Abstract
Request for temporary removal of orthodontic appliances due to medical conditions that require magnetic resonance (MR) imaging is not uncommon in daily practice in the field of orthodontics. This may be at the expense of time and cost. Metal Orthodontic appliances cause more signal loss and image distortion as compared to ceramic and titanium ones. Stainless steel and large brackets in addition to the oriented miniscrews in relation to the axis of magnetic field may cause severe signal loss and image distortion. Moreover, gradient echo and frequency-selective fat saturation MR protocols are more susceptible to metal artifacts. The spin echo and fat-suppression protocols, low magnetic field strength (e.g., 1.5 Tesla vs. 3 Tesla), small field of view, high-resolution matrix, thin slice, increased echo train length and increased receiver band width could be applied to lessen the metal artifacts in MR images. The larger the distance between an appliance and desired location to be imaged, the lower the distortion and signal loss. Decision to remove brackets should be made based on its composition and desired anatomic location. In this review, first the principles of MR imaging are introduced (Part-I) and then the interactions of orthodontic appliances and magnetic field are farther discussed (Part-II).
Keywords: Bracket, Orthodontics, Metal, Artifact, Magnetic resonance imaging
1. A primer on magnetic resonance imaging basics for orthodontists
1.1. Introduction
Removal of orthodontic appliances due to medical purposes that require magnetic resonance (MR) imaging is not uncommon in daily practice. Debonding and rebonding of orthodontic brackets during the treatment course are time consuming, costly, may harm the enamel and lengthen the treatment time, indeed.1 One major drawback is metal artifact induced by the orthodontic appliance that causes signal loss and image distortion. The severity of the signal loss is strongly related to the material composition (ferromagnetic vs. diamagnetic), length, diameter, and shape of material, position of the object in relation to the axis of magnetic field, MR protocols and MR parameters in addition to the anatomic and geometric location of the desired tissues to be imaged.2 Before any decision, the orthodontist should be able to answer three main questions: (1) Would the orthodontic appliance dislodge or move when interacts with the magnetic field with subsequent possibility of tissue damage? (2) Whether and how much the appliance can make imaging artifacts and whether it makes the image undiagnostic or not? (3) How much the appliance would be heated up in the mouth during MR imaging and to what extent it could damage the adjacent oral mucosa?3 This review is aimed to address the above questions, along with a short introduction to the principles of MR imaging.
1.2. Magnetic resonance imaging (MRI)
Each molecule with odd number of electrons surrounding its nucleus spinning at an inert alignment has an internal magnetic moment. When placed in an external magnetic field, interaction of two described magnetic fields and moments may lead to redirection of spinning nuclei (i.e., precession) proportional to the main external magnetic field power.4 Such excitation is achievable via disk-shaped magnets that encircle the patient's body. The precession takes place at a unique frequency (i.e., resonance) for each specific nucleus.4 The excited atoms emit energy by the form of signals as they return to their equilibrium state (i.e., echo) which will be detected by specific receiver coils.
Generally each MRI device works with a particular set of coils including main coil (i.e., produces main magnetic field in the direction of Z-axis, so called B0), gradient coil i.e., (produces magnetization with gradient in specific direction), radiofrequency coil (i.e., produces magnetization vertical to the main magnetization, so called B1), receiver coil (i.e., receives echos from the tissues within the body) and shim coil (improves homogeneity of magnetic field).5
As hydrogen is one of the most abundant atoms within the various body tissues, almost all of current coils are programmed to detect echos of hydrogen atoms.4 Tissue content and texture are responsible for differentiated contrast of each tissue on the final image. This contrast is further improved by different image acquisition techniques and external dye which are discussed later. Gradient coils are responsible for spatial resolution of different tissue parts. Three sets of gradient coil are embedded next to the main coil in Z (longitudinal), Y (transverse), and X (transverse) directions.5 They distort the main field in a predictive manner and make the different protons on a specific direction to resonance with different frequencies. For example, when a transverse coil with a left-to-right gradient is activated, the energy increases; as it reaches the right side of the body, hence right side protons will have different spinning speed and energy and resonance frequency as the matter of position. Likewise, that left side protons will process slower and they tend to lag behind the right side of the body.6
Further 3D reconstruction of captured echos with different spatial and quantitate values is the basis of MRI.4, 7 A Fourier transformation method is applied to discriminate the different echos by breaking the received echo to its constituent frequencies exactly as the inner ear does to discriminate the sounds and the vibrations of hair cells.
1.3. Parameter sequences (TR, TE)
Formation of magnetic echo varies by different methods of RF pulses that perturbs the magnetic field (i.e., different MR sequences or protocols). Radiofrequency pulse may occur at a less than 90- or 180-degree flips which are discussed in detail later.4 TR refers to the interval of two successive 90 degree RF pulses (i.e., relaxation time). TE refers to center of excitation pulse to the peak of detected echo when excited by the 90 degree pulse (i.e., echo time).8 Number of sequential captured echos following a single RF pulse within each TR is called echo train length.6, 7
1.4. Signal timing and weighting (T1, T2, PD)
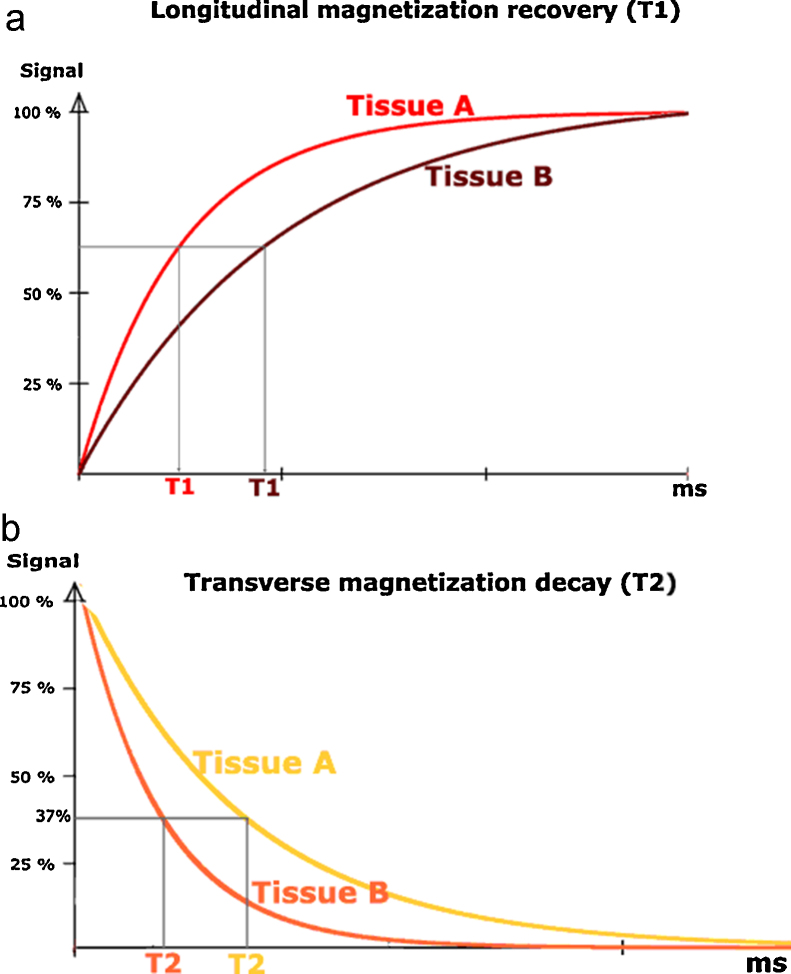
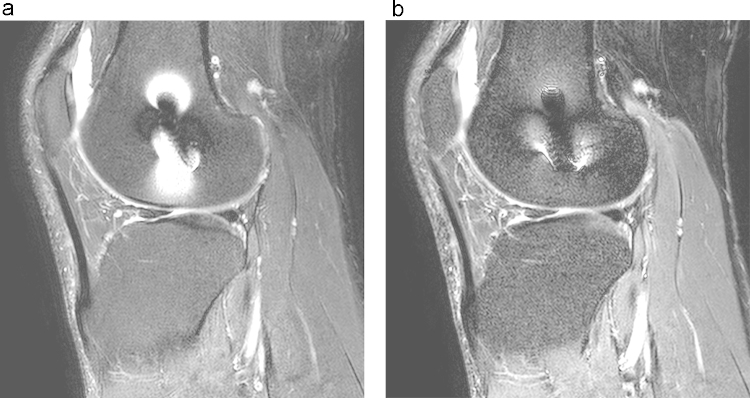
T1 time is defined as the time for the 180 degree longitudinal magnetization (Mz) recovers to 63% of its initial value and spin energy which is obtained from the initial RF is given back to surrounding lattice or environment (spin-lattice) (Fig. 1).8 Longitudinal axis or Z-axis is defined as the vector of magnetization parallel to the main magnetic field vector. T1-weighted images have short TR and TE and have intense fat signals.
Fig. 1.
Longitudinal (T1) and transverse (T2) magnetization. (a) tissue A has shorter T1 time. (b) tissue b has shorter T2 time. (Reprinted with permission, e-MRI, Hoa D, www.imaios.com).
T2 time is defined as transverse magnetization (Mx,y) decays to 37% of its initial value (Fig. 1). This pertains to inter-spin interactions that describe the dephasing of coherent spins which are not parallel to the main magnetic vector (spin-spin).8 T2-weighted images possess relatively longer TR and TE and have intense signal of cerebrospinal fluid and blood.4, 7, 8
Proton density (PD) weighted image has long TR and short TE and is a balanced signal between T1- and T2-weighted signals, in which the effect of T1 and T2 relaxation is minimized that mostly reflects water content.4, 8
TE is always shorter than TR in duration in clinical setups. These parameters could be applied when a specific material, tissue or lesion should be evaluated. For example, fat has short TE and TR that could be best examined in T1-weighted mode, while blood has longer TE and TR which demands T2-weighted image for more accurate interpretation.8 In fact TR and TE modify signals from different tissues. In more details, TR modifies T1-weighting and the longer TR makes an image to be less T1-weighted. TE also modifies T2-weighting in the way that shorter TE makes an image to be less T2-weighted.7
Tissues have different contents of hydrogen atoms, hence different contrast values are depicted in MR imaging. Beside this inherent contrast, an external contrast media or dye could augment the MR signal differences. Contrast dye shortens the TR and TE and is applied in T1-weighted phase.7, 8
1.5. Sequences (protocols) classifications
Heterogeneities of external magnetic field could dephase the spinning alignment of the excited molecules.4 The method of refocusing of dephased nuclei is the basis of main MR protocols. There are two main sequences or protocols in MR imaging: Spin echo (SE) and Gradient echo (GE).7
After main magnetizing field is applied, hydrogen protons will align toward the B0 vector based on the amplitude of Mz. In addition a much weaker transverse magnetizing field is applied at the exact frequency of resonance frequency that aligns the proton at 90° to B0. When the B1 is cut off, they return to precess about B0 again at an angle (i.e., flip angle) which is proportional to B1 amplitude and duration of applying the field. Flip angle dictates the fraction of magnetization in transverse axis in comparison to the remaining magnetization in longitudinal axis.4, 6, 7 This method of magnetization is named a 90° or square magnetization. Simultaneously, gradient coils make the field uniform with subsequent dissimilar precession of protons based on their spatial position. Finally, magnetization of different part will diminish over the time due to out-of-phase move of differently located hydrogen protons with different precession.6 Decay of precession of resonating protons in transverse vector is called free induction decay (FID) which is due to spin-spin relaxation. T2 signals always decay much faster than expected due to inhomogeneity of magnetic field so called T2*.7 Therefore, T2 is tissue related and T2* is a matter magnetic field and is non-tissue related phenomenon.7 How to refocus the out of phase protons to make and perceivable echo is the main difference of aforementioned protocols.6
SE sequence is based on the 180° rephasing RF pulses. In this protocol, the initial 90° RF pulse is applied and after the decay of transverse magnetization, signal coherent is regained (i.e., echo rephrasing) by means of a 180° refocusing RF pulse (Fig. 2).4, 7 The 180° RF pulse reverses dephasing of inhomogeneity (T2* effects) and has no effect on random spin-spin relaxation (T2 effects).7 The 180° RF pulse is applied at TE/2 and is approximately two times the initial 90° RF pulse in terms of power.5, 7
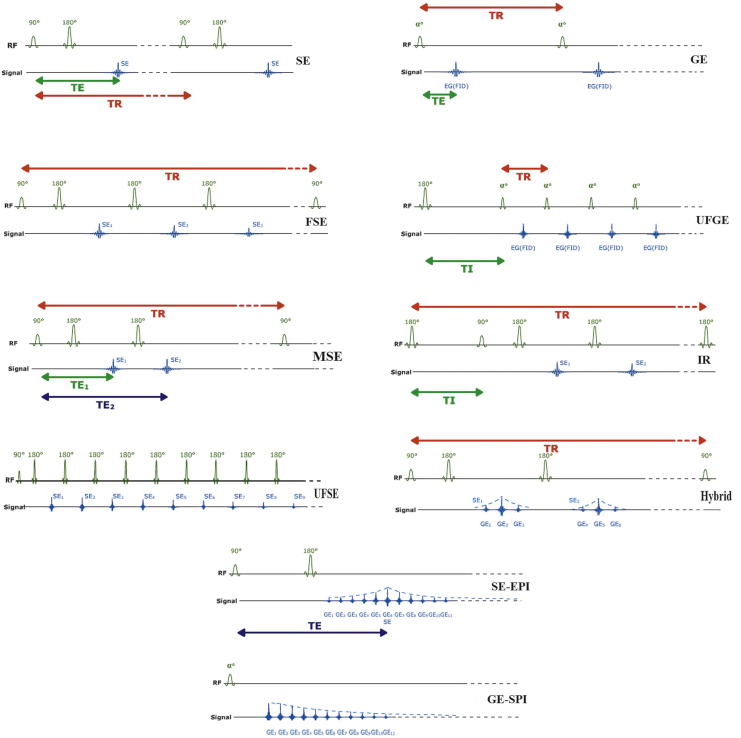
Fig. 2.
Construction of various MR Sequences. α, 90 and 180 indicates partial flip angle, 90° and 180° radiofrequency pulse magnetization, respectively. TI: inversion time, SE: spin echo, FSE: fast spin echo, MSE: multi-echo spin echo, USFE: ultra-fast spin echo, GE: gradient echo, UFGE: ultra-fast gradient echo, IR: inversion recovery, SE-EPI: spin echo echo-planar imaging, GE-EPI: gradient echo echo-planar imaging. (Modified and Reprinted with permission, e-MRI, Hoa D, www.imaios.com).
Fast or Turbo SE (FSE/TSE) applies a number of new 180° refocusing pulse after a single 90° pulse, hence a number of echos could be received during each TR. As mentioned before, the number of echos in this sequence during each TR is called echo train length or turbo factor (Fig. 2).7
This sequence is designed to have infinite TR time and long TE and has reduced repetitions as a single shot option. Of note, signal-to-noise ratio (SNR) is decreased and spatial resolution is attenuated with possibility of blurring of the image.7 Nevertheless, it enables the clinician to study semi mobile anatomic locations and organs such as airways and chest which is almost impossible with other sequences.
Multi-echo SE (MSE) applies a single new 180° pulse after a 90–180° couple pulse, hence offering two echo times (TE). The second new echo is more T2-weighted than the first echo (Fig. 2).7
GE, however, applies no 180° pulse and implements flip angles of less than 90° as compared to the square magnetization in SE. To enter the second phase (refocusing phase), an accelerated decay phase is impelled to squelching the FID. Thereafter, a second reverse rephasing gradient with the same amplitude is applied and revived echo will be used for image reconstruction (Fig. 2, Fig. 3).5, 6 This method offers overall shorter TR and scanning time.4, 7 Such a method is used to rephase the transverse magnetization compared to SE that executes this manner with 180° pulses. GE sequence provides stronger signal of tissues or materials with shorter TR.7
Fig. 3.
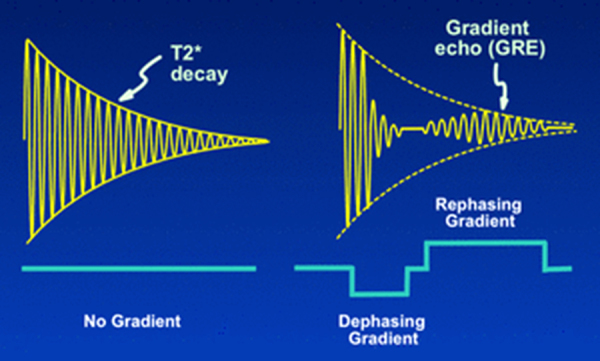
T2* decay and its manipulation to produce the gradient echo. (a) T2* typically occurs due to magnetic field homogeneity. (b) Gradient echo sequence applies two dephasing and rephasing spins after T2 decay to produce signals. (Courtesy Dr. Allen Elster, MRIquestions.com).
Ultra-fast GE (UFGE) sequence offers a very short TR and uses a very small flip angle. So called as another single shot sequence, could be used in contrast-imaging, especially in arterial phase imaging. Due to very short TR, the standard T1 image is poor; therefore a 180 degree inversion pulse is first introduced to restore the T1 image. Consequently, this augmented sequence has the advantage of T1 high resolution 3D image (Fig. 2).7
Fast- and ultra-fast SE protocols could best visualize proton density and T2-weighted images while GE and conventional SE are better protocols for T1-weighted images.7
Inversion recovery (IR) protocol is based on reverting the magnetization vector and measuring the recovery rate of excited molecules till reach the equilibrium. In this protocol, first the tissue is excited by a 180 degree inversion magnetization, followed by a delay and then followed by a 90 degree pulse.4 Hence inversion time (TI) is the interval between two paired inversion (180°) and excitation pulse (90°) (Fig. 2). Its two sub-modalities are short inversion time IR (STIR, TI = 150 ms) and fluid attenuated IR (FLAIR, TI = 2200 ms).5 STIR suppresses the fat signals from the tissues that contains high amount of fat (e.g., parotid glands) and FLAIR attenuates signal of water to enhance the contrast of adjacent tissues (e.g., periventricular area in multiple sclerosis (MS) suspected cases).
The main advantages of IR protocol are that it enables the clinician with selective nullifying the signal of specific tissue. Unless the TI is very short, far below the T1 of tissue of interest, there will be negative signal.6 The clinical correlation is that setting TI at the cross-section point of the curve with the time vector in relation to T1 of that specific tissue allows the signal to exist or to be shade out. For instance, T1 of fat tissue, brain white matter, and CSF are approximately, 240, 780 and 2700 ms, respectively. Therefore, TI of 180 ms will nullify the fat signal in STIR protocol and TI of 2500 ms will suppress CSF signals in FLAIR protocol (Fig. 4).5 Appropriate TI to nullify the signal could be set at %69 the T1 of that tissue. Though offering advantages, increased time of image acquisition is one of the disadvantages of IR protocols.4, 7 Inversion recovery sequence could be applied along with other sequences such as GE and FSE rather than conventional SE sequence.7
Fig. 4.
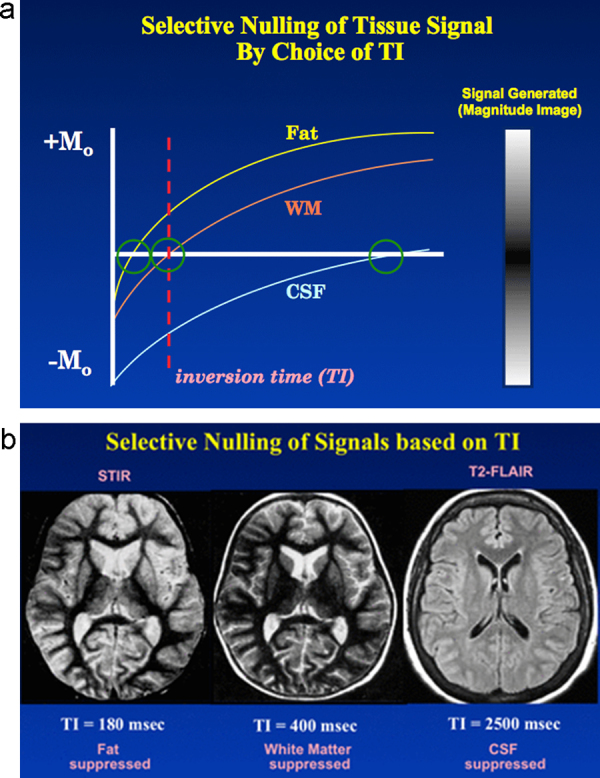
Selective nullification of tissue signals with appropriate selection of inversion time (TI). (a) the value of TI relative to tissue T1 dictates a positive or negative signal. TI < T1 dictates negative signal and vice versa. (b) Selective nullification of fat, white matter and cerebro-spinal fluid (CSF) signals could be accomplished by setting TI longer than T1 of respective tissue. (Courtesy Dr. Allen Elster, MRIquestions.com).
Echo-Planar Image (EPI) is a software technological-derived sequence that makes it possible to trace multiple echos before transverse magnetization decays toward zero, so called free induction decay (FID), in contrast to measuring one echo or a limited number of echos after each RF excitation.6 Hence a continuous readout of signal is possible after the preparative pulse of pulses enables the very fast image acquisition and specifically assesses the tissue dynamic function, so-called functional MRI.7 EPI sequence could be joined with either SE (90–180° magnetization pulse), or GE (α pulse) or IR (180–90° couple pulse) (Fig. 2).7
Hybrid SE/GE sequence is a combination of FSE and GE. It applies a 90–180° magnetization pulse and a multiple read out GE between 180° and 180° repetitions. Therefore, it reduces a number of complementary 180° pulses and acquisition time (Fig. 2).7 Table 1 displays some of the current and widely used MR protocols.5, 7 Detailed description of other various MR sequences is out of scope of this review.
Table 1.
| Sequence | Advantages | Notes |
|---|---|---|
| Spin Echo (SE) | Less susceptible to magnetic susceptible materials | Relative long acquisition time |
| Ultra-Fast SE (UFSE) | Lower acquisition time than SE, investigation of airways, lung, heart | Low spatial resolution, blurring, long TE, hyper echo fat signal |
| Gradient Echo (GE) | Shorter acquisition time than SE, strong signal of material with short TR | Susceptible to magnetic susceptible materials |
| Ultra-fast GE (UFGE) | Low acquisition time, very short TR | Susceptible to magnetic susceptible materials, not quality T1-weighted in standard mode |
| Echo-planar (EPI)a | Lowest acquisition time, Functional MRIb | Low spatial resolution, Susceptible to magnetic susceptible materials |
| Inversion recovery (IR)a | Less susceptible to magnetic susceptible materials, Selective Supersession of CSF or Fat signal | Decreased signal-to-noise ratio and decreased resolution, Long acquisition time, Long TR |
Could be combined with both GE and SE sequences.
Diffusion-weighted EPI and Perfusion-weighted EPI. Note that sequence names may vary between different manufacturers.
1.6. Magnetic susceptibility
When a material is placed in an external magnetic field, it may induce inhomogeneity in magnetic field with subsequent changes in magnetic field gradient. The latter would raise into either hyper- intense signal, signal loss (e.g., void) or intra-voxel dephasing, so called T2* effect (only GE protocols) or geometric distortion (both SE and GE protocols).7, 9 Hence the read-out and constructed pixels may not faithfully display the tissue components and spatial anatomy.10
The ability of an object to induce such changes is referred as magnetic susceptibility which could be classified further as diamagnetic (e.g., water), paramagnetic (e.g., methemoglobin, gadolinium), super-paramagnetic (e.g., lymph nodes, hemosiderin) and ferromagnetic (e.g., certain metals such as iron, cobalt and nickel), in the order of magnitude.8 Diamagnetic materials are relatively inert objects in an external magnetic field which have no unpaired orbital electrons, while the rest have unpaired electrons and are aligned in relation to the external magnetic field axis. The T2-weighted images are more susceptible to magnetic susceptibility effect than T1-weighted images due to longer echo time.2
Of note, metallic property should not be misinterpreted as ferromagnetic property. In other words, not all metals cause signal loss and image distortion. Generally, precious metals (Au, Pt, Ag, Ir, Pd) which are more conductive cause less heterogeneity of RF field and signal loss and encoding distortion happen locally at the distance very close to them.11 On the contrary, Cr–Co and Ni–Cr alloys are less conductive, yet render the undiagnostic image and significant heterogeneity in magnetic field.9 In brief, gold, amalgam and titanium are MR safe.12
1.7. Possible strategies for metal artifacts reduction (MAR)
Eliminating the metal artifact is not virtually possible, nevertheless some strategies are advocated to attenuate such effects that render the image quality labeled as undiagnostic. Generally, MAR strategies could be categorized as object related, parameter related, sequence related and software related factors.
1.7.1. Object related factors
-
a
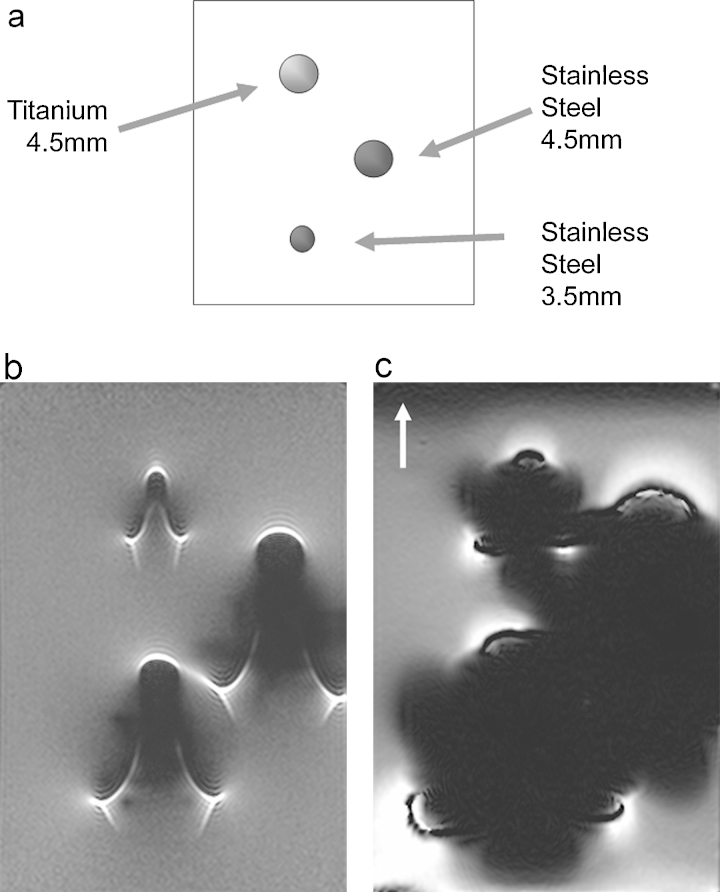
Size, material composition: The larger the diameter of a miniscrew or bracket, the more the artifact size and the signal loss. Many researchers postulated that SS materials possess higher risk for information loss in MR imaging compared to objects made up titanium alloys. (Fig. 5).2
-
b
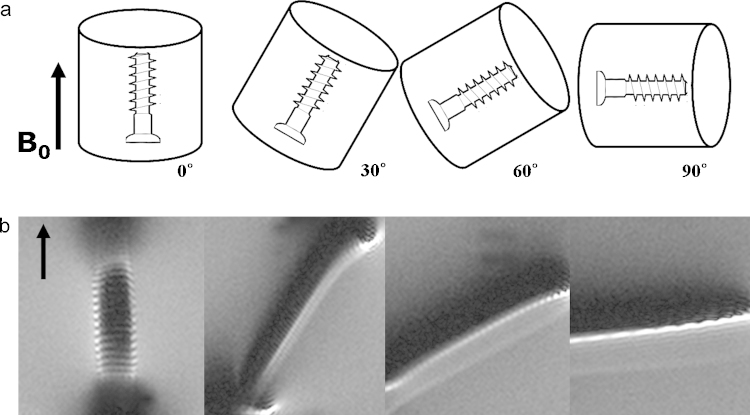
Inclination angle: One of influential factors determining the extent of image distortion is the angle between the long axis of the object, for example a miniscrew, in relation to magnetic field vector. Parallel objects exhibit least distortion, while distortion will be accentuated as the inclination angle increases (Fig. 6).2
Fig. 5.
The effect of material composition and size on metal artifact in MR imaging. (a) One titanium (4.5 mm diameter) and two stainless steel (4.5 mm and 3.5 mm diameters) screws are studied. Regardless of the size, titanium screw induces less artifact. The larger SS screw makes larger artifact. Artifacts in spin echo (SE) sequence (b) are considerably smaller than gradient echo (GE) sequence (c). (Reprinted, with permission, from Lee et al. RadioGraphics 2007;27:791-803. © Radiological Society of North America).
Fig. 6.
The effect of orientation screw on metal artifact. (a) demonstrates the orientation of long axis of screw with regards to constant magnetic field vector (B0). (b) artifacts increases as the inclination angles increases sequentially. (Reprinted, with permission, from Lee et al. RadioGraphics 2007;27:791–803. © Radiological Society of North America).
1.7.2. Protocol related factors
Some MR protocols are less susceptible to metallic effect based on the method of excitation and acquisition of signals. Among current protocols, fast spin echo with short TE is least susceptible sequence and GE and GE-related protocols are most susceptible ones. The reason left behind the refocusing method of GE protocol that applies only a reverse gradient with effect on accelerated decay and not on T2 or T2* signals. Inversion recovery (IR) sequences magnetize the atoms in invert direction compared to SE and measure the recovery rate of atoms to the equilibrium state. Short inversion time IR (STIR) is successfully applied to attenuate metal artifacts (Fig. 7).2
Fig. 7.
The effect of fat -suppression on metal artifact: compare frequency-selective fat saturation (a) with a fat-suppression sequence (STIR) (b). STIR is less susceptible to metal artifact. (Reprinted, with permission, from Lee et al. RadioGraphics 2007;27:791–803. © Radiological Society of North America).
1.7.3. Software related factors
More recently, software are introduced that compensate for signal loss or distortion. They apply either in plane reconstruction (visual angle titling, VAT) or through plane reconstruction by either multi-acquisition variable-resonance image combination (MAVRIC) or slice encoding for metal artifact correction (SEMAC).5, 13 MAVRIC method has been shown promising to reduce metal artifacts due to oral implants in patients suffering from oral cavity tumors. A better result obtained with this new sequence as compared to the fast spin echo sequence and up to 78% artifact reduction was observed.9 In another investigation SEMAC correction in fat-suppressed T2-weighted MR images significantly reduced the metal artifacts related to metallic screws in spine.13
1.7.4. Parameter related factors
Previous investigations depicted that low magnetic field strength (e.g., 1.5 Tesla vs. 3 Tesla), small field of view (with resultant decreased SNR), high-resolution matrix, thin slice, increased echo train length and increased receiver band width, in which the device is designed to receive that particular range of frequencies of excited atoms, will decrease the extent of metal artifacts.2
2. Magnetic resonance in orthodontics with focus on orthodontic appliances metal artifacts
By a recent report by the US Food and Drug Administration (FDA), MRI-related safety issues have raised by 5 times during the period of 2001–2009 of which a major portion of accidents was linked to the burns and projectile mishaps.10 A magnetic field could even make somebody feel his tooth with a nickel post within is being pulled out.14
Back to the main question of the review, one should be decided about the probability of projectile accidents, thermal damage, and artifact problems in MR images when a patient with any kind of metal object in the mouth is requested to refer for an MRI. Motion blur (i.e., patient movement) and metal artifacts are remarkable obstacles to obtain a quality MR image.9
Orthodontic appliances (e.g., fixed or removable orthodontic and maxillofacial orthopedic appliances) constitute the major portion of metal artifacts among other metallic dental objects such as metal crowns and titanium implants with regards to the extent of artifact in brain MRI.10 Generally, hard tissue assessment and metallic artifact due to orthodontic appliances are two major concerns when evaluating MR imaging in orthodontics literature.15, 16 Cortical bone, dentin, and enamel have 0.5 ms, 0.15 ms and 70 μs T2 values, respectively.15 Consequently, the anatomy and degree of maturation of such tissues have not been feasibly investigated by means of earlier generation of MRI devices due to their very short T2 values. More recent modalities such as ultra-short echo sequence grant the clinician the better visualization of the anatomy and degree of calcification of a tooth’ different part that offers TE of 0.05–0.5 ms.15 Excitation of calcium ions may be another solution in the future of this field. In addition to ultra-short TE protocol, sweep image with Fourier transform (SWIFT), zero TE, RVFIS, WAPSI and combined PETRA are other suggested solutions to overcome this drawback.17 Detailed description of mentioned methods are out of the scope of this review. Generally, MR imaging could be applied in cariology, enamel and dentin mineralization, pulpal necrosis, root resorption, demineralization of enamel beneath and adjacent to the orthodontic bracket, reperfusion of transplanted tooth for orthodontic purposes, 3D modeling of dentition, and assessment of impacted and supernumerary teeth (Fig. 8).16, 18 This progress has a particular importance as the hazard of ionizing beams is bypassed especially in young growing children.19 However, these investigations are still in their infancy and they need much more time to be validated and approved in terms of being cost–benefit. It is noteworthy to remind that ultra-fast sequences have relative long TE and should not be mistaken with ultra-short echo sequence with very short TE.15
Fig. 8.
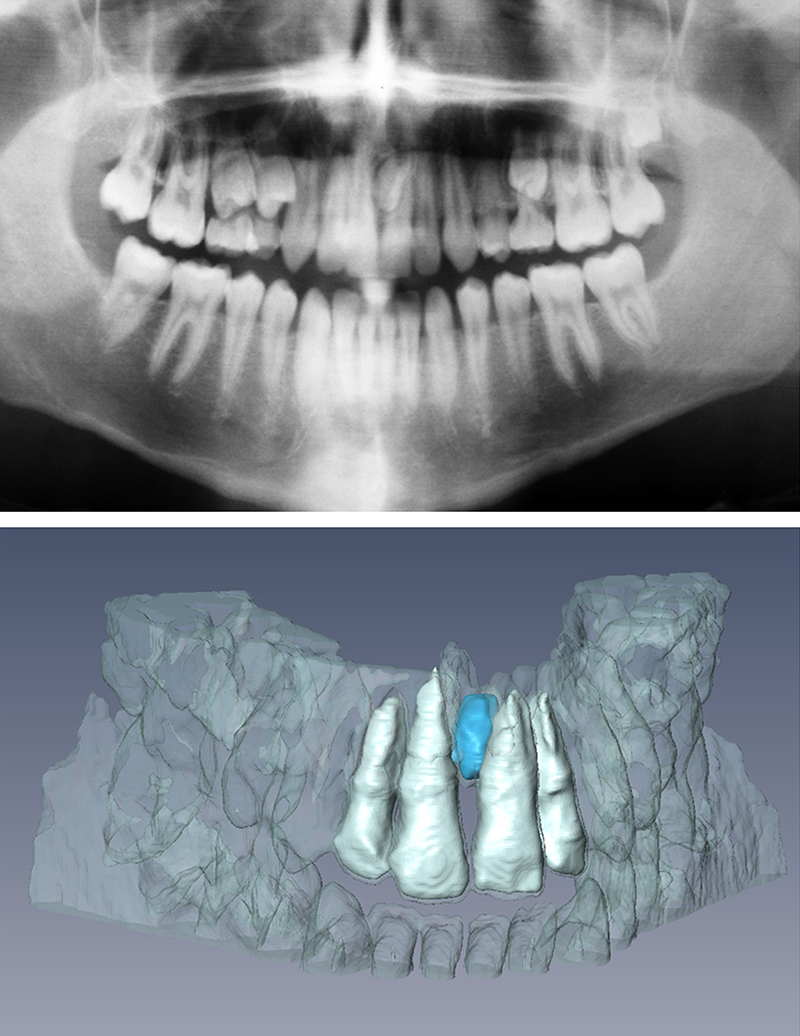
Panoramic radiograph (top) and 3D rendering of MRI data (bottom) showing a mesiodens. (Reprinted, with permission from Elsevier, Tymofiyeva et al., Diagnosis of dental abnormalities in children using 3-dimensional magnetic resonance imaging. J Oral Maxillofac Surg 2013;71:1159–69).
In the past, transmitter and receiver coils were single. Currently, devices are equipped with sophisticated and dedicated patient receiver coils. In dentistry field, there two main receiver coils applied: extra- and intra-oral receivers (Fig. 9, Fig. 10).17, 20 Extra oral coils offer the advantage of less patient discomfort, though low resolution and confounding signals from the adjacent cheek tissue.17 Traditionally, intra-oral coils were placed between cheek and teeth parallel to B0. Very recently, new intra-oral coil has been investigated that is placed parallel and to B1 and is held between the teeth at the level of occlusal plane (Fig. 2). This method provides promising effect of tooth and jaws and excludes unnecessary data from cheek, lip, and tongue.17
Fig. 9.
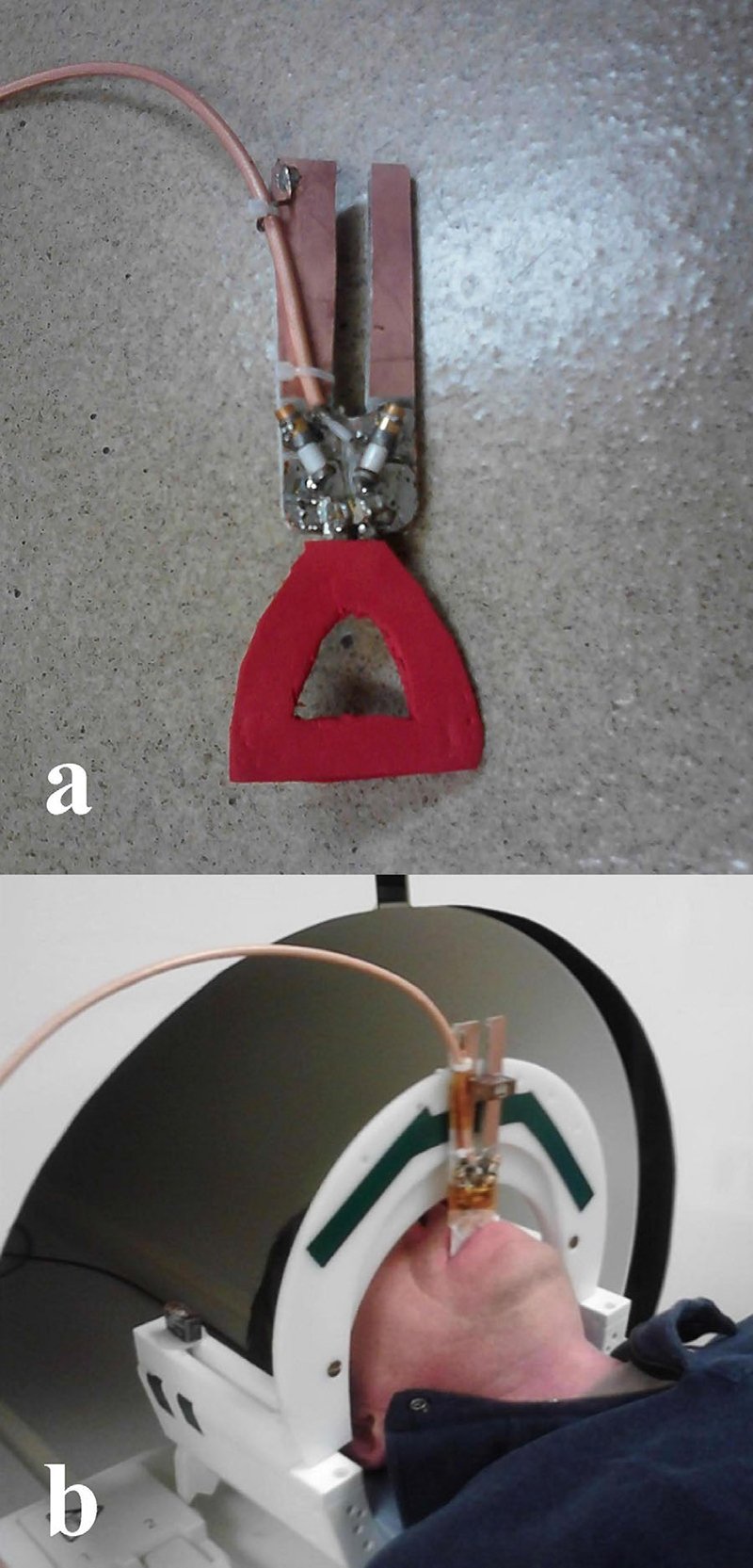
(a) Intraoral dental RF loop coil. (b) and in vivo experimental coil set between the upper and lower jaw bite planes in the occlusal position. (Reprinted, with permission from John Wiley and Sons, Idiyatullin et al., Intraoral approach for imaging teeth using the transverse B1 field components of an occlusally oriented loop coil. Magn Reson Med 2014;72:160–5).
Fig. 10.
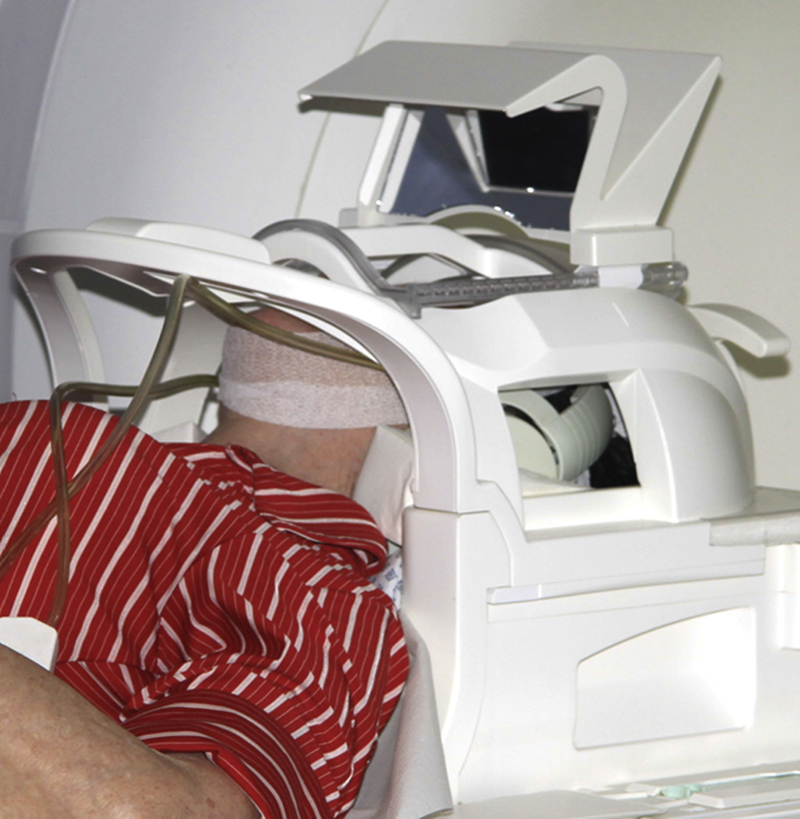
Extra oral head and neck coil. Positioning of the patient with a 20 channel head and neck coil inside the Siemens Skyra 3.0 T MRI. (Reprinted, with permission from Elsevier, Assaf et al., Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J Craniomaxillofac Surg 2014;42:1356–63).
Another issue is the magnetic susceptibility in a magnetic field which is described earlier. Signal loss depends on metal alloy (e.g., 18-8 vs. 17-4) and echo sequence (e.g., SE vs. GE). Elison et al., suggested that of 18-8 SS alloy had lesser signal loss as compared to 17-4 alloy.1 As previously mentioned, IR and SE sequences have less susceptibility to magnetic susceptible materials compared to EPI and GE sequences.1, 7 Metal artifacts are more troublesome in computed tomography (CT) than in MR images. Therefore, MRI may be superior to CT scans when oral implants of any kind are inserted and the oral cavity, especially the soft tissue is requested for medical diagnostic imaging.9, 12, 21
Various orthodontic brackets are available in market in terms of texture including stainless steel (SS), ceramic, ceramic with SS slots, plastic, and titanium brackets.9 Almost invariably, authors have census on severe distortion of MR images in patients with SS brackets; however, decision whether to remove or keep the brackets of other types should be made regarding the desired anatomic location to be imaged.1, 16, 22 As a general rule, the more distance between the bracket and desired location, the less the void and artifact and the less the distortion.1 Tendency of different imaging planes (axial vs. sagittal vs. coronal) varies in different planes. Presence of a metallic dental object may completely obscure the area of interest in one plane, meanwhile affects the other plane just by the acceptable signal voids.23
Clinician should remove SS wires before MRI due to the risk of image artifact, important interaction with magnetic field, and some possible thermal damage, though negligible.24, 25, 26 Preferably, remaining orthodontic devices in patient's mouth should be meticulously checked about their stability including ligature wires, brackets, tubes, and bands.27, 28 All metallic removable appliances need to be removed. If oral cavity MRI is planned, fixed retainers are to be debonded before imaging session.22, 27
It is not uncommon in an orthodontic clinic that a patient asks for removing of the brackets for the MR imaging of lumbosacral pain, knee ligament rupture and central nervous system problems. From the pragmatic scope, it is not possible to predict which patient may have any of possible problems that need MR investigation in future, nonetheless, patients who require periodic MR imaging should be sought at the first appointment. Options for this cohort of patients include ceramic or titanium brackets and bands instead of bonded tube.1 Desired biomechanics, anchorage demands, and treatment protocol, however, render the final clinician decision.
Ceramic brackets may produce low image noise, yet possibility of bracket wing fracture, enamel fracture at the time of debonding, attrition of opposing teeth and higher resistance are drawbacks of such a selection. Titanium brackets are preferred in such situations.1 Ceramic bonded tubes could help lowering the image noises.29 It is wise to trim the rectangular wire ends to enhance the wire play in tube to reduce the frictional resistance. Composites are used to bond bracket on tooth surface because composites contain ferric oxide. This insignificant metal content could only make distortion at the tooth surface area.16 A number of contemporary brackets have various compositions in different parts. For instance, slot composition in ceramic brackets and clip and slot composition in self-ligating systems may vary and decisions should be made based upon each appliance. Table 2 shows detailed suggestions based on current literature.1, 3, 16, 22, 24, 26, 27, 30 It should be mentioned that the degree of image distortion varies inter-individually and highly depends on ferromagnetic content of the alloys and products.22
Table 2.
Suggested guideline for various materials frequently used in orthodontics.1, 3, 16, 22, 24, 26, 27, 30
| Product | Comment |
|---|---|
| SS bracketa,b | Should be removed in head and neck MRI |
| Ceramic bracketb | MRI-safe |
| Ceramic bracket with SS slot | Should be removed in oral cavity MRI |
| Titanium bracketb | Better to be removed in oral cavity MRI |
| Plastic bracket | MRI-safe |
| SS wire | Should be removed in head and neck MRI |
| Ni-Ti wirec | Relatively MRI- compatible |
| Composite wire | Probably MRI-safed |
| Palatal/lingual arch | Should be removed in head and neck MRI |
| Fixed bonded retainer | Should be removed in oral cavity MRI |
|
Ligature wire Miniscrew and Miniplates |
Better to be removed in oral cavity MRI Should be removed in oral cavity MRId,e |
SS: stainless steel.
Self-ligating brackets maybe made of either stainless steel, nickel titanium, nickel-free SS or ceramic, hence decision should be made based on bracket composition and anatomic location of interest and clip and slot material.
Ni-Ti: nickel-titanium.
Needs further investigation.
Miniplates and miniscrews near to TMJ, maxillary sinus and palatal implants may be decided individually.
In conclusion, not all the brackets should be removed before the MR imaging and particular decision should be made individually considering the area of interest to be studied and type of brackets worn. Indeed, future improvements in software-aided reconstruction of affected anatomic areas in MR images may lead to obtain quality MR images even in the presence of stainless steel brackets on teeth.
Conflicts of interest
The authors have none to declare.
Acknowledgements
We would like to express our gratitude to Professor Stuart C. White, DDS PhD, UCLA School of Dentistry for his valuable comments. We also like to thank Dr. Hessam Rahimi, DDS DMSc, owner and orthodontist at Fusion Orthodontics and Children's Dentistry, Dallas, TX for his language editing.
References
- 1.Elison J.M., Leggitt V.L., Thomson M., Oyoyo U., Wycliffe N.D. Influence of common orthodontic appliances on the diagnostic quality of cranial magnetic resonance images. Am J Orthod Dentofacial Orthop. 2008;134:563–572. doi: 10.1016/j.ajodo.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Lee M.F., Kim S., Lee S.A. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multidetector CT. RadioGraphics. 2007;27:791–803. doi: 10.1148/rg.273065087. [DOI] [PubMed] [Google Scholar]
- 3.Cross B., Beckett H. Unwanted effects. Br Dent J. 2006;201:66–67. doi: 10.1038/sj.bdj.4814055. [DOI] [PubMed] [Google Scholar]
- 4.Chernoff D., Stark P. In: Principles of Magnetic Resonance Imaging. Muller N.L., Yeon S.B., editors. 2015. www.uptodate.com/. Last retrieved: January 30. [Google Scholar]
- 5.Elster A.D. 2015. Questions and Answers in MRI. www.mri-q.com/. Last retrieved: March 8. [Google Scholar]
- 6.Hodgson R.J. The basic science of MRI. Orthopedic Trauma. 2011;25:119–130. [Google Scholar]
- 7.Hoa D. e-MRI. Downloaded from: www.imaios.com/en. Last retrieved: December 29, 2014
- 8.Jenkins J.P.R., Jones A.P. Glossary of MR Terms. In: Sutton D, editor. Textbook of Radiology and Imaging. 7th ed. Elsevier; Churchill Livingston: 2003. Appendix II. [Google Scholar]
- 9.Gunzinger J.M., Delso G., Boss A. Metal artifact reduction in patients with dental implants using multispectral three-dimensional data acquisition for hybrid PET/MRI. EJNMMI Physics. 2014;1:102. doi: 10.1186/s40658-014-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew C.A., Maller S., Maheshwaran Interactions between magnetic resonance imaging and dental material. J Pharm Bioall Sci. 2013;5(Suppl S1):113–116. doi: 10.4103/0975-7406.113309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starčuk Z., Bartušek K., Hubálková H., Bachorec T., Starčuková J., Krupa P. Evaluation of MRI artifacts caused by metallic dental implants and classification of the dental materials in use. Meas Sci Rev. 2006;6:24–27. [Google Scholar]
- 12.Eggers G., Rieker M., Kress B., Fiebach J., Dickhaus H., Hassfeld S. Artifacts in magnetic resonance imaging caused by dental material. Magma. 2005;18:103–111. doi: 10.1007/s10334-005-0101-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.H., Lim D., Kim E., Kim S., Song H.T., Suh J.S. Feasibility of fat-saturated T2-weighted magnetic resonance imaging with slice encoding for metal artifact correction (SEMAC) at 3 T. Magn Reson Imaging. 2014;32:1001–1005. doi: 10.1016/j.mri.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Sinkiewicz D. MRI scan hazard. Br Dent J. 2013;214:376. doi: 10.1038/sj.bdj.2013.388. [DOI] [PubMed] [Google Scholar]
- 15.Robson M.D., Gatehouse P.D., Bydder M., Bydder G.M. Magnetic Resonance: An Introduction to Ultrashort TE (UTE) Imaging. J Comput Assist Tomogr. 2003;27:825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Cox R.J., Kau C.H., Rasche V. Three-dimensional ultrashort echo magnetic resonance imaging of orthodontic appliances in the natural dentition. Am J Orthod Dentofacial Orthop. 2012;142:552–561. doi: 10.1016/j.ajodo.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Idiyatullin D., Corum C.A., Nixdorf D.R., Garwood M. Intraoral approach for imaging teeth using the transverse B1 field components of an occlusally oriented loop coil. Magn Reson Med. 2014;72:160–165. doi: 10.1002/mrm.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tymofiyeva O., Rottner K., Gareis D. In Vivo MRI-based dental impression using an intraoral RF receiver coil. Concept Magn Reson B: Magn Reson Eng. 2008;33B:244–325. [Google Scholar]
- 19.Tymofiyeva O., Proff P.C., Rottner K., Düring M., Jakob P.M., Richter E.J. Diagnosis of dental abnormalities in children using 3-dimensional magnetic resonance imaging. J Oral Maxillofac Surg. 2013;71:1159–1169. doi: 10.1016/j.joms.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Assaf A.T., Zrnc T.A., Remus C.C. Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J Craniomaxillofac Surg. 2014;42:1356–1363. doi: 10.1016/j.jcms.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Klinke T., Daboul A., Maron J., Gredes T., Puls R., Jaghsi A. Artifacts in magnetic resonance imaging and computed tomography caused by DentalMaterials. PLoS ONE. 2012;7:e31766. doi: 10.1371/journal.pone.0031766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beau A., Bossard D., Gebeile-Chauty S Magnetic resonance imaging artifacts and fixed orthodontic attachments. Eur J Orthod. 2014 Jul 4 doi: 10.1093/ejo/cju020. pii: cju020 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Costa A.L., Appenzeller S., Yasuda C.L., Pereira F.R., Zanardi V.A., Cendes F. Artifacts in brain magnetic resonance imaging due to metallic dental objects. Med Oral Patol Oral Cir Bucal. 2009;14:E278–E282. [PubMed] [Google Scholar]
- 24.Görgülü S., Ayyildiz S., Kamburoglu K., Gökçe S., Ozen T. Effect of orthodontic brackets and different wires on radiofrequency heating and magnetic field interactions during 3-T MRI. Dentomaxillofac Radiol. 2014;43:20130356. doi: 10.1259/dmfr.20130356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa M., Miyata K., Abe Y., Ishigami T. Radiofrequency heating of metallic dental devices during 3.0 T MRI. Dentomaxillofac Radiol. 2013;42:20120234. doi: 10.1259/dmfr.20120234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okano Y., Yamashiro M., Kaneda T., Kasai K. Magnetic resonance imaging diagnosis of the temporomandibular joint in patients with orthodontic appliances. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:255–263. doi: 10.1067/moe.2003.37. [DOI] [PubMed] [Google Scholar]
- 27.Patel A., Bhavra G.S., O’Neill J.R.S. MRI scanning and orthodontics. J Orthod. 2006;33:246–249. doi: 10.1179/146531205225021726. [DOI] [PubMed] [Google Scholar]
- 28.Sadowsky P.L., Bernreuter W., Lakshminarayanan A.V., Kenney P. Orthodontic appliances and magnetic resonance imaging of the brain and temporomandibular joint. Angle Orthod. 1988;58:9–20. doi: 10.1043/0003-3219(1988)058<0009:OAAMRI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Harris T.M., Faridrad M.R., Dickson J.A. The benefits of aesthetic orthodontic brackets in patients requiring multiple MRI scanning. J Orthod. 2006;33:90–94. doi: 10.1179/146531205225021465. [DOI] [PubMed] [Google Scholar]
- 30.Tymofiyeva O., Vaegler S., Rottner K. Influence of dental materials on dental MRI. Dentomaxillofac Radiol. 2013;42:20120271. doi: 10.1259/dmfr.20120271. [DOI] [PMC free article] [PubMed] [Google Scholar]