Abstract
Background
Relapsing fever (RF) is an acute infectious disease caused by arthropod-borne spirochetes of the genus Borrelia. The disease is characterized by recurrent episodes of fever that concur with spirochetemia. The RF borrelioses include louse-borne RF caused by Borrelia recurrentis and tick-borne endemic RF transmitted by argasid soft ticks and caused by several Borrelia spp. such as B. crocidurae, B. coriaceae, B. duttoni, B. hermsii, B. hispanica and B. persica. Human infection with B. persica is transmitted by the soft tick Ornithodoros tholozani and has been reported from Iran, Israel, Egypt, India, and Central Asia.
Methods
During 2003–2015, five cats and five dogs from northern, central and southern Israel were presented for veterinary care and detected with borrelia spirochetemia by blood smear microscopy. The causative infective agent in these animals was identified and characterized by PCR from blood and sequencing of parts of the flagellin (flab), 16S rRNA and glycerophosphodiester phosphodiestrase (GlpQ) genes.
Results
All animals were infected with B. persica genetically identical to the causative agent of human RF. Phylogenetic analysis indicated that DNA sequences from these pet carnivores clustered together with B. persica genotypes I and II from humans and O. tholozani ticks and distinctly from other RF Borrelia spp. The main clinical findings in cats included lethargy, anorexia, anemia in 5/5 cats and thrombocytopenia in 4/5. All dogs were lethargic and anorectic, 4/5 were febrile and anemic and 3/5 were thrombocytopenic. Three dogs were co-infected with Babesia spp. The animals were all treated with antibiotics and the survival rate of both dogs and cats was 80 %. The cat and dog that succumbed to disease died one day after the initiation of antibiotic treatment, while survival in the others was followed by the rapid disappearance of spirochetemia.
Conclusions
This is the first report of disease due to B. persica infection in cats and the first case series in dogs. Infection was associated with anemia and thrombocytopenia. Fever was more frequently observed in dogs than cats. Domestic canines and felines suffer from clinical disease due to B. persica infection and may also serve as sentinels for human infection.
Keywords: Relapsing fever, Borreliosis, Borrelia persica, Feline, Canine
Background
Relapsing fever (RF) is an acute infectious disease caused by arthropod-borne spirochetes of the genus Borrelia. The disease in humans is characterized by recurrent episodes of fever, which usually concur with spirochetemia and is considered from a historical perspective the first disease linked to a specific microbial causative infectious agent [1]. The RF borrelioses can be grouped into two forms: louse-borne epidemic RF caused by B. recurrentis and tick-borne endemic RF commonly transmitted by argasid soft ticks with the exception of Borrelia miyamotoi, transmitted by several species of Ixodes hard ticks [2, 3].
Human infection with B. persica also known as Persian RF has been reported from Iran, Israel, Egypt, Pakistan, and former USSR Asian republics including Uzbekistan [4–8].
B. persica is transmitted by the soft tick Ornithodoros tholozoni whose distribution includes the Middle East, Central Asia and Northern India [9]. Ornithodoros tholozani feeds on warm-blooded animals and commonly lives in caves, ruins, rock crevices and man-made shelters where livestock is housed [10]. Human RF borreliosis is a reportable disease in Israel and the annual incidence average in civilians declined from 0.35 cases per 100,000 inhabitants from 1975 to 1985 to 0.11 cases per 100,000 inhabitants from 1986 to 2003. However, the incidence among Israeli soldiers is considerably higher with an average of 6.4/100,000 persons [11]. Although human RF borreliosis due to B. persica in the Middle East is usually not associated with mortality, severe infections with the acute respiratory syndrome and fatal infections have been reported in Israel [12, 13]. Three different genotypes of B. persica have been described from humans and O. tholozani ticks based on DNA sequences of the flagellin (flaB) gene [9].
Disease in domestic animals due to RF borreliae was rarely described. In this context, borreliosis with two species of RF borreliae, B. turicatae and B. hermsii, has been reported to cause disease in dogs in the USA [14–17]. Borrelia persica infection has recently been reported in a young spirochetemic puppy from Iran [18]. However, to our best knowledge no descriptions of disease caused by RF borreliae have been reported in domestic felines except for the isolation of B. persica from an Israeli cat, which is one of the cases described in this study [19].
Methods
Naturally-infected animals
Spirochetemia was detected during microscopic examination of blood smears stained by Romanowsky staining solutions from animals whose blood was submitted for a complete blood count (CBC) to veterinary diagnostic laboratories in Israel or performed in-house by veterinarians during the years 2003 to 2015. Blood anticoagulated in EDTA from these animals was submitted for molecular identification and genotyping to the Koret School of Veterinary Medicine at the Hebrew University.
PCR and genetic analysis
DNA was extracted from 200 μl of EDTA-anticoagulated blood samples from spirochetemic animals using the Illustra blood genomicPrep Mini Spin Kit (GE Health care, Buckinghamshire, UK), following the manufacturer’s instructions. PCRs were performed with primers targeting three different genes of RF borreliae (Table 1). An approximately 515 bp fragment of the Borrelia 16S ribosomal RNA (16S rRNA) gene was amplified using primers rec4 and rec9 as previously described [20]. An approximately 346 bp fragment of the flab gene was amplified from extracted DNA samples as previously reported for other Borrelia species using primers Bfpbu and Bfpcr [21], and an approximately 212 bp fragment of the glycerophosphodiester phosphodiesterase (GlpQ) gene, which is specific for RF borreliae, was amplified using primers 128f and 340r [22]. DNA from a borrelia-negative dog and from a human case of B. persica infection were used as negative and positive controls, respectively. A non-template negative control (NTC) was also included in each PCR run. Positive DNA amplicons were purified (EXO-Sap, New England Biolabs Inc., Ipswich, USA) and sequenced in the Center for Genomic Analyses at the Hebrew University (Jerusalem, Israel) using the BigDye Terminator cycle from Applied Biosystems ABI3700 DNA Analyzer. The ABI Data Collection and Sequence Analysis software (ABI, Carlsbad, USA) was used for analysis. DNA sequences were compared for similarities to other sequences in GenBank using the BLAST program hosted by NCBI, National Institutes of Health, USA (http://www.ncbi.nlm.nih.gov) and new DNA sequences from Borrelia-infected dogs and cats were deposited in GenBank.
Table 1.
Target genes and primers used for PCR to detect and characterize Borrelia persica in blood samples from cats and dogs
PCRs for Ehrlichia canis and for Babesia spp. were performed on DNA from the dog blood samples using primers 16S-F and 16S-R and the Piroplasmid-F and Piroplsamid-R primers, respectively, as previously described [23, 24]. PCR for hemotrophic Mycoplasma species was performed using primers HBT-F and HBT-R as previously described [25]. Serology for feline leukemia virus (FeLV) and for feline immunodeficiency virus (FIV) was performed using a commercial assay (SNAP FIV/FeLV Combo Test, IDEXX Laboratories, Westbrook, Maine, USA).
Phylogenetic analysis
A phylogenetic analysis, which included DNA sequences from this study, was carried out to compare these sequences to other Borrelia spp. that had previously been deposited in GenBank. Sequences were analyzed using the MEGA version 6.1 (http://www.megasoftware.net/) and phylogenetic trees were constructed by the maximum likelihood algorithms using the Tamura-3-Parameter model [26]. Bootstrap replicates were performed to estimate the node reliability, and values were obtained from 1000 randomly selected samples of the aligned sequence data.
Results
The demographic and clinical findings from the ten spirochetemic cats and dogs are presented in detail in Tables 2 and 3. The five cats were from cities (Jerusalem and Arad) and from rural villages (Kfar Adumim, Matzuba and Kfar Oranim) in southern, central and northern Israel and all had outdoor access. The five dogs were all from small towns and villages (Yavne’el, Meitar, Hashmonaim, Karmei Yosef and Amatzia) located also in southern, central and northern Israel.
Table 2.
Demographic and clinical characteristics of cats infected with Borrelia persica included in the study
| Cat number (sample ID) | 1 (6812) | 2 (42798) | 3 (9727) | 4 (213813) | 5 (8738) |
|---|---|---|---|---|---|
| Location | Kfar Adumim | Jerusalem | Arad | Matzuba | Kfar Oranim |
| Year of diagnosis | 2003 | 2003 | 2010 | 2012 | 2015 |
| Outdoor access | + | + | + | + | +; stray |
| Sex; age in years | F; 2 | M; 1 | Male; ND | F; 7.5 | F; 1 |
| Fever | – | – | – | - | 39.8 °C |
| Lethargy | + | + | + | + | ND |
| Anorexia | + | + | + | + | ND |
| Pale mucous membranes | – | – | + | + | + |
| Icterus | – | – | + | + | - |
| Anemia | + | + | + | + | + |
| Hematocrit L/L | 0.208 | 0.116 | 0.113 | 0.109 | 0.270 |
| MCV fL | 40.5 | 42.1 | 31.2 | 39.3 | ND |
| MCHC g/L | 336 | 288 | 389 | 390 | ND |
| Leukocytosis; WBC number × 109/L | –; 11.6 | –; 5.86 | +; 33.88 | +; 21.33 | -; Est WNL |
| Thrombocytopenia; PLT number × 109/L | +; 35 | +; 119 | –; 227 | +; 71 | +; Est low |
| Co-infection: PCR for hemotrophic Mycoplasma spp. Serology FeLV; FIV | – | – | – | - | - |
| (–; –) | (–; –) | ND | (-; +) | (-; -) | |
| Antibiotic treatment | amoxicillin/clavulanic acid | amoxicillin/clavulanic acid | long acting tetracycline and amoxicillin/clavulanic acid | doxycycline | doxycycline |
| Survival and response to treatment | survived; spirochetemia not evident one day after treatment initiation | died one day after treatment initiation | survived | survived; clinical improvement reported 12 h after initial doxycycline; clinical recovery in 4 days | survived; recovered clinically and no spirocehtemia evident on follow-up 21 days after treatment initiation |
ND not determined, Est estimated from blood smear, WNL within normal limits; Hematocrit, reference range 0.277–0.468 L/L; MCV mean cell volume, range 41–53 fL; MCHC mean corpuscular hemoglobin concentration, range 270–330 g/L; WBC white blood cells, range 6.3–19.6 × 109/l; PLT: platelets, range 156–626 × 109/l
Table 3.
Demographic and clinical characteristics of dogs infected with Borrelia persica included in the study
| Dog number (sample ID) | 1 (61887) | 2 (8726) | 3 (4663) | 4 (9835) | 5 (8692) |
|---|---|---|---|---|---|
| Location | Yavne' el | Meitar | Hashmonaim | Karmei Yosef | Amatzia |
| Year of diagnosis | 2003 | 2012 | 2012 | 2013 | 2015 |
| Sex; age in years | M; 1.5 | F; 3 | M; 4 | F; 12.5 | M; 2.5 |
| Breed | Siberian Husky | Mixed breed | Labrador | Mixed breed | German Shepherd |
| Fever | 40.8 ° C | 41.2 °C | 39.5 °C | –; (38.9 ° C) | 39.4 °C |
| Lethargy | + | + | + | + | + |
| Anorexia | + | + | + | + | + |
| Pale mucous membranes | – | + | + | + | - |
| Icterus | – | – | + | – | - |
| Anemia | + | + | + | + | - |
| Hematocrit L/L | 0.271 | 0.340 | 0.189 | 0.270 | 0.375 |
| MCV fL | 75.3 | 77.7 | 66.7 | 69.2 | 67.5 |
| MCHC g/l | 305 | 305 | 337 | 353 | 381 |
| Leukocytosis | – | – | – | – | - |
| WBC number × 109/l | 10.69 | 11.80 | 12.80 | 9.13 | 7.70 |
| Thrombocytopenia | – | + | – | + | + |
| PLT number × 109/l | 168 | 41 | 171 | 86 | 39 |
| Co-infection: | |||||
| PCR for E. canis | – | – | – | – | - |
| PCR for Babesia sp. | – | + | + | + | - |
| Antibiotic treatment | ciprofloxacin for 2 days and then doxycycline | doxycycline | long acting tetracycline and amoxicillin/clavulanic acid | amoxicillin initially, continued with doxycycline | doxycycline |
| Survival and response to treatment | survived; no spirochetemia at recheck 7 days after treatment initiation | survived; no spirochetemia at recheck 11 days after treatment initiation; treated also with imidocarb dipropionate for babesiosis | died one day after treatment initiation | survived; improved a day after initial treatment with amoxicillin; treated also with imidocarb dipropionate for babesiosis | survived; recovered clinically at recheck 4 days after treatment initiation |
Hematocrit, reference range 0.371–0.57 L/L; MCV mean cell volume, range 58.8–71.2 fL; MCHC mean corpuscular hemoglobin concentration, range 310–362 g/l; WBC white blood cells, range 5.2–13.9 × 109/L; PLT: platelets, range 150–400 × 109/l
The five spirochetemic cats included three females and two males with an age range of one to 7.5 years. Only one of the cats had fever (> 39.0 °C). All four cats with owners presented with lethargy and anorexia. Three of the five cats had pale mucous membranes and two were icteric. Blood for CBC was available from four cats (#1–4) and only hematocrit and blood smear evaluation were recorded from cat # 5. All cats were anemic (hematocrit < 0.277 L/L) with microcytic anemia in three cats. Thrombocytopenia was evident in 4/5 cats (platelets < 156 × 109/l) and confirmed by blood smear evaluation, while leukocytosis (> 19.6 × 109/l) was evident in 2/5. All cats were negative for hemotrophic Mycoplasma species by PCR. Four of the cats (#1, 2, 4 and 5) were tested for infection with FeLV and FIV and cat #4 was found positive for FIV. Additional blood was not available to test cat # 3 for infection with these retroviruses. All cats received antibiotic treatment; three were treated with amoxicillin/clavulanic acid, two with doxycycline and one with a combination of amoxicillin/clavulanic acid and long acting injectable tetracycline. The cats that survived the infection were treated for variable durations ranging from one to four weeks. One cat (#2) died one day after treatment initiation, whereas the remaining four cats survived and recovered. Cat # 1 did not have evident spirochetemia when evaluated by blood smear one day after treatment initiation; the owners of cat # 4 reported a clinical improvement twelve hours after initial antibiotic administration and return to normal activity within four days; and cat #5 also recovered clinically rapidly and no spirochetemia was detected on a follow-up 21 days after treatment initiation.
The five spirochetemic dogs included three males and two females with an age range of 1.5 to 12.5 years. Four had fever (> 39.0 °C) and the other dog had a borderline fever of 38.9 °C. All dogs presented lethargy and anorexia. Four of the five dogs had pale mucous membranes and one was icteric. Four dogs were anemic (hematocrit < 0.371 L/L) with macrocytic anemia in two. Thrombocytopenia (< 150 ×109/l) was evident in three animals. All dogs were negative for E. canis infection tested by PCR and three (#2,3 and 4) were positive for Babesia spp. by PCR and blood smear evaluation. All dogs received antibiotic treatment: two were treated with doxycycline alone, one with ciprofloxacin for two days and then switched to doxycycline, another one received a combination of long acting tetracycline and amoxicillin/clavulanic acid, and one received amoxicillin initially and was later switched to doxycycline. Two dogs with babesiosis (#2 and 4) were treated also with imidocarb dipropionate injections. The dogs that survived the infection were treated with doxycycline for 21 days. One dog (#3) co-infected with Babesia sp. died one day after treatment initiation, whereas the four other dogs survived and fully recovered. Dogs #1 and #2 did not have evident spirochetemia in their blood smears at recheck seven and eleven days after treatment initiation, respectively. In dogs #4 and #5, clinical improvement was noted one and four days, respectively, after initial antibiotic administration.
Numerous spirochetes were noted in blood smears from all dogs and cats with 4 to 10 spirochetes per microscopic field at 500× magnification (Fig. 1). Cat #2 which died in the course of infection had an overwhelmingly high spirochetemia (Figs. 2 and 3) and blood obtained from this cat was used for culture of B. persica as previously described [19]. Spirochetes seen in the blood smears occasionally formed aggregates with platelets (Fig. 4) or encircled erythrocytes (Fig. 5).
Fig. 1.
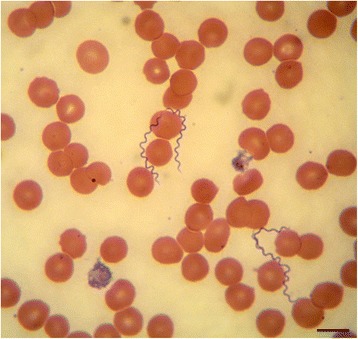
Spirochetemia with Borrelia persica in a blood smear from dog no. 4. Romanowsky stain. Scale-bar: 10 μM
Fig. 2.

Prominent Borrelia persica spirochetemia in cat no. 2. Romanowsky stain. Scale-bar: 20 microns
Fig. 3.

Higher magnification of spirochetemia with Borrelia persica in cat no. 2. Romanowsky stain. Scale-bar: 10 μM
Fig. 4.
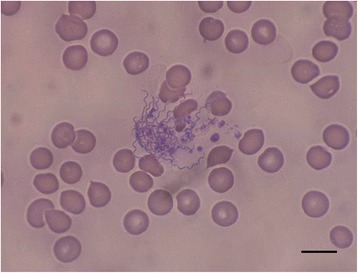
Borrelia persica spirochetes aggregating with platelets in a blood smear from dog no. 1. Romanowsky stain. Scale-bar: 10 μM
Fig. 5.
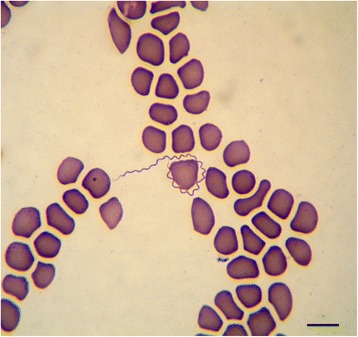
Borrelia persica spirochete encircling a canine erythrocyte in blood smear from dog no. 2. Romanowsky stain. Scale-bar: 10 μM
PCR of blood from all cats and dogs was positive for Borrelia sp. by all three PCR protocols amplifying fragments of the 16S rRNA, flaB and GlpQ genes, except for cat # 2. This animal was not tested with the GlpQ PCR, because no further DNA was available for the test (Table 4). DNA sequences amplified from all of the animals with the three PCR protocols were 99–100 % identical to B. persica sequences already deposited in GenBank and B. persica was constantly the first match in all BLAST searches. Twenty-nine DNA sequences from this study were deposited in GenBank and assigned accession numbers (Table 4).
Table 4.
GenBank accession number of Borrelia persica from cats and dogs included in the study
| Animal number | 16S rRNA | flagellin (flab) | glycerophosphodiester phosphodiesterase (GlpQ) |
|---|---|---|---|
| Cat 1 (6812) | DQ207601 | KT895509 | KT895516 |
| Cat 2 (42798) | DQ207603 | DQ211356 | ND |
| Cat 3 (9727) | KT895504 | KT895511 | KT895518 |
| Cat 4 (213813) | KT895503 | KT895510 | KT895517 |
| Cat 5 (8738) | KU565880 | KU565881 | KU932019 |
| Dog 1 (61887) | DQ207600 | DQ207604 | KU932020 |
| Dog 2 (8726) | KT895505 | KT895512 | KT895519 |
| Dog 3 (4663) | KT895506 | KT895513 | KT895520 |
| Dog 4 (9835) | KT895507 | KT895514 | KT895521 |
| Dog 5 (8692) | KT895508 | KT895515 | KT895522 |
ND not done
The Babesia PCR products amplified by the Piroplasmid PCR from dogs #2, 3 and 4 yielded sequences that differed from other known Babesia spp. in GenBank and therefore were termed Babesia sp. in this study and await further characterization.
Phylogenetic analysis of the flaB gene sequences (Fig. 6) indicated that the sequences from the B. persica infected dogs and cats clustered together with each other and with sequences of other B. persica organisms recovered from humans and O. tholozani ticks. Most of the cat and dog sequences clustered together with a B. persica genotype 1 sequence (DQ679907) from a human individual, whereas one cat sequence clustered together with a B. persica genotype 2 from a tick (DQ6795509), while none of our sequences clustered closely with B. persica genotype 3 from a human (DQ679906). All B. persica sequences clustered separately from other Old World RF Borrelia spp. including B. duttonii, B. crociduare, B. hispanica and B. recurrentis. RF species from the American continent including B. parkeri, B. turicatae and B. hermsii also clustered separately and together with B. miyamotoi which was described from the American continent and also from Asia and Europe.
Fig. 6.
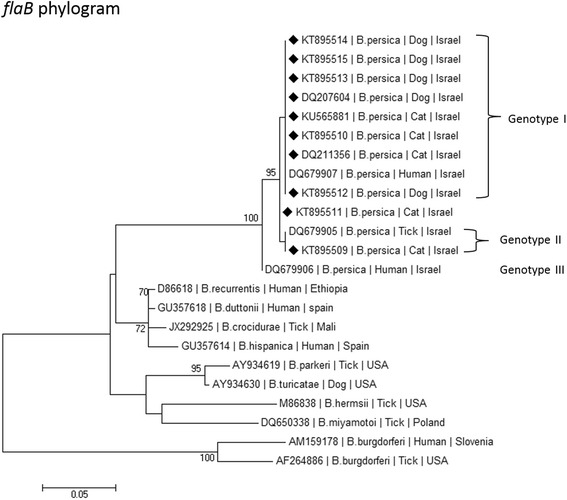
A maximum likelihood phylogram comparing 267 bp DNA sequences of the flaB gene from the cats and dogs included in the study to sequences from other B. persica GenBank accessions and from other Borrelia spp. New sequences derived from this study are marked with black diamond squares. Note the division into B. persica genotypes marked in Roman numerals. The GenBank accession numbers, species of infected host and country of origin are included for each sequence. The Tamura-3-Parameter model was used in the construction of this phylogram and bootstrap values higher than 70 % are indicated
Phylogenetic analysis of GlpQ sequences (Fig. 7) also indicated that the sequences from the B. persica-infected dogs and cats clustered together with each other and with other B. persica sequences from a human and an O. tholozani tick. As in the flaB phylogram, B. persica GlpQ sequences clustered separately from Old World RF Borrelia spp. including B. hispanica, B. duttonii, B. crocidurae and B. recurrentis. The Old World species including B. persica also clustered separately from American RF Borrelia spp. namely B. hermsii, B. parkeri and B. turicatae.
Fig. 7.

A maximum likelihood phylogram comparing 133 bp DNA sequences of the GlpQ gene from the cats and dogs included in the study to sequences from other Borrelia persica GenBank accessions and from other Borrelia spp. New sequences derived from this study are marked with black diamond squares. The GenBank accession numbers, species of infected host and country of origin are included for each sequence. The Kimura-2-Parameter model was used in the construction of this phylogram and bootstrap values higher than 70 % are indicated
Discussion
This study describes clinical disease associated with B. persica infection in domestic cats and dogs. The infected animals had profound spirochetemia, were lethargic, anorectic and suffered from anemia and frequent thrombocytopenia. Infected dogs were febrile or borderline febrile, whereas fever was noted in only one infected cat. Treated dogs and cats mostly recovered rapidly with antibiotic treatment, and spirochetemia apparently cleared as found in follow up blood tests. Nevertheless, the fact that two of the infected animals (20 %) had died indicates that this disease is not benign and is potentially fatal in pets. The Jarisch–Herxheimer reaction with fever, sweating, anorexia and occasional death upon initiation of antibiotic treatment for RF borreliosis and bacterial decay, has been associated with the increase of circulating levels of tumor necrosis factor α (TNF-α), interleukin-6, and interleukin-8 in humans [27]. Death due to a similar reaction may have occurred also in the dog and cat in this study that died soon after the beginning of antibiotic treatment. No evidence of cyclic spirochetemia with relapsing episodes of fever, as shown in infected people, was reported for the infected dogs and cats. Yet, this may be due to the difficulty of getting a thorough history on these pets, the possibility that the infected animals were treated and not given the opportunity to develop recurrent fever episodes, which could be recorded by their veterinarians, or simply due to lack of close monitoring.
The only previous report of a dog with infection caused by B. persica was described in a 2-week old puppy from Teheran, Iran, presenting anorexia, pale mucous membranes, diarrhea, vomiting and anemia [18]. Another species, B. hispanica has been shown to experimentally infect a dog by rat bite [28]. Two other species of RF borreliae have been reported to infect dogs in North America. Dogs with B. turicatae infection reported from Texas and Florida [14–16] were febrile, lethargic, anorectic, anemic and thrombocytopenic. A single case of canine infection with B. hermsii was reported from Washington State, USA. This dog presented with lethargy, anorexia, fever, anemia, leukopenia and thrombocytopenia [17]. These reports from dogs infected with B. turicatae, B. hermsii and the B. persica-infected puppy from Iran indicate that different RF Borrelia spp. are able to infect dogs with similar clinical manifestations including lethargy, anorexia, fever, anemia and thrombocytopenia.
Disease caused by B. persica does not seem to be frequent in Israeli cats and dogs as the cases included in this series, although probably not the only cases of this disease in Israel during the period of case collection, had been recorded over a 12-year period. Furthermore, the lack of apparent geographical clustering of cases and the wide distribution of disease locations from the southern part of the country to its north, suggests that infection is sporadic.
The common treatment for human B. persica RF is with doxycycline [29, 30] although treatment with amoxicillin has also been recommended [9]. Doxycycline is also recommended as the major drug for post-exposure prevention [31, 32]. Although almost all the dogs and cats in this study, which recovered from disease were treated with doxycycline or another tetracycline, cat #1 also recovered and was apparently non-spirochetemic one day after treatment initiation with amoxicillin and clavulanic acid, whereas dog #4 was treated initially with amoxicillin, reported to improve one day later, and only then was continued with doxycycline. This suggests that several antibiotics may be effective against B. persica infection in cats and dogs. Nevertheless, due to the apparent efficacy of doxycycline evident in this study and based on the recommendation for human treatment and prophylaxis with this drug [29, 31], it would be sensible to recommend doxycycline as a first line antibiotic for canine and feline B. persica infection.
Co-infection with babesiosis potentially contributed to the severity and clinical manifestations of B. persica infection in the three co-infected dogs. Canine babesiosis is also associated with fever, anemia and thrombocytopenia [33]. Nevertheless, the two dogs with no detectable babesiosis were also febrile, thrombocytopenic (dog # 5) or borderline thrombocytopenic (dog # 1) and anemic (dog #1) or borderline anemic (dog #5). Thus, babesiosis by itself cannot account for all the clinical findings in the dogs with B. persica infection. Furthermore, no cats were positive for hemotrophic mycoplasmas and only one of the four cats tested for FeLV and FIV was positive for FIV (#4). Therefore, no clear association of feline B. persica infection with hemortophic Mycoplasma or immune-suppressive viral infections such as FeLV and FIV is apparent.
The genetic analysis of B. persica from cats and dogs based on three different genes strongly suggests that the pathogen from these animals is identical to the cause of RF in humans in Israel and other countries. The B. persica organisms detected in the animals belonged to two of the three known B. persica genotypes described from humans in Israel [5, 9]. Since the same infectious agent exists in humans and domestic animals, the infection and disease might be considered a zoonosis. Nevertheless, the question of transmission and life-cycle of B. persica is complex as transovarial transmission of this infection from the adult female tick via the eggs to its offspring has been reported [34], and thus this infection might not need an animal reservoir. Consequently, the roles of animals or humans in the life-cycle of this pathogen may only be the supply of a blood meal for the host tick. Despite this, this bacterium is adapted to growth in medium containing human serum [19] and also to propagation in animals as shown experimentally in laboratory mice [35]. Therefore, B. persica may easily infect animals and hence vertebrates may play an important role in its life-cycle as reservoir hosts.
The tick O. tholozani is typically found in caves, ruins and archeological sites in Israel where human infection with B. persica has often been reported to be acquired, and the disease is frequently referred to as cave fever [9, 11]. Since O. tholozani ticks feed rapidly and do not commonly attach to their host for more than 20–30 min [9], ticks are usually not found on human patients and are also not reported on animal hosts in Israel. Therefore, it would be unlikely to find cats and dogs infested with O. tholozani. Furthermore, in perspective of the nature of the tick’s habitat, it seems more probable that wildlife animals would serve as reservoirs of this infection, as cats and dogs are not expected to reach remote cave locations, which are far from their typical environment. Such potential wildlife reservoirs may nevertheless be related to dogs or cats, e.g. wild canids such as jackals, foxes or wolves, or wild feline species.
Although we have documented severe disease with profound B. persica spirochetemia in dogs and cats, chronic persistent sub-clinical low level spirochetemia undetected by conventional blood smear microscopy may exist in animals, which could serve as reservoirs for tick infection. Such a possibility should be evaluated by PCR surveys. Such surveys of animal infection with B. persica could be of public health importance as animals may serve as sentinels for human infection.
Conclusions
This study describes severe clinical potentially fatal disease associated with B. persica infection, a causative agent of human RF, in domestic cats and dogs. Infection was associated with lethargy, anorexia, anemia and thrombocytopenia in both species while fever was more frequent in infected dogs than in cats. This infection therefore endangers both humans and domestic carnivores, and in the context of One Health, animal infection may serve as a sentinel for human infection.
Animal ethics statement
This study was carried out in accordance with the Hebrew University ethic regulations for experimentation in animals. The study involved exclusive use of residual blood samples taken as a part of the animals diagnostic procedure by attending veterinarians.
Acknowledgments
The authors thank the veterinarians who treated the cats and dogs reported in this study: Drs Almagor, Haimowitz, Kalechman, Volnasky, Cohensius, Oren, Ashuach, Wainberg, Shaani and Blum for assistance in this study. We also thank Dr. Eyal for her laboratory assistance. The study was funded by support from the USAID MERC program grant no. TA-MOU-12-M32-038 and grant 2014.52146 from the Netherlands Ministry of Foreign Affairs, The Hague, Netherlands. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 11th CVBD World Forum Symposium.
Abbreviations
- 16S rRNA
16S ribosomal RNA gene
- CBC
complete blood count
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- flab
flagellin gene
- GlpQ
glycerophosphodiester phosphodiestrase gene
- RF
relapsing fever
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GB and RKS planned and conceived the study and wrote the manuscript. YNB, TH, YH and GK performed the genetic analyses and participated in writing the manuscript. ZA, EL and IA participated in conceiving the study, diagnosing and managing of the animal cases, and writing the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Obermeier O. Vorkommen feinster, eine Eigenbewegung zeigender Fäden im Blute von Recurrenskranken. Centralblatt f d Medicinischen Wissenschaften. 1873;10:145–7. [Google Scholar]
- 2.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 3.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;7:631–9. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arshi S, Majidpoor A, Sadeghi H, Asmar M, Emdadi D, Drakhshan MH. Relapsing fever in Ardabil, a northwestern province of Iran. Arch Iran Med. 2002;5:141–5. [Google Scholar]
- 5.Safdie G, Farrah IY, Yahia R, Marva E, Wilamowski A, Sawalha SS, et al. Molecular characterization of Borrelia persica, the agent of tick borne relapsing fever in Israel and the Palestinian Authority. PLoS ONE. 2010;5:e14105. [DOI] [PMC free article] [PubMed]
- 6.Davis GE, Hoogstraal H. Biology of the spirochete Borrelia persica, found in the tick Ornithodrus tholozani (Argasinae) collected in the governorate of the western Egyptian desert; comments on the distribution and ecology of the vector tick. Ann Parasitol Hum Comp. 1956;31:147–54. [PubMed] [Google Scholar]
- 7.Zamani Z, Arjmand M, Oreiz F, Soleimani M, Hosseini SH, Assmar M, et al. Culture of Borrelia persica and its flagellar antigen in vitro. Pak J Biol Sci. 2014;176:190–7. [DOI] [PubMed]
- 8.Elbir H, Larsson P, Normark J, Upreti M, Korenberg E, Larsson C, et al. Genome sequence of the Asiatic species Borrelia persica. Genome Announc. 2014;2:e01127-13. [DOI] [PMC free article] [PubMed]
- 9.Assous MV, Wilamowski A. Relapsing fever borreliosis in Eurasia-forgotten, but certainly not gone! Clin Microbiol Infect. 2009;15:407–14. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 10.Estrada-Pena A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. doi: 10.1023/A:1006241108739. [DOI] [PubMed] [Google Scholar]
- 11.Sidi G, Davidovitch N, Balicer RD, Anis E, Grotto I, Schwartz E. Tickborne relapsing fever in Israel. Emerg Infect Dis. 2005;11:1784–6. doi: 10.3201/eid1111.050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagupsky P, Moses S. Neonatal Borrelia species infection (relapsing fever) Am J Dis Child. 1985;139:74–6. doi: 10.1001/archpedi.1985.02140030076034. [DOI] [PubMed] [Google Scholar]
- 13.Yossepowitch O, Gottesman T, Schwartz-Harari O, Soroksky A, Dan M. Aseptic meningitis and adult respiratory distress syndrome caused by Borrelia persica. Infection. 2012;40:695–7. doi: 10.1007/s15010-012-0296-8. [DOI] [PubMed] [Google Scholar]
- 14.Breitschwerdt EB, Nicholson WL, Kiehl AR, Steers C, Meuten DJ, Levine JF. Natural infections with Borrelia spirochetes in two dogs from Florida. J Clin Microbiol. 1994;32:352–7. doi: 10.1128/jcm.32.2.352-357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS, et al. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol. 2005;43:3851–9. [DOI] [PMC free article] [PubMed]
- 16.Whitney MS, Schwan TG, Sultemeier KB, McDonald PS, Brillhart MN. Spirochetemia caused by Borrelia turicatae infection in three dogs in Texas. Vet Clin Pathol. 2007;36:212–6. [DOI] [PubMed]
- 17.Kelly AL, Raffel SJ, Fischer RJ, Bellinghausen M, Stevenson C, Schwan TG. First isolation of the relapsing fever spirochete, Borrelia hermsii, from a domestic dog. Ticks Tick Borne Dis. 2014;5:95–9. doi: 10.1016/j.ttbdis.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirani D, Rakhshanpoor A, Cutler SJ, Ghazinezhad B, Naddaf SR. A case of canine borreliosis in Iran caused by Borrelia persica. Ticks Tick Borne Dis. 2016;7:424–6. doi: 10.1016/j.ttbdis.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzer S, Margos G, Overzier E, Fingerle V, Baneth G, Straubinger RK. Borrelia persica: In vitro cultivation and characterization via conventional PCR and multilocus sequence analysis of two strains isolated from a cat and ticks from Israel. Ticks Tick Borne Dis. 2015;6:751–7. doi: 10.1016/j.ttbdis.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Ras NM, Lascola B, Postic D, Cutler SJ, Rodhain F, Baranton G, et al. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol. 1996;6:859–65. [DOI] [PubMed]
- 21.Fukunaga M, Ushijima Y, Aoki LY, Talbert A. Detection of Borrelia duttonii, a tick-borne relapsing fever agent in central Tanzania, within ticks by flagellin gene-based nested polymerase chain reaction. Vector Borne Zoonotic Dis. 2001;1:331–8. doi: 10.1089/15303660160025949. [DOI] [PubMed] [Google Scholar]
- 22.Halperin T, Orr N, Cohen R, Hasin T, Davidovitch N, Klement E, et al. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Trop. 2006;98:189–95. [DOI] [PubMed]
- 23.Peleg O, Baneth G, Eyal O, Inbar J, Harrus S. Multiplex real-time qPCR for the detection of Ehrlichia canis and Babesia canis vogeli. Vet Parasitol. 2010;173:292–9. doi: 10.1016/j.vetpar.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Tabar MD, Altet L, Francino O, Sánchez A, Ferrer L, Roura X. Vector-borne infections in cats: molecular study in Barcelona area (Spain) Vet Parasitol. 2008;151:332–6. doi: 10.1016/j.vetpar.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. 2003;93:307–17. doi: 10.1016/S0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negussie Y, Remick DG, DeForge LE, Kunkel SL, Eynon A, Griffin GE. Detection of plasma tumor necrosis factor, interleukins 6, and 8 during the Jarisch-Herxheimer Reaction of relapsing fever. J Exp Med. 1992;175:1207–12. doi: 10.1084/jem.175.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horrenberger R. Experimental transmission of the Spirochaeta hispanica to the dog by rat bite. C R Seances Soc Biol Fil. 1955;149:1432–4. [PubMed]
- 29.Colin de Verdiere N, Hamane S, Assous MV, Sertour N, Ferquel E, Cornet M. Tickborne relapsing fever caused by Borrelia persica, Uzbekistan and Tajikistan. Emerg Infect Dis. 2011;17:1325–7. doi: 10.3201/eid1707.101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs I, Tarabin S, Kafka M. Relapsing fever: Diagnosis thanks to a vigilant hematology laboratory. Vector Borne Zoonotic Dis. 2015;15:446–8. [DOI] [PubMed]
- 31.Hasin T, Davidovitch N, Cohen R, Dagan T, Romem A, Orr N, et al. Postexposure treatment with doxycycline for the prevention of tick-borne relapsing fever. N Engl J Med. 2006;355:148–55. [DOI] [PubMed]
- 32.Moran-Gilad J, Levine H, Schwartz E, Bartal C, Huerta-Hartal M, Schwaber MJ, et al. Postexposure prophylaxis of tick-borne relapsing fever: lessons learned from recent outbreaks in Israel. Vector Borne Zoonotic Dis. 2013;13:791–7. [DOI] [PubMed]
- 33.Solano-Gallego L, Baneth G. Babesiosis in dogs and cats-expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60. [DOI] [PubMed]
- 34.Burgdorfer WM, Varma GR. Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol. 1996;12:347–76. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzer S, Overzier E, Hermanns W, Baneth G, Straubinger RK. Borrelia persica infection in immunocompetent mice - A new tool to study the infection kinetics in vivo. PLoS Negl Trop Dis. 2016;10(2):e0004404. [DOI] [PMC free article] [PubMed]


