Abstract
Objective
To analyse the impact of postprocedural mitral regurgitation (MR), in an interaction with aortic regurgitation (AR), on mortality following transcatheter aortic valve implantation (TAVI).
Methods
To assess the interaction between MR and AR, we compared the survival rate of patients (i) without both significant MR and AR versus (ii) those with either significant MR or significant AR versus (iii) with significant MR and AR, all postprocedure. 381 participants of the Polish Transcatheter Aortic Valve Implantation Registry (166 males (43.6%) and 215 females (56.4%), age 78.8±7.4 years) were analysed. Follow-up was 94.1±96.5 days.
Results
Inhospital and midterm mortality were 6.6% and 10.2%, respectively. Significant MR and AR were present in 16% and 8.1% patients, including 3.1% patients with both significant MR and AR. Patients with significant versus insignificant AR differed with respect to mortality (log rank p=0.009). This difference was not apparent in a subgroup of patients without significant MR (log rank p=0.80). In a subgroup of patients without significant AR, there were no significant differences in mortality between individuals with versus without significant MR (log rank p=0.44). Significant MR and AR had a significant impact on mortality only when associated with each other (log rank p<0.0001). At multivariate Cox regression modelling concomitant significant MR and AR were independently associated with mortality (OR 3.2, 95% CI 1.54 to 5.71, p=0.002).
Conclusions
Significant MR or AR postprocedure, when isolated, had no impact on survival. Combined MR and AR had a significant impact on a patient's prognosis.
Introduction
Mitral regurgitation frequently coexists with aortic stenosis. Its presence is associated with increased surgical risk and a worse long-term outcome in patients undergoing aortic valve replacement.1 There seems to be little doubt that preprocedural mitral regurgitation adversely impacts prognosis in transcatheter aortic valve implantation (TAVI) patients as well.2 However, in some patients, the severity of mitral regurgitation is reduced following TAVI.3 Data concerning the potential clinical benefit of such an improvement with long-term follow-up are more limited.2 Recently, Khawaja et al4 demonstrated that mitral regurgitation worsening is associated with a poorer survival rate. Persistent or even worsening significant mitral regurgitation may be especially important in patients who develop a significant paravalvular leak following TAVI, leading to additional volume overload and making these patients vulnerable to haemodynamic decompensation. However, the impact of this adverse interaction between mitral and aortic regurgitation on survival rates following TAVI has not yet been separately studied.
The Polish Transcatheter Aortic Valve Implantation Registry (POL-TAVI) 2013 included 105 patients qualified for TAVI with significant mitral regurgitation. This provided an opportunity to supplement existing data concerning the impact of preprocedural and postprocedural mitral regurgitation on mortality following TAVI and its interaction with aortic regurgitation.
Methods
POL-TAVI is an obligatory prospective registry of all patients undergoing TAVI in Polish hospital centres.5 The registry database contains detailed demographic and clinical characteristics, results of imaging studies including echocardiography and CT, laboratory assessment, procedural results and the results of a short-term and midterm follow-up (1 month, 6 months and 1 year). The current analysis included patients who underwent TAVI in the year 2013, regardless of the access site and valve type.
Echocardiographic study
Transthoracic echocardiographic studies were performed prior to the procedure, immediately postprocedure and at a 1-month follow-up. Mitral regurgitation assessment involved assessment of multiple indices including valve morphology, colour Doppler and continuous-wave Doppler of the regurgitant jet, vena contracta width, regurgitant volume and effective orifice area (when available), according to current guidelines.6 7 Mitral regurgitation was considered ‘significant’ if graded as moderate (grade 3) or severe (grade 4). Changes in mitral regurgitation severity were assessed between the baseline study, postprocedure and at a 1-month follow-up. They were classified as improvement/no change and worsening of mitral regurgitation by at least one grade. Paravalvular aortic regurgitation was graded according to the Valve Academic Research Consortium (VARC)-2 criteria.8
The follow-up period was defined from the date of the procedure to either death or the last available follow-up visit. The primary end-point was death from any cause. Mortality data were obtained from the clinical follow-up data or from the National Statistics Office (PESEL system, PL), when necessary.
Statistical analysis
Statistical analysis was performed by SPSS V 17.0 (IBM, New York, USA). The results were presented as mean (±SD) or median (range). Differences among the groups were assessed with Student's t or Wilcoxon tests. Survival curves were created using the Kaplan–Meier method for the following subgroups with: (i) insignificant versus significant mitral regurgitation at baseline, (ii) immediately postprocedure and (iii) at a 1-month follow-up, as well as for subgroups of patients with (iv) no change versus improvement versus worsening of mitral regurgitation at a 1-month follow-up, compared with the baseline study.
To assess the interaction between the presence of aortic and mitral regurgitation, we compared the survival of patients (i) without significant mitral and aortic regurgitation versus (ii) those with either significant aortic or significant mitral regurgitation alone versus (iii) those with both significant mitral and aortic regurgitation postprocedure.
Univariate Cox proportionate hazards modelling was performed for each covariate using an unadjusted model. Subsequently multivariate models using a forward elimination method and entry criteria of p≤0.05 were constructed.
The study complied with the Declaration of Helsinki, and the Institutional Ethics Committee of the Institute of Cardiology approved the research protocol. Patients gave oral informed consent to participate in the registry.
Results
In the year 2013, 381 patients were enrolled in the POL-TAVI registry following the TAVI procedure, including 166 males (43.6%) and 215 females (56.4%) aged 78.8±7.4 years (range 35–85 years). A Medtronic CoreValve prosthesis was implanted in 209 patients (54.9%), Edwards-Sapien XT in 133 (34.9%) patients, Edwards-Sapien in 2 patients (4.5%) and other valve types in 22 patients (5.8%). The follow-up period was 94.1±96.5 days. Inhospital and overall mortality were 6.6% and 10.2% (25 and 39 patients, respectively). The demographic and clinical data are presented in table 1 and baseline echocardiographic characteristics in table 2.
Table 1.
Baseline demographic and clinical characteristics
| Age, years (SD) | 78 75±7,4 |
| Male sex, n (%) | 166 (43.6) |
| Logistic EuroSCORE (SD) | 19.8 (14.3) |
| New York Heart Association class (NYHA), n (%) | |
| I | 3 (0.8) |
| II | 76 (19.9) |
| III | 265 (69.6) |
| IV | 37 (9.7) |
| Significant coronary artery disease, n (%) | 122 (31.9) |
| Prior myocardial infarction, n (%) | 100 (26.2) |
| Prior CABG, n (%) | 77 (20.2) |
| Prior PCI (last 12 months), n (%) | 96 (25.2) |
| Prior aortic balloon valvuloplasty, n (%) | 9 (2.4) |
| Prior cerebrovascular disease, n (%) | 51 (13.4) |
| Chronic obstructive pulmonary disease, n (%) | 63 (16.5) |
| Creatinine >200 μmol/L, n (%) | 27 (7.1) |
| Atrial fibrillation, n (%) | 132 (34.6) |
| Diabetes, n (%) | 139 (36.5) |
| Prior pacemaker, n (%) | 51 (13.4) |
| Pulmonary hypertension, n (%) | 66 (17.3) |
| Multimorbidity, n (%) | 257 (67.5) |
| Porcelain aorta, n (%) | 45 (11.8) |
| Chest deformity, n (%) | 12 (3.1) |
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Table 2.
Baseline echocardiographic characteristics
| Aortic valve area, cm2 (SD) | 0.67 (0.18) |
| Peak aortic gradient, mm Hg (SD) | 81.9 (27.2) |
| Mean aortic gradient, mm Hg (SD) | 53.1 (20.1) |
| Left ventricular ejection fraction, % (SD) | 52.4 (12.2) |
| Right ventricular systolic pressure, mm Hg (SD) | 46.5 (15.10) |
| Mitral regurgitation grade, n (%) | |
| None | 20 (5.2) |
| 1 | 47 (12.3) |
| 2 | 209 (54.9) |
| 3 | 91 (23.9) |
| 4 | 14 (3.7) |
| Aortic regurgitation grade, n (%) | |
| None | 14 (3.7) |
| 1 | 85 (22.3) |
| 2 | 214 (55.9) |
| 3 | 60 (15.7) |
| 4 | 9 (2.4) |
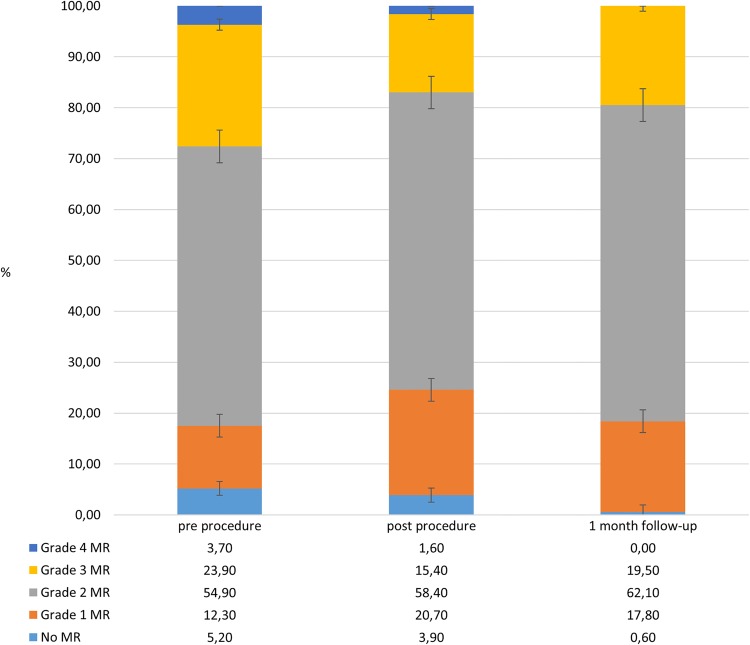
Significant (grade 3 or 4) mitral regurgitation was present in 27.6% at baseline examination, in 16% of patients prior to discharge, in 8.7% of patients at a 1-month follow-up and in 2.9% of patients at a 6-month follow-up (p≤0.0001 for all vs baseline). Changes in the mitral regurgitation grades during follow-up are presented in figure 1.
Figure 1.
Mitral regurgitation (MR) grades preprocedure, postprocedure and at a 1-month follow-up.
Patients with a Medtronic CoreValve prosthesis had a greater degree of mitral regurgitation prior to discharge than patients with the Edwards-Sapien or Edwards-Sapien XT valves (2.0±0.8 vs 1.8±0.7, p≤0.005). The same pertained to paravalvular aortic regurgitation (1.7±0.6 vs 1.5±0.6, p≤0.01).
Significant mitral regurgitation at different time points and mortality
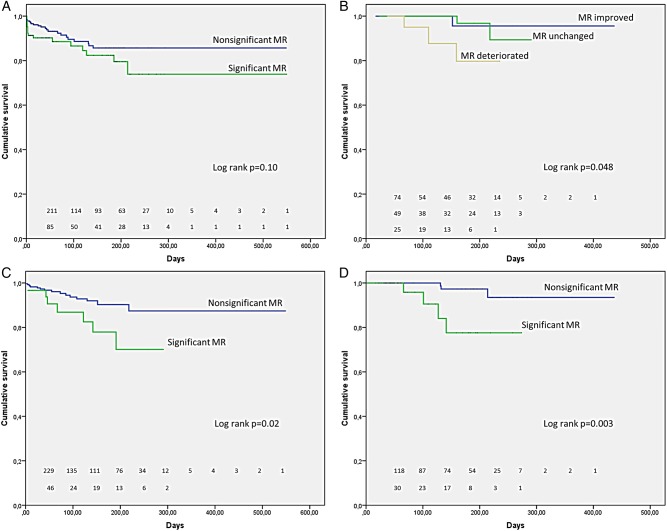
Kaplan–Meier analysis revealed that patients with significant versus insignificant preprocedural mitral regurgitation did not differ with respect to mortality (log rank Mantel–Cox p=0.10) (figure 2A). There were significant differences in mortality however when preprocedural mitral regurgitation deteriorated versus remained unchanged or improved at the 1-month follow-up (log rank Mantel–Cox p=0. 048) (figure 2B). Regardless of preprocedural mitral regurgitation, significant differences in mortality were apparent in patients with significant versus insignificant mitral regurgitation postprocedure and at the 1-month follow-up (log rank Mantel–Cox p=0.02 and p=0.003, respectively) (figure 2C, D).
Figure 2.
Kaplan–Meier curves demonstrating the association between mitral regurgitation (MR) and mortality in the overall group. (A) No significant difference in total mortality between patients with significant versus insignificant preprocedural mitral regurgitation (log rank Mantel–Cox p=0.10); (B) significant difference in mortality in patients in whom preprocedural mitral regurgitation deteriorated versus remained unchanged or improved at a 1-month follow-up; (C) significant differences in mortality in patients with significant versus insignificant mitral regurgitation postprocedure and (D) at a 1-month follow-up.
Interaction between mitral regurgitation and aortic regurgitation postprocedure
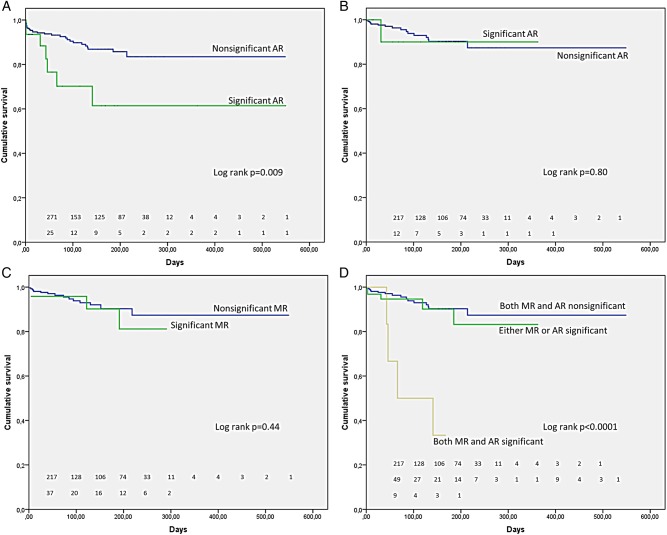
Thirty-one patients (8.1%) had significant aortic regurgitation postprocedure including 12 patients with both significant mitral and aortic regurgitation (3.1%). Patients with significant versus insignificant aortic regurgitation, immediately postprocedure, differed significantly with respect to mortality (log rank Mantel–Cox p=0.009) (figure 3A). This difference was no longer apparent when a subgroup of patients without significant mitral regurgitation postprocedure was selected (log rank Mantel–Cox p=0.80) (figure 3B). Similarly in a subgroup of patients without significant aortic regurgitation postprocedure, there were no significant differences in mortality between individuals with versus without significant mitral regurgitation postprocedure (log rank Mantel–Cox p=0.44) (figure 3C). Significant mitral and aortic regurgitation postprocedure had a significant impact on mortality only when associated with each other (log rank Mantel–Cox p<0.0001) (figure 3D).
Figure 3.
Kaplan–Meier curves demonstrating the relationship between mitral regurgitation (MR) and aortic regurgitation (AR) postprocedure. (A) Significant difference in mortality between patients with significant versus insignificant AR immediately postprocedure; (B) no difference in mortality between patients with significant versus insignificant AR postprocedure in a subgroup of patients without significant MR postprocedure; (C) no difference in mortality between patients with significant versus insignificant MR postprocedure in a subgroup of patients without significant AR postprocedure; (D) significant difference in mortality in patients with associated mitral and AR postprocedure.
Cox regression analysis
Univariate Cox regression analysis revealed that preprocedure mitral regurgitation had no significant association with mortality (OR 1.71, 95% CI 0. 91 to 3.23, p=0.10). Factors significantly, and at borderline significance, associated with mortality in the overall population at univariate Cox proportionate hazards modelling are presented in table 3.
Table 3.
Univariate Cox proportionate hazards modelling
| Covariate | OR | 95% CI | p Value |
|---|---|---|---|
| Concomitant significant mitral and aortic regurgitation postprocedure | 2.45 | 1.37 to 4.34 | 0.002 |
| Significant mitral regurgitation at 1-month follow-up | 2.54 | 1.09 to 5.89 | 0.03 |
| New York Heart Association Class III or IV | 5.22 | 1.26 to 21.65 | 0.02 |
| Significant aortic regurgitation postprocedure | 2.84 | 1.25 to 6.44 | 0.01 |
| Significant mitral regurgitation postprocedure | 2.54 | 1.10 to 5.89 | 0.03 |
| Ejection fraction <0.50 | 1.89 | 1.02 to 3.60 | 0.045 |
| EuroSCORE II | 1.05 | 1.01 to 1.09 | 0.01 |
| Systolic pulmonary artery pressure | 1.03 | 1.001 to 1.06 | 0.04 |
At multivariate Cox regression modelling in the overall group concomitant significant mitral and aortic regurgitation postprocedure and EuroSCORE II were independently associated with mortality (OR 3.21, 95% CI 1.54 to 5.71, p=0.002 and OR 1.07, 95% CI 1.02 to 1.11, p=0.003).
Discussion
The current study demonstrated that there was a significant interaction between the presence of a paravalvular leak following TAVI and persistent mitral regurgitation. Of practical importance, while there was a significant difference in mortality between patients with significant versus insignificant aortic regurgitation immediately postprocedure, this difference was no longer apparent in a subgroup of patients without significant mitral regurgitation postprocedure. Conversely, significant mitral regurgitation, as expected, had an impact on mortality, but this difference was not apparent in a subgroup of patients without significant aortic regurgitation. Concomitant significant mitral and aortic regurgitation postprocedure led to a sevenfold increase in mortality, independently of other risk factors.
The incidence of paravalvular aortic regurgitation is decreasing, but is still substantial. It has been identified as an independent risk factor of short-term and long-term mortality.9 While some authors suggest that the risk attributed to a paravalvular aortic regurgitation may be in part related to confounding factors, volume overload caused by a regurgitant jet should not be ignored. The interaction with mitral valve function was demonstrated by Hayashida et al10—significant paravalvular leaks were associated with the lack of regression or even further aggravation of mitral regurgitation. In such circumstances, the harm resulting from combined volume overload may clearly prevail over the benefits of pressure overload correction with TAVI.
Management of significant mitral regurgitation in patients with severe aortic stenosis is a challenge. Since significant mitral regurgitation is associated with increased perioperative risk and worse long-term survival, some authors recommend double-valve replacement/repair.11 The benefit of mitral valve repair with aortic valve surgery was demonstrated, for example, by Coutinho et al12 who reported that patients who underwent combined mitral and aortic valve surgery experienced more pronounced reverse left ventricular remodelling and greater clinical benefit (New York Heart Association functional (NYHA) functional classes III to IV). The lack of improvement in mitral regurgitation severity in patients who underwent isolated aortic valve surgery was associated with nearly fivefold increase in late mortality. We observed greater mortality only in patients in whom mitral regurgitation deteriorated. In the propensity-matched analysis of patients with significant mitral regurgitation performed by McCarthy et al13, patients undergoing double-valve surgery and TAVI had comparable perioperative outcomes. However, as expected, mitral regurgitation was more significantly reduced in surgical than TAVI patients and midterm survival was better in surgical patients. Given these considerations in the cornerstone Placement of Aortic Transcatheter Valve (PARTNER) trial, which laid the ground for the percutaneous TAVI, concomitant significant mitral regurgitation constituted one of the exclusion criteria.14 Nevertheless, a subgroup of patients with significant mitral regurgitation was also included in the PARTNER trial and its presence was associated with a doubling of 30-day mortality.15
Presently, an increasing number of high-risk patients with aortic stenosis and concomitant mitral regurgitation undergo TAVI beyond classical indications.16 The decisions to intervene in one or both valves in high-risk patients with aortic stenosis and concomitant mitral regurgitation involve a quadruple choice between single-valve, double-valve surgery, isolated TAVI and TAVI with sequential/simultaneous percutaneous mitral valve intervention in selected patients.17 The decisions are usually based on the assessment, mitral valve morphology and function as well as the probability of mitral regurgitation improvement after isolated aortic valve intervention.18
However, the prediction of mitral regurgitation improvement following TAVI is somewhat elusive. The results of clinical observations are conflicting and a reproducible set of factors that can be reliably used to predict improvement of mitral regurgitation following isolated aortic valve intervention has yet to be equivocally defined.11 19 Therefore, in patients with mitral regurgitation, special precautions should be taken to avoid paravalvular aortic regurgitation and resulting combined volume overload following the procedure.
Of notice, paravalvular leaks were more prevalent following the use of self-expandable valves as compared with balloon-expandable valves.20 Moreover, the use of the self-expandable valves was associated with a less prominent mitral regurgitation improvement compared with the balloon-expandable valves.21 We also observed a greater degree of mitral and aortic regurgitation postprocedure following self-expandable valve implantation. It is therefore interesting to note that mitral valve regurgitation was an independent risk factor for late death in the registry studies where most patients were treated with the CoreValve system. This association was much weaker or not apparent in the registry studies where the CoreValve system was used in a minority of patients.2 These observations may be explained by the more frequent adverse interactions between mitral and aortic regurgitation in patients who received balloon-expandable valves.
The study limitations
This study has several limitations. While the diagnosis of mitral and aortic regurgitation criteria was unified throughout the centres, according to the current guidelines, there was no core echocardiographic laboratory and quantitative assessment was not universally unified. The registry did not provide data on the aetiology of concomitant mitral regurgitation, therefore we were not able to substratify patients according to the mechanism of a regurgitant jet. The subgroup of patients with concomitant mitral and aortic regurgitation was limited in number and an analysis needs to be replicated in the meta-analysis of available data.
Conclusions and practical implications
The current study demonstrated that there was a significant interaction between the occurrence of paravalvular leak following TAVI and persistent mitral regurgitation. Of practical importance while there was a significant difference in mortality between patients with significant versus insignificant aortic regurgitation immediately postprocedure, this difference was no longer apparent in a subgroup of patients without significant mitral regurgitation postprocedure. Conversely, significant mitral regurgitation, as expected, exerted an impact on mortality but this difference was not apparent in a subgroup of patients without significant aortic regurgitation. Concomitant significant mitral and aortic regurgitation postprocedure led to a significant increase in mortality, independent of other risk factors. It remains to be established whether in patients for whom a percutaneous approach is chosen, preprocedural mitral regurgitation should influence the prosthesis choice. Undoubtedly, it warrants consideration of managing physicians since clearly, patients with significant paravalvular leak following TAVI in whom significant mitral regurgitation persists or worsens are at greater risk of haemodynamic complications and have a worse midterm prognosis. In such patients, a second step intervention—either a paravalvular leak closure or percutaneous edge-to-edge mitral valve repair (or both)—should be considered by a multidisciplinary heart team.
Key messages.
What is already known on this subject?
In some patients, significant preprocedural mitral regurgitation (MR) improves while in the others, it worsens following transcatheter aortic valve implantation (TAVI). Persistent significant MR may be especially important in patients who develop a significant paravalvular leak.
What might this study add?
It demonstrated that there was a significant interaction between the presence of a paravalvular leak and persistent MR postprocedure that led to an increased mortality independent of other risk factors. Of importance however, significant MR had no impact on mortality in a subgroup of patients without significant aortic regurgitation and significant paravalvular leak had no impact on mortality in a subgroup of patients without significant MR.
How might this impact on clinical practice?
The results of the study may influence management approach to paravalvular leaks following TAVI, depending on the presence or absence of significant MR postprocedure.
Acknowledgments
The authors would like to thank Robin Krauze and Professor Tadeusz Krauze for their support.
Footnotes
Contributors: All authors contributed significantly to the planning, conduct and reporting of the work described in the article.
Competing interests: None declared.
Ethics approval: Institute of Cardiology Warsaw.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.David TE, Armstrong S, Maganti M, et al. Clinical outcomes of combined aortic root replacement with mitral valve surgery. J Thorac Cardiovasc Surg 2008;136:82–7. 10.1016/j.jtcvs.2008.02.038 [DOI] [PubMed] [Google Scholar]
- 2.Nombela-Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol 2014;63:2643–58. 10.1016/j.jacc.2014.02.573 [DOI] [PubMed] [Google Scholar]
- 3.Hekimian G, Detaint D, Messika-Zeitoun D, et al. Mitral regurgitation in patients referred for transcatheter aortic valve implantation using the Edwards Sapien prosthesis: mechanisms and early postprocedural changes. J Am Soc Echocardiogr 2012;25:160–5. 10.1016/j.echo.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 4.Khawaja MZ, Williams R, Hung J, et al. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart 2014;100:1799–803. 10.1136/heartjnl-2014-305775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zembala M, Wilczek K, Przybylski R, et al. [POL-TAVI First--Polish report on transcatheter aortic valve implantation (TAVI) of Edwards-Sapien prosthesis in the first 19 high risk patients with severe aortic stenosis and comorbidities]. Kardiol Pol 2009;67:936–40. [PubMed] [Google Scholar]
- 6.Lancellotti P, Moura L, Pierard LA, et al. European association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–32. 10.1093/ejechocard/jeq031 [DOI] [PubMed] [Google Scholar]
- 7.Szymański P, Śpiewak M, Miszalski-Jamka T, et al. [Imaging in organic mitral regurgitation. Expert consensus statement of the polish clinical forum for cardiovascular imaging] . Kardiol Pol 2013;71:976–85. 10.5603/KP.2013.0238 [DOI] [PubMed] [Google Scholar]
- 8.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Valve Academic Research Consortium (VARC)-2. Eur J Cardiothorac Surg. 2012;42:S45–60. 10.1093/ejcts/ezs533 [DOI] [PubMed] [Google Scholar]
- 9.Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585–95. 10.1016/j.jacc.2013.01.047 [DOI] [PubMed] [Google Scholar]
- 10.Hayashida K, Lefèvre T, Chevalier B, et al. Impact of post-procedural aortic regurgitation on mortality after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2012;5:1247–56. 10.1016/j.jcin.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Barreiro CJ, Patel ND, Fitton TP, et al. Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: impact on survival and functional outcome. Circulation 2005;112:I443–7. 10.1161/CIRCULATIONAHA.104.526046 [DOI] [PubMed] [Google Scholar]
- 12.Coutinho GF, Correia PM, Pancas R, et al. Management of moderate secondary mitral regurgitation at the time of aortic valve surgery. Eur J Cardiothorac Surg 2013;44:32–40. 10.1093/ejcts/ezs676 [DOI] [PubMed] [Google Scholar]
- 13.McCarthy FH, Desai ND, Herrmann HC, et al. Aortic and mitral valve replacement versus transcatheter aortic valve replacement in propensity-matched patients. Ann Thorac Surg 2014;98:1267–73. 10.1016/j.athoracsur.2014.05.075 [DOI] [PubMed] [Google Scholar]
- 14.Leon MB, Smith CR, Mack M, et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 15.Toggweiler S, Boone RH, Rodés-Cabau J, et al. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol 2012;59:2068–74. 10.1016/j.jacc.2012.02.020 [DOI] [PubMed] [Google Scholar]
- 16.Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010;122:62–9. 10.1161/CIRCULATIONAHA.109.907402 [DOI] [PubMed] [Google Scholar]
- 17.Kische S, D'Ancona G, Paranskaya L, et al. Staged total percutaneous treatment of aortic valve pathology and mitral regurgitation: institutional experience. Catheter Cardiovasc Interv 2013;82:E552–63. 10.1002/ccd.24809 [DOI] [PubMed] [Google Scholar]
- 18.Unger P, Dedobbeleer C, Van Camp G, et al. Mitral regurgitation in patients with aortic stenosis undergoing valve replacement. Heart 2010;96:9–14. 10.1136/hrt.2009.165548 [DOI] [PubMed] [Google Scholar]
- 19.Absil B, Dagenais F, Mathieu P, et al. Does moderate mitral regurgitation impact early or mid-term clinical outcome in patients undergoing isolated aortic valve replacement for aortic stenosis? Eur J Cardiothorac Surg 2003;24:217–22. 10.1016/S1010-7940(03)00251-3 [DOI] [PubMed] [Google Scholar]
- 20.Généreux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol 2013;61:1125–36. 10.1016/j.jacc.2012.08.1039 [DOI] [PubMed] [Google Scholar]
- 21.Nombella-Franco L, Eltchaninoff H, Zahn R, et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: a meta-analysis. Heart 2015;101:1395–405. 10.1136/heartjnl-2014-307120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2015-308842supp_appendix.pdf (116.6KB, pdf)