45.45) | 6 (54.54) |
| UGI bleed | 17 (13.82) | 12 (70.58) | 5 (41.66) | 7 (58.33) |
| Hyponatremia | 81 (65.85) | 58 (71.6) | 18 (31.03) | 40 (68.96) |
N: number; AD: acute decompensation; HBV: hepatitis B virus; HE: hepatic encephalopathy; SBP: spontaneous bacterial peritonitis; UGI: upper gastrointestinal.
Table 5.
Short-Term (3 Months) Mortality in ACLF Cases as per Acute Predisposing Event.
| Acute predisposing agent | Total cases predisposed, N (%) | Followed up cases, N (%) | Cases died, N (%) |
|---|---|---|---|
| Single insult | 88 (71.54) | 62 (70.45) | 40 (66.12) |
| Alcohol intake | 30 (34.09) | 22 (73.33) | 21 (95.45) |
| HAV infection | 6 (6.81) | 6 (4.87) | 3 (50) |
| HBV flare | 7 (7.95) | 5 (71.42) | 3 (60) |
| Infection | 22 (25) | 16 (69.56) | 6 (37.5) |
| Hepatotoxic medication | 6 (6.81) | 3 (50) | 1 (33.33) |
| Variceal bleed | 2 (2.27) | 1 (50) | 0 (0) |
| Unknown predisposition | 15 (17.04) | 9 (60) | 7 (77.77) |
| Multiple insults (Probable sequential order of individual acute insults) | 35 (28.45) | 27 (77.14) | 22 (81.48) |
| Alcohol intake → Infection | 12 (34.28) | 10 (37.03) | 9 (90) |
| Alcohol intake → Indigenous drugs | 6 (17.14) | 4 (14.81) | 2 (50) |
| Infections → Indigenous drugs | 4 (11.42) | 2 (7.4) | 2 (100) |
| Infections → Variceal bleed | 3 (8.57) | 2 (7.4) | 1 (50) |
| Infection → Falciparum malaria | 3 (8.57) | 2 (7.4) | 1 (50) |
| Alcohol intake → Acute HAV infection | 2 (5.71) | 2 (7.4) | 2 (100) |
| Alcohol intake → Variceal bleed | 1 (2.85) | 1 (3.7) | 1 (100) |
| Alcohol intake → HBV Flare → Antitubercular drugs | 2 (5.71) | 2 (7.4) | 2 (100) |
| Acute HEV Infection → Indigenous drugs → Infection | 1 (2.85) | 1 (3.7) | 1 (100) |
| Acute HAV infection → Indigenous drugs → Infection | 1 (2.85) | 1 (3.7) | 1 (100) |
N: number; →: followed by; HAV: hepatitis A virus; HBV: hepatitis B virus; HEV: hepatitis B virus.
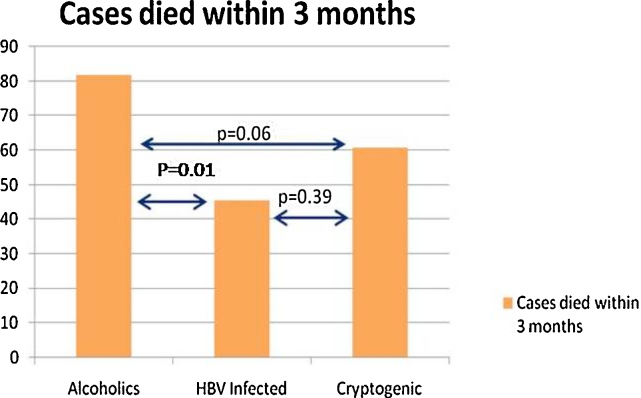
Figure 1.
Comparative short-term mortality (3 months) in ACLF cases basing on etiology of chronic liver disease. In-between alcoholics and cryptogenic cases: P = 0.06; alcoholics and hepatitis B virus (HBV) related cases: P = 0.01; HBV-related and cryptogenic cases: P = 0.01.
Discussion
ACLF is a distinct entity with potential reversibility, i.e. possibility of returning back to the baseline clinical status if the acute event and clinical consequences recover.3 Both, hepatic and nonhepatic insults, may lead to ACLF.16 Studies from the West suggest that nonhepatic insults like acute variceal bleed, nonhepatotropic infections, and sepsis can act as acute insults while data from Southeast Asia and the Indian subcontinent support the role of acute hepatic insults as major acute events.8, 10, 11, 12, 15, 17, 18, 19 In the APASL 2009 consensus meet, there was opposition to inclusion of variceal bleed in the list of acute insults3; however, others favor inclusion of variceal bleed as an acute insult leading to ACLF.2, 20 Jalan et al. suggested that nonhepatic insults like gastrointestinal bleeding or sepsis may initiate a cascade resulting in end-organ dysfunction and liver failure in patients with stable cirrhotic status.21 They hypothesized that upregulated cytokine activity and disturbances in systemic hemodynamics may act as etiopathogenetic factors in these patients.21 Besides, patients with ACLF have decreased cellular immunity resulting in increased sepsis-related morbidities and mortality.22 Sepsis is defined as the presence of systemic inflammatory response syndrome (SIRS) in presence of infection with or without the presence of multiorgan dysfunction. In India, infections other than hepatitis viruses are common causes of hepatic dysfunction. Although an Indian study reported that in tropical countries falciparum malaria, dengue fever, and enteric fever may contribute to acute liver failure,23 the role of these infections in causing ACLF is not yet clear. As per APASL 2009 consensus, any infectious agent leading to acute hepatocellular dysfunction, by directly affecting the liver in a patient with CLD, should be considered as an acute precipitant.3 In our study, we found three cases had falciparum malaria associated with other acute precipitants. However, basing on the small numbers and presence of other associated acute precipitants, their causal role is difficult to be established; their association with ACLF might merely be coincidental. Most (65.04%) ACLF cases in our study had alcoholic liver disease (ALD) as underlying CLD, which was also observed in the CANONIC study4 and study by Duseja et al.20 Patients with ALD have increased risk of suffering from infections and sepsis,24 due to defective neutrophil functions.25 The APASL consensus recommendations opposed inclusion of nonhepatotropic infections as acute precipitants, because ACLF cases were at an increased risk of suffering from infections due to depressed cellular immunity due to underlying CLD process.3 We included only those cases of ACLF with infection and hepatic impairment who otherwise fulfilled the definition of ACLF as per modified APASL consensus. Acute variceal bleeding would not qualify as an acute event, if there is no jaundice. We only included patients presenting with jaundice and other components of ACLF according to modified APASL criteria following variceal bleed. In our study, in 6 of 123 ACLF cases, variceal bleeding precipitated AD, which was similarly observed in previous studies.11 While data from South-east Asia have implicated acute flare of HBV as a common precipitant of ACLF,18, 19 other Indian studies have reported that superadded hepatotropic viral infections, such as acute HEV infection, are the dominant acute precipitants.8, 10, 17, 26, 27 Saraswat et al. reported ACLF in 121 (3.75%) of 3220 patients over a period of >6 years. The acute event was attributed to HEV in 80 (61.1%), HAV in 33 (27.2%), and combined HEV and HAV infection in 8 (6.1%) patients.26 However, in our study, HEV and HAV infections were less frequent causes of AD. Besides, it is difficult to compare our results with the other Indian studies because of variable criteria used for diagnosis of ACLF. The clinical presentation of our patients was similar to other studies, with majority of cases having ascites, hepatic encephalopathy, renal failure, and Child's C status. High short-term mortality of 30–50% in 1 month has been reported in patients with ACLF.11 Comparative patients’ characteristics between the current study and prior studies are shown in Table 6. We observed a very high short-term mortality in ACLF cases in our study, which was also observed in CANONIC study,4 studies by Duseja et al.,20 and Jha et al.28 In our study there was no significant difference in mortality between the cases presenting with leukocytosis compared to cases without leukocytosis, in contrast to CANONIC study.4 We observed statistically nonsignificant trend of higher mortality in patients with multiple acute insults compared to cases with single acute predisposition, as reported by Jha et al.28 We found significantly higher mortality in patients with alcoholic predisposition compared to cases with nonalcoholic acute insults, as reported in CANONIC study.4 However, we observed a statistically nonsignificant trend of higher mortality in ACLF cases with history of prior AD compared to newly diagnosed ACLF cases, in contrast to the CANONIC study.4 Alcohol intake was the most common etiology of CLD in our study, as reported in the CANONIC study4 and study by Duseja et al.20 In our study, 71.2% cases died within a period of 3 months, similar to CANONIC study.4 The in-hospital mortality rate in our study was 39.1%, which was similar to the figures reported in other studies.4, 29 Recent studies by Duseja et al.30 and Agrawal et al.31 have reported that Acute Physiology and Chronic Health Evaluation (APACHE II) score and Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score were better than SOFA, CTP, and MELD scores in predicting short-term mortality in ACLF patients, respectively.
Table 6.
Comparative Patients’ Characteristics Between the Current Study and Prior Studies.
| Patients characteristics | Current study | Study by Duseja et al.20 | Study by Jha et al.28 | CANONIC study4 |
|---|---|---|---|---|
| Total no. of cases | 123 | 102 | 52 | 303 |
| Mean age ± SD (years) | 45.83 ± 12.05 | 44 ± 12.5 | 38.6 ± 16.7 | 57.2 ± 12.2 |
| Male:Female | 7.78:1 | 5:1 | 3.72:1 | 1.8:1 |
| Acute precipitants (single) | Cases (%) | Cases (%) | Cases (%) | Cases (%) |
| Active alcohol drink | 34 | 29 | – | 24.5 |
| Infection | 25 | 47 | 24.9 | 32.6 |
| HEV infection | 0 | 4 | 9.6 | □ |
| HAV infection | 6.81 | 2 | 11.5 | □ |
| HBV flare | 7.95 | 1 | 9.6 | □ |
| Acute variceal bleed | 2.27 | 12 | □ | 13.2 |
| Surgical intervention | 0 | 1 | 1.92 | 8.6 |
| Hepatotoxic medication | 6.81 | 1 | 7.6 | □ |
| Unknown | 17.04 | 1 | 11.5 | 43.6 |
| Multiple predisposition | 28.45 | □ | 17.1 | 13.5 |
| Short-term mortality | 71.26 | 46 | 53.8 | 51.2 |
| CTP Score | 12.26 ± 1.27 | 10.8 ± 2.4 | 11.69 ± 1.48 | 9.7 ± 2.1 |
SD: standard deviation; HEV: hepatitis E virus; □: not found/evaluated; HAV: hepatitis A virus; HBV: hepatitis B virus; CTP: Child Turcotte Pugh.
Our study has some limitations inherent to small sample size and lack of long-term follow-up. We could not study the roles of various inflammatory cytokines, hemodynamics, and liver histology in our study. Besides, we could not use important relevant prognostic scoring systems, such as APACHE II, SOFA, or CLIF-SOFA scores in the assessment of short-term natural course. A significant proportion of cases were lost to follow-up affecting the accuracy of the mortality estimates in our study.
Conclusion
ACLF patients in our population had high short-term mortality rates with most of the deaths occurring in the alcohol-related cirrhotic. Active alcohol drinking followed by bacterial infections was mainly responsible for AD in our study. Further prospective long-term studies with large number of cases are required to validate our findings. The high mortality highlights the fact that there is need for liver transplantation in the management of these patients, who would otherwise succumb unless transplanted.
Conflicts of Interest
The authors have none to declare.
References
- 1.Ohnishi H., Sugihara J., Moriwaki H., Muto Y. Acute-on-chronic liver failure. Ryoikibetsu Shokogun Shirizu. 1995;(7):217–219. [PubMed] [Google Scholar]
- 2.Jalan R., Gines P., Olson J.C. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Sarin S.K., Kumar A., Almeida J.A. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau R., Jalan R., Gines P. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Olson J.C., Wendon J.A., Kramer D.J. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- 6.Kohrt H.E., Ouyang D.L., Keeffe E.B. Antiviral prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis. 2007;11:965–991. doi: 10.1016/j.cld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Sera T., Hiasa Y., Michitaka K. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern Med. 2006;45:721–724. doi: 10.2169/internalmedicine.45.1590. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran J., Eapen C.E., Kang G. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–138. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 9.Monga R., Garg S., Tyagi P., Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol. 2004;23:50–52. [PubMed] [Google Scholar]
- 10.Kumar M., Sharma B.C., Sarin S.K. Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus-related chronic liver disease. J Gastroenterol Hepatol. 2008;23(6):83–87. doi: 10.1111/j.1440-1746.2007.05243.x. [DOI] [PubMed] [Google Scholar]
- 11.Laleman W., Wilmer A., Evenepoel P. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. doi: 10.1186/cc4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen S., Davies N.A., Mookerjee R.P. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109–1119. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 13.Lee K.H., Lee M.K., Sutedja D.S., Lim S.G. Outcome from molecular adsorbent recycling system (MARS) liver dialysis following drug-induced liver failure. Liver Int. 2005;25:973–977. doi: 10.1111/j.1478-3231.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- 14.Mattéi A., Rucay P., Samuel D., Feray C., Reynes M., Bismuth H. Liver transplantation for severe acute liver failure after herbal medicine (Teucrium polium) administration. J Hepatol. 1995;22:597. doi: 10.1016/0168-8278(95)80458-7. [DOI] [PubMed] [Google Scholar]
- 15.Sen S., Williams R., Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22(suppl 2):5–13. doi: 10.1034/j.1600-0676.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Malik R., Mookerjee R.P., Jalan R. Infection and inflammation in liver failure: two sides of the same coin. J Hepatol. 2009;51:426–429. doi: 10.1016/j.jhep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Acharya S.K., Sharma P.K., Singh R. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Cui Y.L., Yan F., Wang Y.B. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci. 2010;55:2373–2380. doi: 10.1007/s10620-010-1257-7. [DOI] [PubMed] [Google Scholar]
- 19.Yu J.W., Sun L.J., Zhao Y.H., Li S.C. Prediction value of model for end-stage liver disease scoring system on prognosis in patients with acute-on-chronic hepatitis B liver failure after plasma exchange and lamivudine treatment. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1242–1249. doi: 10.1111/j.1440-1746.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- 20.Duseja A., Chawla Y.K., Dhiman R.K., Kumar A., Choudhary N., Taneja S. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF) Dig Dis Sci. 2010;55:3188–3192. doi: 10.1007/s10620-010-1377-0. [DOI] [PubMed] [Google Scholar]
- 21.Jalan R., Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 22.Wasmuth H.E., Kunz D., Yagmur E. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Deepak N.A., Patel N.D. Differential diagnosis of acute liver failure in India. Ann Hepatol. 2006;150:6. [PubMed] [Google Scholar]
- 24.Jalan R., Stadlbauer V., Sen S. Natural history of acute decompensation of cirrhosis: the basis of definition, prognosis and pathophysiology of acute on chronic liver failure. Hepatology. 2006;44(suppl 1):371A. [Google Scholar]
- 25.Mookerjee R.P., Stadlbauer V., Lidder S. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 26.Radha Krishna Y., Saraswat V.A., Das K. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009;29:392–398. doi: 10.1111/j.1478-3231.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A., Aggarwal R., Naik S.R., Saraswat V., Ghoshal U.C., Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004;23:59–62. [PubMed] [Google Scholar]
- 28.Jha A.K., Nijhawan S., Rai R.R., Nepalia S., Jain P., Suchismita A. Etiology, clinical profile, and in-hospital mortality of acute-on-chronic liver failure: a prospective study. Indian J Gastroenterol. 2013;32(2):108–114. doi: 10.1007/s12664-012-0295-9. [DOI] [PubMed] [Google Scholar]
- 29.Katoonizadeh A., Laleman W., Verslype C. Early features of acute-on-chronic liver failure: a prospective cohort study. Gut. 2010;59:1561–1569. doi: 10.1136/gut.2009.189639. [DOI] [PubMed] [Google Scholar]
- 30.Duseja A., Choudhary N.S., Gupta S., Dhiman R.K., Chawla Y. APACHE II score is superior to SOFA, CTP and MELD in predicting the short-term mortality in patients with acute-on-chronic liver failure (ACLF) J Dig Dis. 2013;14(September (9)):484–490. doi: 10.1111/1751-2980.12074. [DOI] [PubMed] [Google Scholar]
- 31.Dhiman R.K., Agrawal S., Gupta T., Duseja A., Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20(October (40)):14934–14941. doi: 10.3748/wjg.v20.i40.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]