Abstract
Background
Assess clinicopathological features of patients with keratocystic odontogenic tumor (KCOT) associated with Gorlin–Goltz syndrome in our institution from 2004 to 2015.
Method
After histopathological analyses of KCOT related to Gorlin–Goltz syndrome, 7 patients were assessed. These patients presented a total of 15 primary and 2 recurrent KCOT.
Results
All patients presented a multiple KCOT, and 13 lesions were located in mandible (77%) and 4 (23%) in maxilla. Most of the tumors presented a unilocular pattern (71%) and had tooth association (88%). Four patients (57%) were in the age group of 10–19 years and three patients (43%) were in the age group of 20–29 years. There were four male and three female patients.
Conclusion
KCOT is a frequent manifestation of Gorlin–Goltz syndrome and can be its first sign, mainly in young patients. The four patients presented with two lesions (57%) and three lesions in three patients (43%).
Keywords: Gorlin–Goltz syndrome, Keratocyst, Keratocystic odontogenic tumor, Nevoid basal cell carcinoma syndrome
1. Introduction
Multiple OKCs may occur in association with jaw cyst basal cell nevus syndrome or Gorlin–Goltz syndrome. In 1960, Gorlin and Goltz studied the main features of this disorder, hence the name. It is characterized by disorders affecting multiple systems including skeletal, cutaneous, ophthalmic, reproductive and nervous systems.1 The pathogenesis of the syndrome is attributed to abnormalities in the long arm of chromosome 9 (q22.3–q31) and loss of, or mutations of human patched gene (PTCH1 gene). Diagnosis is based upon established major and minor clinical and radiological criteria and ideally confirmed by deoxyribo nucleic acid (DNA) analysis. This syndrome is also named as Gorlin syndrome, multiple nevoid basal cell epithelioma, jaw cyst bifid rib syndrome, or multiple nevoid BCC syndrome.2 The frequency of the syndrome varies according to the country where the study has been carried out. On an average, the incidence of Gorlin–Goltz syndrome has been reported to be 1 in 50,000 to 150,000 in general population.
Treatment modalities may differ for small and large cysts. Small cysts can be enucleated, whereas large cysts can be marsupialized. Because of aggressive nature and high rate of recurrence,4 there should be periodic follow-up at regular intervals of 6 months till 5 years, followed by once annually for the entire life.
The purpose of this paper is to describe clinical and histopathological aspects of KCOTs associated with Gorlin–Goltz syndrome and report other manifestations associated with it as OKC are the most common manifestations of the syndrome.3
2. Material and methods
This study consisted of a retrospective analysis of patients diagnosed with KCOT and related to Gorlin–Goltz syndrome. The seven patients presenting KCOT related to Gorlin–Goltz syndrome were evaluated from 2004 to 2015. These patients presented a total of 15 primary and 2 recurrent KCOT. Data including age, gender, number of KCOT, location, signs, symptoms, radiographic features, treatment, and recurrence were analyzed.
2.1. Histopathological analysis
The hematoxylin/eosin (HE)-stained slides of KCOTs were retrieved and submitted to histopathological examination. All slides were reviewed by an oral pathologist and the findings were confirmed by a general pathologist.
3. Results
3.1. Clinicopathological features of 17 KCOT lesions in 7 patients
All patients had KCOT as first manifestation of the syndrome. The patient's age at KCOT diagnosis ranged from 10 to 24 years (Table 2). The four patients (57%) were in the age group of 10–19 years and three patients (43%) were in the age group of 20–29 years (Table 2). There were four male (57%) and three female (43%) patients (Table 2). All patients presented with multiple KCOT. The four patients presented with two lesions (57%) and three lesions in three patients (43%). Besides the 15 primary KCOTs, 13 lesions were located in mandible (77%) and 4 (23%) in maxilla (Table 3). Most tumors presented unilocular pattern and association with a tooth (Table 3). All patients presented with calcification of the falx cerebri. In addition palmar pits, abnormal ribs were also present in some patients. The clinical manifestations are listed in Table 1. At initial evaluation, all patients complained of swelling. The time of complaint ranged between 20 days and 7 months. On examination, 1 patient presented facial asymmetry and one patient reported with pathological fracture (Fig. 11). Radiographic analysis of 17 KCOTs demonstrated that 13 lesions were located in the mandible and 4 in the maxilla. Most of the tumors presented a unilocular pattern (71%) and had tooth association (88%). The size of the lesions ranged from 2 to 6 cm (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9). According to KCOT treatment, enucleation associated with curettage was performed in 18% cases and the other cases were treated by marsupialization and curettage in 82% (Table 3).
Table 2.
Clinical features of seven patients of Gorlin–Goltz syndrome.
| Variable | Category | n | % |
|---|---|---|---|
| Age | 10–19 | 4 | 57 |
| 20–29 | 3 | 43 | |
| Gender | Male | 4 | 57 |
| Female | 3 | 43 | |
| Number of KCOT | 2 | 4 | 57 |
| 3 | 3 | 43 | |
| Total | 7 | 100 | |
Table 3.
Radiographic features of 17 KCOT in 7 Gorlin–Goltz patients.
| Variable | Category | n | % |
|---|---|---|---|
| Location | Mandible posterior | 9 | 52.94 |
| Mandible anterior | 4 | 23.52 | |
| Total | 13 | 76.46 | |
| Maxilla posterior | 2 | 11.76 | |
| Maxilla anterior | 2 | 11.76 | |
| Total | 4 | 23.52 | |
| Radiographic pattern | Multilocular | 5 | 29.41 |
| Unilocular | 12 | 70.58 | |
| Tooth association | Yes | 15 | 88.23 |
| No | 2 | 11.76 | |
| Treatment | Enucleation with curettage | 3 | 17.64 |
| Marsupialization and curettage | 14 | 82.35 | |
| Recurrence | Yes | 2 | 11.76 |
| No | 15 | 88.23 |
Table 1.
2 Major or 1 major and 2 minor criteria should be satisfied for positive diagnosis.
| Major criteria |
| 1. More than two BCCs or one BCC under the age of 20 years |
| 2. Histologically proven odontogenic keratocyst of the jaw |
| 3. Three or more cutaneous palmar or plantar pits |
| 4. Bifid, fused or markedly splayed ribs |
| 5. First degree relative with nevoid basal cell carcinomas |
| Minor criteria |
| This consists of any one of the following features |
| 1. Proven macrocephaly, after adjustment for height |
| 2. One of the several orofacial congenital malformations: cleft lip or palate, frontal bossing, ‘coarse face’, moderate or severe hypertelorism |
| 3. Other skeletal abnormalities: sprengel deformity, marked pectus deformity, marked syndactyly of the digits |
| 4. Radiological abnormalities: bridging of the sella turcica, vertebral anomalies such as hemivertebrae, fusion or elongation of the vertebral bodies, modeling defects of the hands and feet, or flame shaped lucencies of the hands or feet |
| 5. Ovarian fibroma |
| 6. Medulloblastoma |
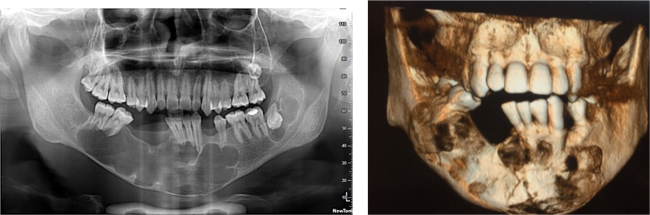
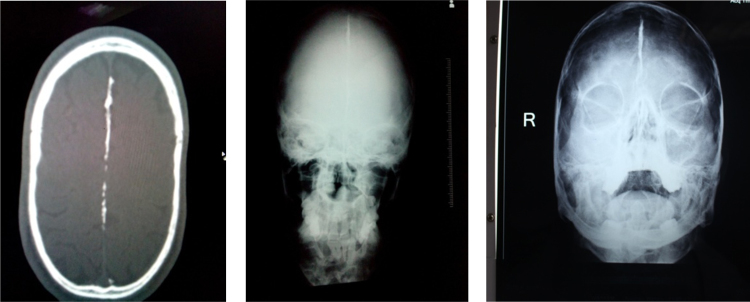
Fig. 11.
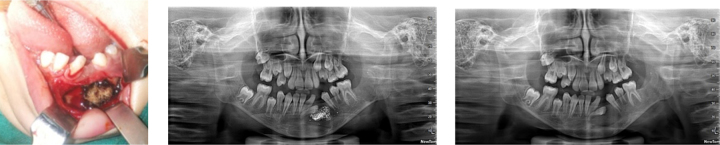
Panoramic radiograph revealing multiple well defined unilocular pericoronal radiolucencies in relation to impacted 28, 38 and CBCT image showing pathological fracture of midline.
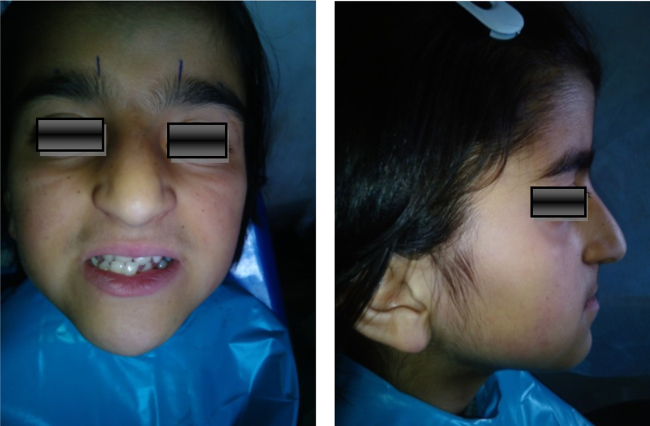
Fig. 1.
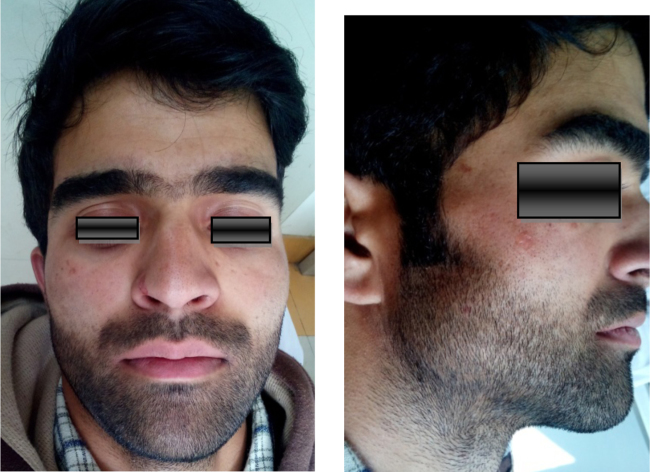
Extraoral photograph showing mild frontal bossing, wide and depressed nasal bridge hypertelorism, and mandibular prognathism.
Fig. 2.
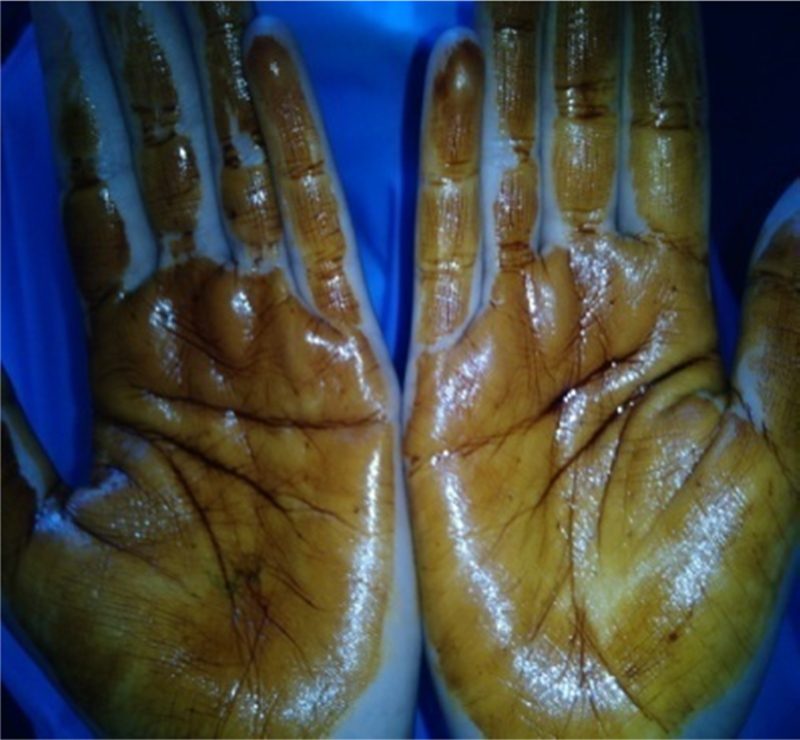
Palmar pits.
Fig. 3.
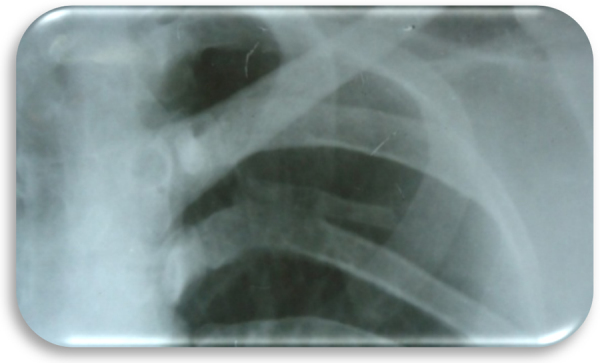
Bifid-rib anomaly was evident with respect to left fifth rib on chest X-ray.
Fig. 4.
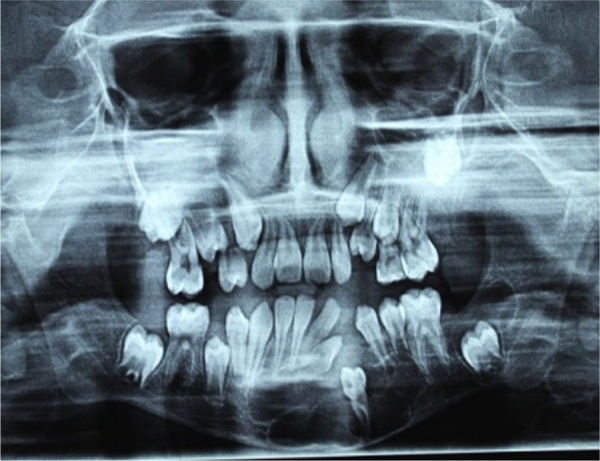
Preoperative OPG showing multiple radiolucencies (3) in the mandible (2) and maxilla (1). Tooth buds of 27, 33, 37 appear displaced.
Fig. 5.
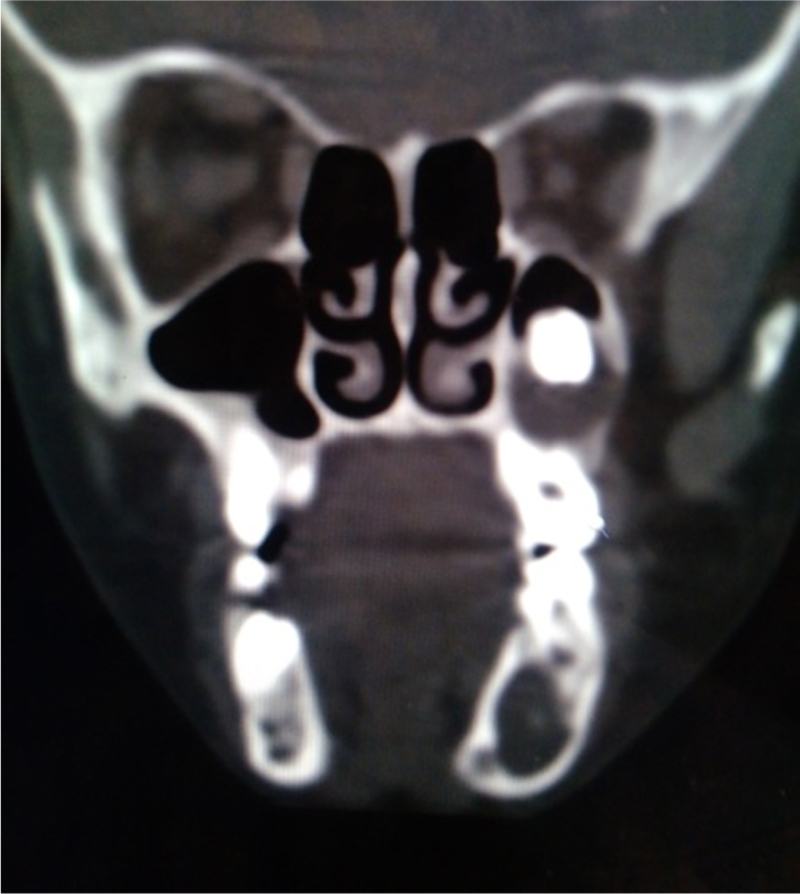
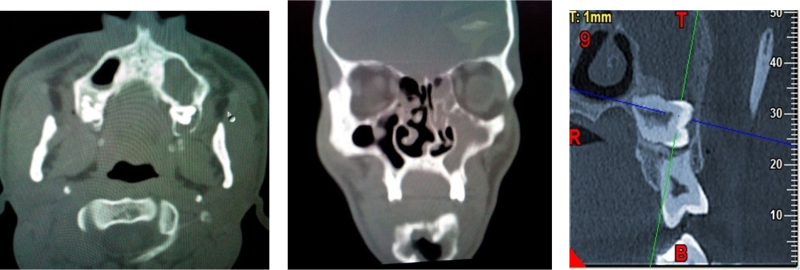
Computerized tomography scan showed 2 well defined non enhancing radiolucent lesions involving left maxilla and mandible.
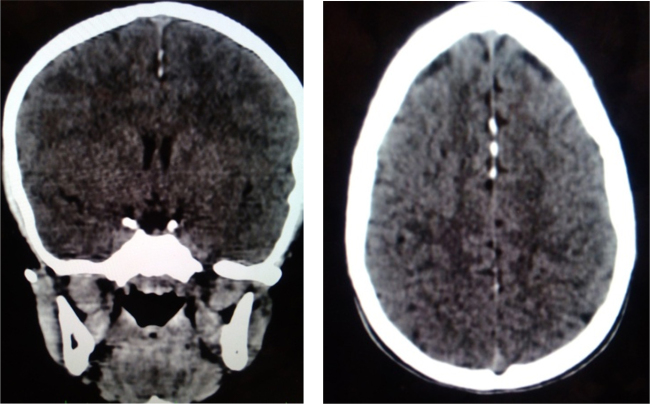
Fig. 6.
Falx cerebri calcifications.
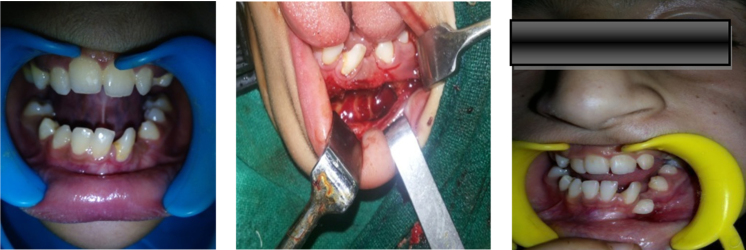
Fig. 7.
Preoperative intraoral, intraoperative and follow up intraoral picture.
Fig. 8.
Marsupialization and postoperative OPG.
Fig. 9.
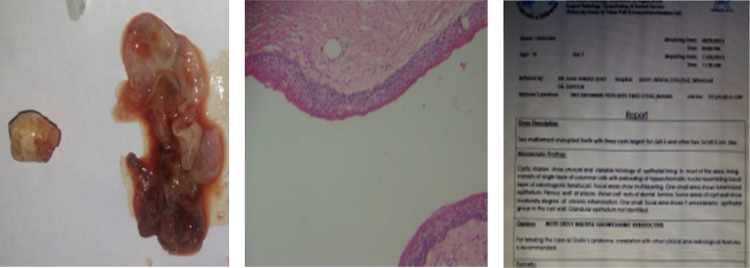
Cystic lining: histopathological report showed a cystic capsule lined by a corrugated epithelium having uniform thickness showing proliferation in some areas.
4. Discussion
KCOT is the main feature observed in patients with Gorlin–Goltz syndrome.3 The diagnostic criteria for nevoid BCC were established by Evans et al., and modified by Kimonis et al. in 1973.11, 24 According to them, diagnosis of Gorlin–Goltz syndrome can be established when two major or one major and two minor criteria are present which are described in Table 1. Kimonis et al. evaluated clinical and radiological data of 105 persons with NBCCS. Pits, BCCs, jaw cysts, and falx calcification were the most common anomalies, and according to their results the authors suggested some major and minor criteria for Gorlin–Goltz syndrome diagnosis.11, 24 Comparing these criteria with the First International Colloquium on NBCCS criteria, there were two important alterations. The latter suggested changing rib anomalies to minor criterion and MB to major criterion. Interestingly, Amlashi et al. evaluated 76 patients with MBs and three of them had syndromic MBs. Additionally, the authors reviewed the literature and found other 33 patients with syndromic MBs. The mean age of syndromic MBs was 4 years (earlier than sporadic MBs) and most syndromic patients were younger than 2 years.12 Only one of these patients developed MB at 3 years (Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17). At 17 years, he presented 3 synchronous KCOTs, and at 18 years calcification of the falx cerebri. It is worthy of note that calcification of the falx cerebri had been previously investigated in this patient. Kimonis et al. evaluated the falx cerebri calcification in 82 individuals with Gorlin–Goltz syndrome.11 This calcification was present in 23 out of 29 (79%) individuals over the age of 40, 20 out of 26 (77%) individuals between the ages of 20 and 40 and 10 out of 27 (37%) individuals under the age of 20. In the present study, all patients reported falx cerebri calcification. In the stomatological system, besides KCOT, other benign and malignant tumors have been described in Gorlin–Goltz syndrome patients such as ameloblastoma, myxoma, fibrosarcoma, squamous cell carcinoma, adenoid cystic carcinoma, and lymphoma. Furthermore, development defects such as cleft lip/palate, dental ectopy/heterotopy, impacted teeth, dental agenesis, malocclusion, mandibular prognathism, high-arched palate, skeletal open bite, and hyperplasia of mandibular coronoid process have also been reported.1, 10, 13, 14, 15, 16 Interestingly, Ponti et al.17 evaluated 41 ameloblastomas and two of them were related to Gorlin–Goltz syndrome. In addition, PTCH 1 germline mutations were also detected in both cases and negative in the others. The authors suggested including ameloblastoma as a criterion for syndrome identification. In the present study, patient presenting with Gorlin–Goltz syndrome had KCOT as the first sign of the syndrome in all patients. In a similar study, Lo Muzio et al.18 evaluated 37 individuals with NBCCS, and 34 of them had KCOT (92%). In these patients, the first manifestation of the syndrome was KCOT. In general, most of the patients with Gorlin–Goltz syndrome are females and KCOT occurs in the second decade of life with a mean age ranging from 17 to 26 years.18, 19, 20 In the present study, 43% patients were females. In the largest series in English literature, Woolgar et al. evaluated 164 KCOTs in syndromic patients and 379 KCOTs not associated with Gorlin–Goltz syndrome.19 It was observed that the posterior area of the mandible was the main affected site, followed by the maxillary molar region in both groups. Since the syndromic patients almost always have more than one tumor, it is to be expected that more maxillary tumors are present in these patients. Such data were also demonstrated in the present study, in which multiple lesions were found in all seven patients and accounted for 17 tumors (13 in the mandible and 4 in the maxilla). In general, the mandible was the main location of the lesions (77%), 9 being in the body/ramus and 4 in the anterior area, in addition to those (23%) in the posterior and anterior region of the maxilla. Other studies have also shown multiple lesions affecting syndromic patients. Kimonis et al. reported that 78 (74%) NBCCS patients presented KCOT with number of tumors ranging from 1 to 28.11 However, the authors did not clarify which tumors were primary or recurrent. Furthermore, it was also shown that 5 individuals had more than 10 KCOTs in their lifetime. Ahn et al. reviewed 33 well-documented case reports of NBCCS published between 1981 and 2002. Out of the total, 30 patients (90.9%) had KCOT and the number of lesions per patient ranged from 1 to 6 (mean 2.7 lesions).20 In a recent NBCCS case series reported in Indian patients, all 6 patients developed multiple KCOT (range 3–6)21; similarly, all of the patients in this study had multiple KCOT. The above-mentioned research involving patients with KCOT related to Gorlin–Goltz syndrome.5, 6, 7, 8, 9, 10 Regarding the treatment of KCOT, Zecha et al. demonstrated that 58 patients who did not have an NBCCS diagnosis and were treated with enucleation alone had recurrence in 20.7% of the case.22 Recurrence was observed in 2 tumors which corresponded to 1 patient. This patient has been asymptomatic for 9 years. Recurrences usually manifest within the first 5–7 years. However, Zhao et al. demonstrated recurrence after 13 years of follow-up.23 Similarly, our study demonstrated 1 patient who had recurrence after 10 years.
Fig. 10.
Extra oral photograph showing mild frontal bossing, wide and depressed nasal bridge, hypertelorism, mandibular prognathism, and eyebrows meeting in midline.
Fig. 12.
Computerized tomography scan showing well defined non enhancing radiolucent lesions involving left maxilla with associated impacted tooth 18.
Fig. 13.
Falx cerebri calcifications.
Fig. 14.
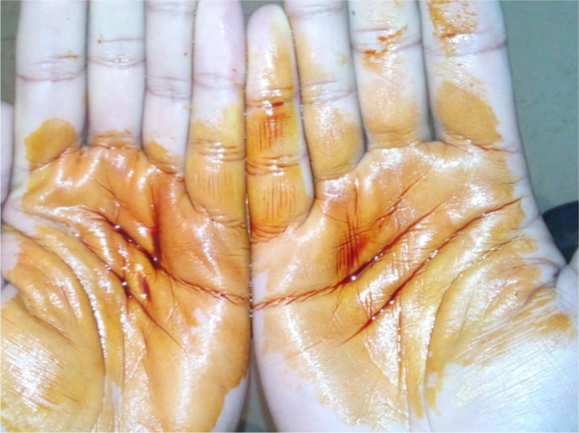
Palmar pits.
Fig. 15.

Histopathology report.
Fig. 16.
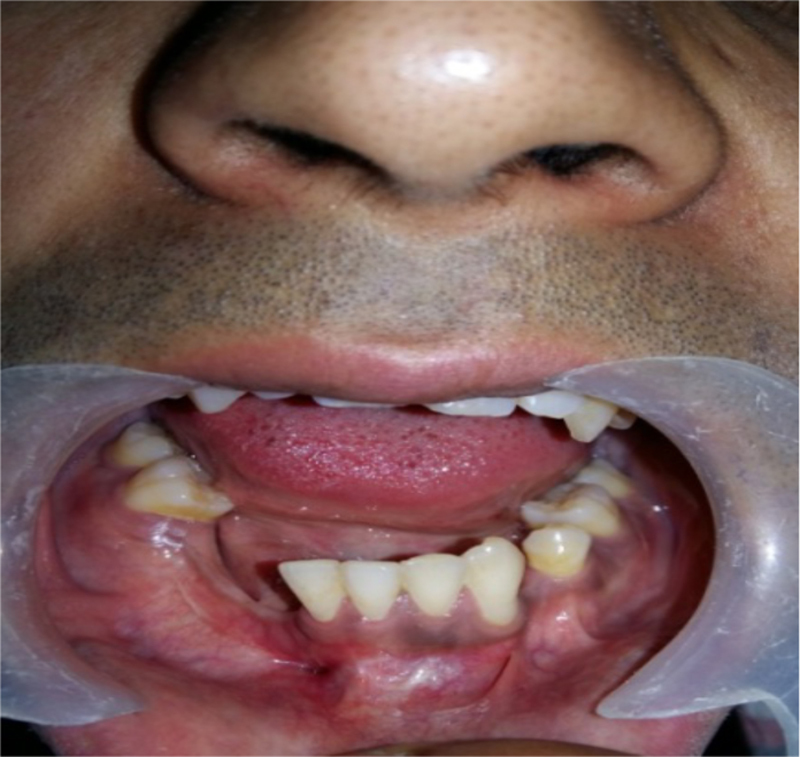
Postoperative intraoral picture.
Fig. 17.
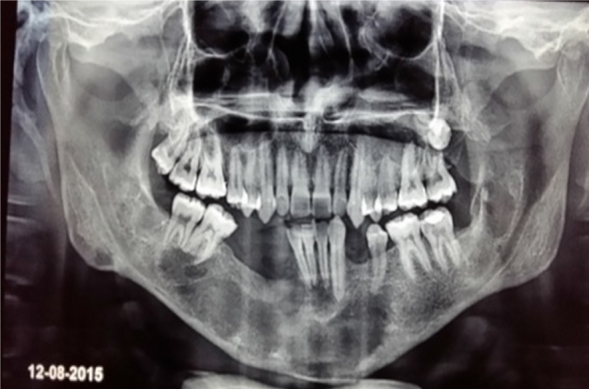
Postoperative OPG.
In summary, Gorlin–Goltz syndrome is a condition which has a predisposition of cancer and it is very important to diagnose and treat the existing problems early. Thorough extraoral and intraoral examinations along with OPG, skull and chest radiographs help in proper diagnosis of the condition. Odontogenic keratocysts of the jaws, which can cause disfigurement of the face, mobility, and even loss of teeth, can be avoided by early detection and treatment of the problems. Interestingly, in this series, all patients had multiple lesions. Early diagnosis of the syndrome and a long follow-up period are important due to the severity of clinical manifestations. Moreover, a multidisciplinary team is required, including dentists, dermatologists, geneticists, and neurologists to improve the diagnosis and quality of life.
Conflicts of interest
The authors have none to declare.
References
- 1.Gorlin R.J. Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 1987;66:98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bree A.F., Shah M.R., BCNS Colloquium Group Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS) Am J Med Genet A. 2011;155A:2091–2097. doi: 10.1002/ajmg.a.34128. [DOI] [PubMed] [Google Scholar]
- 3.Evans D.G., Ladusans E.J., Rimmer S., Burnell L.D., Thakker N., Farndon P.A. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993;30:460–464. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agaram N.P., Collins B.M., Barnes L. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004;128:313–317. doi: 10.5858/2004-128-313-MATDTO. [DOI] [PubMed] [Google Scholar]
- 5.Tincani A.J., Martins A.S., Andrade R.G., Franco J.R.E.F.M., Camargo M.A.B., Martins A.S. Nevoid basal-cell carcinoma syndrome: literature review and case report in a family. Sao Paulo Med J. 1995;113:917–921. doi: 10.1590/s1516-31801995000300006. [DOI] [PubMed] [Google Scholar]
- 6.Melo E.S., Kawamura J.Y., Alves C.A., Nunes F.D., Jorge W.A., Cavalcanti M.G. Imaging modality correlations of an odontogenic keratocyst in the nevoid basal cell carcinoma syndrome: a family case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:232–236. doi: 10.1016/j.tripleo.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Lopes N.N., Caran E.M., Lee M.L., Silva N.S., Rocha A.C., Macedo C.R. Gorlin–Goltz syndrome and neoplasms: a case study. J Clin Pediatr Dent. 2010;35:203–206. doi: 10.17796/jcpd.35.2.x01248284w166485. [DOI] [PubMed] [Google Scholar]
- 8.Visioli F., Martins C.A., Heitz C., Rados P.V., Sant’Ana Filho M. Is nevoid basal cell carcinoma syndrome really so rare? Proposal for an investigative protocol based on a case series. J Oral Maxillofac Surg. 2010;68:903–908. doi: 10.1016/j.joms.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Casaroto A.R., Loures D.C., Moreschi E. Early diagnosis of Gorlin–Goltz syndrome: case report. Head Face Med. 2011;2:1–5. doi: 10.1186/1746-160X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira C.M., Lopes A.P., Meneghini A.J., Silva A.F., Botelho T.L. Oral diffuse B-cell non-Hodgkin's lymphoma associated to Gorlin–Goltz syndrome: a case report with one year follow-up. Indian J Pathol Microbiol. 2011;54:388–390. doi: 10.4103/0377-4929.81649. [DOI] [PubMed] [Google Scholar]
- 11.Kimonis V.E., Goldstein A.M., Pastakia B. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299–308. [PubMed] [Google Scholar]
- 12.Amlashi S.F., Riffaud L., Brassier G., Morandi X. Nevoid basal cell carcinoma syndrome: relation with desmoplastic medulloblastoma in infancy. A population-based study and review of the literature. Cancer. 2003;98:618–624. doi: 10.1002/cncr.11537. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K., Amagasa T., Shioda S., Kayano T. Basal cell nevus syndrome with squamous cell carcinoma of the maxilla: report of a case. J Oral Maxillofac Surg. 1989;47:629–633. doi: 10.1016/s0278-2391(89)80081-3. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz B., Goldberg L.H., Schechter N.R., Kemp B.L., Ruiz H. Basal cell nevus syndrome concurrent with adenoid cystic carcinoma of salivary gland. J Am Acad Dermatol. 2003;48:S64–S66. doi: 10.1067/mjd.2003.164. [DOI] [PubMed] [Google Scholar]
- 15.Eslami B., Lorente C., Kieff D., Caruso P.A., Faquin W.C. Ameloblastoma associated with the nevoid basal cell carcinoma (Gorlin) syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e10–e13. doi: 10.1016/j.tripleo.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;25:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponti G., Pastorino L., Pollio A. Ameloblastoma: a neglected criterion for nevoid basal cell carcinoma (Gorlin) syndrome. Fam Cancer. 2012;11:411–418. doi: 10.1007/s10689-012-9529-3. [DOI] [PubMed] [Google Scholar]
- 18.Lo Muzio L., Nocini P.F., Savoia A. Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin Genet. 1999;55:34–40. doi: 10.1034/j.1399-0004.1999.550106.x. [DOI] [PubMed] [Google Scholar]
- 19.Woolgar J.A., Rippin J.W., Browne R.M. The odontogenic keratocyst and its occurrence in the nevoid basal cell carcinoma syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:727–730. doi: 10.1016/0030-4220(87)90176-9. [DOI] [PubMed] [Google Scholar]
- 20.Ahn S.G., Lim Y.S., Kim D.K., Kim S.G., Lee S.H., Yoon J.H. Nevoid basal cell carcinoma syndrome: a retrospective analysis of 33 affected Korean individuals. Int J Oral Maxillofac Surg. 2004;33:458–462. doi: 10.1016/j.ijom.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S.R., Jaetli V., Mohanty S., Sharma R., Gupta A. Nevoid basal cell carcinoma syndrome in Indian patients: a clinical and radiological study of 6 cases and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:99–110. doi: 10.1016/j.tripleo.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Zecha J.A., Mendes R.A., Lindeboom V.B., van der Waal I. Recurrence rate of keratocystic odontogenic tumor after conservative surgical treatment without adjunctive therapies – A 35-year single institution experience. Oral Oncol. 2010;46:740–742. doi: 10.1016/j.oraloncology.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y.F., Wei J.X., Wang S.P. Treatment of odontogenic keratocysts: a follow-up of 255 Chinese patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:151–156. doi: 10.1067/moe.2001.125694. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed N., Salman M., Mansoor M.A. Gorlin–Goltz syndrome. J Coll Physicians Surg Pak. 2007;17:568–569. [PubMed] [Google Scholar]