Abstract
Background
In Southeast Asia, the canine vector-borne pathogens Babesia spp., Ehrlichia canis, Anaplasma platys, Hepatozoon canis, haemotropic mycoplasmas and Dirofilaria immitis cause significant morbidity and mortality in dogs. Moreover, dogs have also been implicated as natural reservoirs for Rickettsia felis, the agent of flea-borne spotted fever, increasingly implicated as a cause of undifferentiated fever in humans in Southeast Asia. The objective of this study was to determine the prevalence and diversity of canine vector-borne pathogens in 101 semi-domesticated dogs from rural Cambodia using molecular diagnostic techniques.
Results
The most common canine vector-borne pathogens found infecting dogs in this study were Babesia vogeli (32.7 %) followed by Ehrlichia canis (21.8 %), Dirofilaria immitis (15.8 %), Hepatozoon canis (10.9 %), Mycoplasma haemocanis (9.9 %) and “Candidatus Mycoplasma haematoparvum” (2.9 %). A high level of co-infection with CVBD agents (23.8 %) was present, most commonly B. vogeli and E. canis. Naturally occurring R. felis infection was also detected in 10.9 % of dogs in support of their role as a natural mammalian reservoir for flea-borne spotted fever in humans.
Conclusions
This study reports for the first time, the prevalence and diversity of CVBD pathogens in dogs in Cambodia. In total, five species of CVBD pathogens were found infecting semi-domesticated dogs and many were co-infected with two or more pathogens. This study supports the role of dogs as natural mammalian reservoirs for R. felis, the agent of flea-borne spotted fever in humans.
Keywords: Canine, Vector-borne, Dogs, Cambodia, Babesia, Ehrlichia, Dirofilaria, PCR, Mycoplasma, Hepatozoon
Background
In Southeast Asia, canine vector-borne diseases (CVBD) are a significant cause of morbidity and mortality in dogs. Dogs infected with vector-borne pathogens may develop either a sub-clinical or clinical infection, which may lead to fatal outcomes in some cases. In addition, dogs have also been implicated as natural mammalian reservoirs for R. felis, the agent of cat-flea-typhus or flea-borne spotted fever (FSF) in humans [1]. Despite the growing number of studies on the prevalence and distribution of CVBD in Asia, there is a paucity of information available on these agents in Cambodia.
In Southeast Asia, canine babesiosis caused by the piroplasms Babesia vogeli and Babesia gibsoni are vectored by Rhipicephalus sanguineus and Haemaphysalis longicornis, respectively. The latter may also be transmitted by blood exchange in fighting dogs [2] and vertically [3]. Ehrlichia is an alpha-proteobacterium genus belonging to the family Anaplasmataceae. Canine monocytic ehrlichiosis or tropical canine pancytopenia caused by Ehrlichia canis and vectored by R. sanguineus is widely distributed throughout Southeast Asia [4–6] and an important cause of mortality in dogs. Canine hepatozoonosis is a mild tick-borne protozoan disease caused by the apicomplexan parasite Hepatozoon canis in Asia. Transmission of H. canis to dogs occurs by ingestion of an infected R. sanguineus tick, rather than a tick bite [7]. Co-infection of H. canis with other infectious agents such as Ehrlichia and parvovirus is common [8, 9] and may contribute to or exacerbate clinical disease. Three species, Mycoplasma haemocanis, “Candidatus Mycoplasma haematoparvum” and “Candidatus Mycoplasma haemobos” have been reported in dogs and the clinical effects of these pathogens varies from asymptomatic to the stimulation of a hemolytic syndrome [10]. In Southeast Asia evidence to support the importance of Anaplasma platys as a cause of canine infectious cyclic thrombocytopenia is lacking, owing primarily to a paucity of published data [11, 12]. Single infections with A. platys are generally clinically unapparent but pathogenicity appears to be increased in co-infections [10]. Canine dirofilariosis is a mosquito-borne disease caused by Dirofilaria immitis. It is a chronic, progressive and potentially lethal disease whose primary lesions occur in the pulmonary arteries and lung parenchyma, leading to congestive heart failure [13].
Rickettsia felis is a globally emerging zoonotic pathogen responsible for flea-borne spotted fever (FSF) in humans. Worldwide, Ctenocephalides felis and in India, Ctenocephalides felis orientis have been implicated the most likely biological vectors for R. felis Cal2 and R. felis-like species RF2125, respectively. The agent is being increasingly implicated as an important cause non-specific febrile illness in humans in Laos [14], Bangladesh [15], Kenya [16] and Senegal [17]. In addition it may lead to severe multi-systemic disease owing to disseminated vasculitis [18–20]. Dogs [1, 21] and more recently cats [22] have been implicated as natural mammalian reservoirs for infection based on the detection of circulating R. felis DNA in peripheral blood.
The aim of this study was to investigate the occurrence of the primary CVBD of veterinary and public health importance in Cambodia using molecular diagnostic techniques.
Methods
Sampling and DNA extraction
Blood samples were collected into sodium citrate blood tubes from 101 semi-domesticated, free-roaming dogs owned by residents belonging to 37 households in Dong Village, Rovieng district, Preah Vihear province, Cambodia (Fig. 1). Dogs in these communities lacked access to veterinary care, including vaccination, sterilisation, deworming and ectoparasite treatment. All blood samples were subjected to proteinase digestion in the presence of sodium dodecyl sulphate (SDS) followed by phenol-chloroform extraction of proteins and ethanol/salt precipitation of DNA as described elsewhere [23]. Final elution of DNA was made in 100 μl of TE buffer (10 mMTris-Cl, 0.5 mM EDTA, pH 9.0). The extracted DNA was stored at -20 °C until required for PCR amplification.
Fig 1.
Map of Cambodia with Rovieng District, Preah Vihear Province highlighted in red. (Google Earth)
PCR analysis and sequencing
All extracted DNA were subjected to previously described PCR assays for the detection of CVBD and spotted fever rickettsiae, as shown in Table 1. Samples positive for R. felis on the ompB gene were also subjected to a species-specific nested PCR targeting a 654 bp region of the gltA gene (Table 1). Genomic DNA of following organisms were used as positive controls for each PCR assay: B. vogeli, A. platys, R. felis, M. haemocanis, H. canis, D. immitis and E. canis. Nuclease-free water was used as a negative control. All the positive PCR products were purified using PureLink quick PCR purification kit (Invitrogen, Eugene, USA) according to the manufacturer’s protocol and submitted to the University of Queensland Animal Genetic Laboratory for DNA sequencing. All sequences were viewed using Finch TV 1.4.0 (Geospiza Inc.) and compared with known sequences from the GenBank™ database using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1.
Oligonucleotide primers used to detect vector borne protozoan and rickettsial pathogens
| Pathogen | Specific | Gene target (bp) | Reference |
|---|---|---|---|
| Babesia spp. | Genus | 18S rRNA (422–440) | [44] |
| Hepatozoon spp. | Genus | 18S r RNA (666) | [45] |
| Mycoplasma spp. | Genus | 16S rRNA (595) | [46] |
| Ehrlichia spp. | Genus | 16S rRNA (345) | [47] |
| A. platys | Species | 16S r RNA (678) | [47] |
| SFG rickettsiae | Group | ompB (252) | [48] |
| R. felis | Species | gltA (654) | [21] |
| D. immitis | Genus | 5.8S-ITS2-28S (430–664) | [49] |
Phylogenetic analysis
DNA sequences of the partial gltA gene of samples positive for R. felis were viewed using Finch TV 1.4.0 (Geospiza, Inc.) and aligned using BioEdit version 7.2.0 [24] together with the gltA gene of the following rickettsiae species: R. felis-like species RF2125, R. felis Cal 2 strain, R. australis, R. typhi, R. prowazekii, R. asiatica, R. conorii, R. rickettsia, R. honei, R. marmionii (GenBank accession nos. AF516333, CP000053, U59718, U59714, M17149, AB297812, HM050292, DQ150689, AF018074, and AY737684, respectively). Neighbor-joining analyses were conducted with Tamura-Nei parameter distance estimates, and trees were constructed using Mega 6 software (www.megasoftware.net). Bootstrap analyses were conducted using 1000 replicates.
Statistical analysis
Statistical analysis was conducted using STATA version 12.1 [25]. The association between CVBD agent positivity and the age and sex of dogs were evaluated using X2 test. Statistical significant was consider at P-value <0.05 with 95 % confidence interval (CI).
Animal ethics
Written informed consent was obtained from each dog owner prior to sample collection. This study adhered to strict guidelines outlined by the European Convention for the “Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes”. In addition, permission was gained from the Ministry of Agriculture, Forestry and Fisheries, Cambodia who were present for sampling and personally oversaw that animals were handled with respect according to the laws on experimental animal care in Cambodia.
Results
Detection of canine vector-borne pathogens
Of 101 dogs sampled, 43 were male and 58 female. The sampled group consisted of 43 juveniles (12 weeks – 1 year), 56 adults (> 1–7 years) and 2 geriatric (> 7 years) dogs. Although a detailed physical examination was not conducted, all dogs were of mixed local breed and appeared bright and alert. In total, 72 dogs (71.3 %) were positive for at least one CVBD pathogen. DNA of B. vogeli, E. canis, D. immitis, H. canis, M. haemocanis and “Candidatus M. haematoparvum” was detected in 32.7 % (33/101), 21.8 % (22/101), 15.8 % (16/101), 10.9 % (11/101), 9.9 % (10/101) and 2.9 % (3/101) dogs, respectively (Table 2). Multiple infections were detected in 20 (19.8 %) of these infected dogs (Table 2). Additionally, one dog (0.9 %) was co-infected with four vector-borne pathogens in this study. In total, 10.9 % (11/101) dogs were found positive for R. felis. In three households, more than one dog was found harboring R. felis (Fig. 1).
Table 2.
Prevalence of canine vector borne pathogens detected by PCR
| Pathogen | Dogs (n = 101) |
|---|---|
| Number positive (%) | |
| Babesia vogeli (total) | 33 (32.7) |
| Ehrlichia canis (total) | 22 (21.8) |
| Hepatozoon canis (total) | 11 (10.9) |
| Anaplasma platys (total) | 0 (0.0) |
| Mycoplasma haemocanis (total) | 10 (9.9) |
| “Candidatus Mycoplasma haematoparvum” (total) | 3 (2.9) |
| Dirofilaria immitis (total) | 16 (15.8) |
| Rickettsia felis (total) | 11 (10.9) |
| B. vogeli and M. haemocanis | 1 (0.9) |
| B. vogeli and “Candidatus Mycoplasma haematoparvum” | 1 (0.9) |
| B. vogeli and H. canis | 1 (0.9) |
| B. vogeli and E. canis | 5 (4.9) |
| B. vogeli and R. felis | 1 (0.9) |
| B. vogeli and D. immitis | 1 (0.9) |
| E. canis and R. felis | 2 (1.9) |
| E. canis and M. haemocanis | 1 (0.9) |
| H. canis and D. immitis | 1 (0.9) |
| R. felis and D. immitis | 1 (0.9) |
| B. vogeli, M. haemocanis and D. immitis | 1 (0.9) |
| B. vogeli, E. canis and H. canis | 1 (0.9) |
| B. vogeli, E. canis and R. felis | 2 (1.9) |
| B. vogeli, E. canis and M. haemocanis | 1 (0.9) |
| B. vogeli, E. canis and D. immitis | 2 (1.9) |
| B. vogeli, H. canis and D. immitis | 1 (0.9) |
| B. vogeli, H. canis, M. haemocanis and D. immitis | 1 (0.9) |
| Total infected dogs | 72 (71.3) |
| Total co-infected dogs | 24 (23.8) |
Phylogenetic analysis
DNA sequences of all positive PCR products were > 99–100 % homologous to those available in the GenBank™ database for all CVBD pathogens identified. Subsequent R. felis-specific nested PCR was successful for all eleven SFG-positive dogs. Resulting DNA sequences were > 99 % homologous to the R. felis Cal 2 strain on the gltA gene (GenBank: CP000053) (Fig. 2) and all eleven isolates clustered with R. felis Cal2 strain (99 % bootstrap support).
Fig. 2.
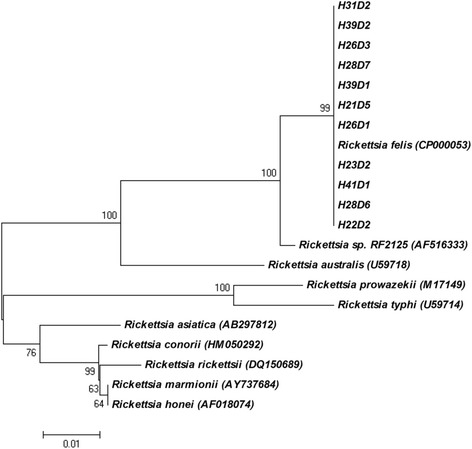
Phylogenetic tree obtained from neighbor-joining analysis of the gltA gene amplicons (600 bp) of Rickettsia felis detected in eleven dogs clustered with R. felis Cal 2 strain (CP000053)
Statistical analysis
There were no significant associations between the dogs’ sex and age and the presence of CVBD infections in this study.
Discussion
This study represents the first report of CVBD infections in dogs from Cambodia. Our results indicate that CVBD pathogens B. vogeli, E. canis, D. immitis, H. canis, M. haemocanis and “Candidatus M. haematoparvum” are highly endemic in this area of Cambodia. Concurrent infection with two or more CVBD pathogens (19.8 %) was common among dogs and B. vogeli and E. canis constituted the most common dual infections. Co-infection with two or more CVBD pathogens is known to be associated with greater pathogenicity. Co-infection in dogs infected with tick-borne pathogens including E. canis, B. vogeli, A. platys and H. canis have been associated with a greater degree of anemia for example, than single infections [26]. This consequence has important implications on the health of dogs in these communities, although this clinical aspect was not further explored in this study. Our results also highlight the importance of screening for more than one pathogen in veterinary practice in Cambodia. Although not quantified, a high burden of ticks and flea infestation was observed on dogs during blood collection.
The absence of A. platys in this study is surprising. A. platys shares a common vector with all reported CVBD pathogens in this study and has been reported infecting 4/30 refuge dogs in nearby Malaysia and in 2.3 % of ticks in neighboring Thailand [11]. Babesia gibsoni has also been reported in dogs in nearby Malaysia at a rate of 17.1 % [27]. Numerous studies in neighboring Thailand, however, have not detected the pathogen in either ticks or dogs to date. In Thailand, this may be due to the reported absence to date of the tick vector H. longicornis [28, 29].
Canine heartworm or D. immitis is highly endemic in Southeast Asia with a reported prevalence ranging between 18.2 and 25.8 % in stray and client-owned dogs residing in Thailand, South Korea, China, Taiwan and Malaysia [30–34]. PCR assays can only detect DNA of circulating microfilaria in blood [35]. Therefore, the reported prevalence of heartworm infection in these community dogs of 15.8 % may be a gross underestimation. In heartworm endemic regions of India [36] and Australia [37], occult infections that have reported to comprise up to 30 % of infections and therefore future use of antigen-based tests would be of great advantage in gaining a more accurate estimate of infection levels in this community.
Nearly 11 % of dogs sampled in this study were positive for R. felis, adding evidence to suggest that dogs are the primary mammalian reservoir hosts for this emerging rickettsial zoonosis. The strain of R. felis detected in this study was 100 % homologous with the R. felis Cal 2 strain, hypothesized to have a co-evolutionary adaptive relationship with C. felis felis [38, 39]. In India and neighboring Thailand and Myanmar, Rickettsia felis-like species/strains, R. felis-like species RF2125 and R. felis-like species RF31 were detected in C. felis orientis, the most common flea species infesting dogs and cats in India and South East Asia [38, 40, 41]. Therefore, it is surprising that dogs in this Cambodian village harbor R. felis Cal 2 strain. Unfortunately, fleas were not sampled for species identification in this study, but future identification of fleas and the R. felis-like strains/species they harbor, will assist unravelling this hypothesis.
No significant risk factors were found associated with CVBD infections in this study. Of interest was that in three households, two of the resident dogs were found infected with R. felis, suggesting a household-level clustering effect. The finding of R. felis in this study suggests that dogs may act as a potential source of human rickettsial infection [42]. Scrub typhus and murine typhus have been reported in patients with unknown febrile illness in Cambodia [43]. However, since antibodies to murine typhus may cross-react with those of R. felis, it is plausible that some of these cases may be attributed to flea-borne spotted fever. Following the detection of R. felis in dogs in rural Cambodia, local health authorities should be made aware of the clinical consequences of FSF and include the agent as part of their routine diagnostic screening for illnesses ranging from undifferentiated febrile disease to those resembling spotted fevers/typhus.
Conclusion
This study reports for the first time the prevalence and diversity of CVBD pathogens in dogs in Cambodia. In total five species of CVBD pathogens were found infecting semi-domesticated dogs and many were co-infected with two or more pathogens. This study supports the role of dogs as natural mammalian reservoirs for R. felis, the agent of flea-borne spotted fever in humans.
Acknowledgements
This paper has been sponsored by Bayer Animal Health in the framework of the 11th CVBD World Forum Symposium. We would like to acknowledge staff of The National Center for Parasitology, Entomology and Malaria Control, Ms. Daream Sok from Department of Fisheries Post-Harvest Technologies and Quality Control (DFPTQ), Fisheries Administration, Cambodia, Ms. Supanun Boonchob and Ms. Pacharathorn Simking, staff of The Faculty of Veterinary Medicine, Kasetsart University, Thailand and all the participants for their assistance in sample collection from Dong village in Cambodia. This project was financially supported by Kasetsart University Research and Development Institute (KURDI).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TI participated in the design of the study, preformed the experiments, analyzed the results and wrote the manuscript. WC assisted with the field work and data collection. SFH and RJT assisted with study design, interpretation of the results and critically appraising and writing the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Hii SF, Kopp SR, Abdad MY, Thompson MF, O'Leary CA, Rees RL, Traub RJ. Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis. Vector-Borne Zoonotic Dis. 2011;11:1007–12. doi: 10.1089/vbz.2010.0270. [DOI] [PubMed] [Google Scholar]
- 2.Jefferies R, Ryan UM, Jardine J, Broughton DK, Robertson ID, Irwin PJ. Blood, bull terriers and babesiosis: further evidence for direct transmission of Babesia gibsoni in dogs. Aust Vet J. 2007;85:459–63. doi: 10.1111/j.1751-0813.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 3.Fukumoto S, Suzuki H, Igarashi I, Xuan X. Fatal experimental transplacental Babesia gibsoni infections in dogs. Int J Parasit. 2005;35:1031–5. doi: 10.1016/j.ijpara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Irwin PJ, Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004;20:27–34. doi: 10.1016/j.pt.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Nazari M, Lim SY, Watanabe M, Sharma RSK, Cheng NABY, Watanabe M. Molecular detection of Ehrlichia canis in dogs in Malaysia. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinyoowong D, Jittapalapong S, Suksawat F, Stich RW, Thamchaipenet A. Molecular characterization of Thai Ehrlichia canis and Anaplasma platys strains detected in dogs. Infect Genet Evol. 2008;8:433–8. doi: 10.1016/j.meegid.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Baneth G, Mathew JS, Shkap V, Macintire DK, Barta JR, Ewing SA. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003;19:27–31. doi: 10.1016/S1471-4922(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Baneth G, Aroch I, Presentey B. Hepatozoon canis infection in a litter of Dalmatian dogs. Vet Parasitol. 1997;70:201–6. doi: 10.1016/S0304-4017(96)01152-1. [DOI] [PubMed] [Google Scholar]
- 9.Baneth G, Harrus S, Gal A, Aroch I. Canine vector-borne co-infections: Ehrlichia canis and Hepatozoon canis in the same host monocytes. Vet Parasitol. 2015;208:30–4. doi: 10.1016/j.vetpar.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Hii S, Traub R, Thompson M, Henning J, O'Leary C, Burleigh A, McMahon S, Rees R, Kopp S. Canine tick-borne pathogens and associated risk factors in dogs presenting with and without clinical signs consistent with tick-borne diseases in northern Australia. Aust Vet J. 2015;93:58–66. doi: 10.1111/avj.12293. [DOI] [PubMed] [Google Scholar]
- 11.Foongladda S, Inthawong D, Kositanont U, Gaywee J. Rickettsia, Ehrlichia, Anaplasma, and Bartonella in ticks and fleas from dogs and cats in Bangkok. Vector Borne Zoonotic Dis. 2011;11:1335–41. doi: 10.1089/vbz.2010.0174. [DOI] [PubMed] [Google Scholar]
- 12.Mokhtar AS, Lim SF, Tay ST. Molecular detection of Anaplasma platys and Babesia gibsoni in dogs in Malaysia. Trop Biomed. 2013;30:345–8. [PubMed] [Google Scholar]
- 13.McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich S, Phommasone K, Anantatat T, Panyanivong P, Slesak G, Blacksell SD, Dubot-Peres A, Castonguay-Vanier J, Stenos J, Newton PN, Paris DH. Rickettsia felis infections and Comorbid conditions, Laos, 2003–2011. Emerg Infect Dis. 2014;20:1402–4. doi: 10.3201/eid2008.131308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdouse F, Hossain MA, Paul SK, Ahmed S, Mahmud MC, Ahmed R, Haque AK, Khan MN, Ghosh S, Urushibara N, Kobayashi N. Rickettsia felis infection among humans, Bangladesh, 2012–2013. Emerg Infect Dis. 2015;21:1483–5. doi: 10.3201/eid2108.150328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, Cleaveland S, Ng'ang'a Z, Junghae M, Breiman RF, Richards AL, Njenga MK. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. 2012;18:328–31. doi: 10.3201/eid1802.111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, Trape JF, Raoult D. Rickettsia felis-associated Uneruptive Fever, Senegal. Emerg Infect Dis. 2010;16:1140–2. doi: 10.3201/eid1607.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindblom A, Severinson K, Nilsson K. Rickettsia felis infection in Sweden: report of two cases with subacute meningitis and review of the literature. Scand J Infect Dis. 2010;42:906–9. doi: 10.3109/00365548.2010.508466. [DOI] [PubMed] [Google Scholar]
- 19.Williams M, Izzard L, Graves SR, Stenos J, Kelly JJ. First probable Australian cases of human infection with Rickettsia felis (cat-flea typhus) Med J Aust. 2011;194:41–3. doi: 10.5694/j.1326-5377.2011.tb04145.x. [DOI] [PubMed] [Google Scholar]
- 20.Zavala-Castro J, Zavala-Velazquez J, Walker D, Perez-Osorio J, Peniche-Lara G. Severe human infection with Rickettsia felis associated with hepatitis in Yucatan, Mexico. Int J Med Microbiol. 2009;299:529–33. doi: 10.1016/j.ijmm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hii SF, Kopp SR, Thompson MF, O'Leary CA, Rees RL, Traub RJ. Molecular evidence of Rickettsia felis infection in dogs from northern territory, Australia. Parasit Vectors. 2011;4:198. doi: 10.1186/1756-3305-4-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed R, Paul SK, Hossain MA, Ahmed S, Mahmud MC, Nasreen SA, Ferdouse F, Sharmi RH, Ahamed F, Ghosh S, Urushibara N, Aung MS, Kobayashi N. Molecular detection of Rickettsia felis in humans, cats, and cat fleas in Bangladesh, 2013–2014. Vector Borne Zoonotic Dis. 2016;16(5):356–8. doi: 10.1089/vbz.2015.1886. [DOI] [PubMed] [Google Scholar]
- 23.Bremer WG, Schaefer JJ, Wagner ER, Ewing SA, Rikihisa Y, Needham GR, Jittapalapong S, Moore DL, Stich RW. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95–105. doi: 10.1016/j.vetpar.2005.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids SympSer. 1999;41:95–8. [Google Scholar]
- 25.StataCorp . Stata statistical software: release 12. College Station: StataCorp LP; 2011. [Google Scholar]
- 26.Rojas A, Rojas D, Montenegro V, Gutiérrez R, Yasur-Landau D, Baneth G. Vector-borne pathogens in dogs from Costa Rica: first molecular description of Babesia vogeli and Hepatozoon canis infections with a high prevalence of monocytic ehrlichiosis and the manifestations of co-infection. Vet Parasitol. 2014;199:121–8. doi: 10.1016/j.vetpar.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Rajamanickam C, Wiesenhutter E, Zin FM, Hamid J. The incidence of canine haematozoa in Peninsular Malaysia. Vet Parasitol. 1985;17:151–7. doi: 10.1016/0304-4017(85)90101-3. [DOI] [PubMed] [Google Scholar]
- 28.Parola P, Sanogo OY, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez JP, Miller RS, Telford SR, 3rd, Wongsrichanalai C, Raoult D. Identification of Rickettsia spp. and Bartonella spp. in fleas from the Thai-Myanmar border. Ann N Y AcadSci. 2003;990:173–81. doi: 10.1111/j.1749-6632.2003.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 29.Tanskull P, Inlao I. Keys to the adult ticks of Haemaphysalis Koch, 1844, in Thailand with notes on changes in taxonomy (Acari: Ixodoidea: Ixodidae) J Med Entomol. 1989;26:573–601. doi: 10.1093/jmedent/26.6.573. [DOI] [PubMed] [Google Scholar]
- 30.Boonyapakorn C, Srikitjakarn L, Morakote N, Hoerchner F. The epidemiology of Dirofilaria immitis infection in outpatient dogs at Chiang Mai University Small Animal Hospital, Thailand. Southeast Asian J Trop Med Public Health. 2008;39:33–8. [PubMed] [Google Scholar]
- 31.Song KH, Park JE, Lee DH, Lee SH, Shin HJ. Serological update and molecular characterization of Dirofilaria immitis in dogs. South Korea Res Vet Sci. 2010;88:467–9. doi: 10.1016/j.rvsc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Hou H, Shen G, Wu W, Gong P, Liu Q, You J, Cai Y, Li J, Zhang X. Prevalence of Dirofilaria immitis infection in dogs from Dandong, China. Vet Parasitol. 2011;183:189–93. doi: 10.1016/j.vetpar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Wu CC, Fan PC. Prevalence of canine dirofilariasis in Taiwan. J Helminthol. 2003;77:83–8. doi: 10.1079/JOH2002150. [DOI] [PubMed] [Google Scholar]
- 34.Retnasabapathy A, San KT. Incidence of canine heartworm (Dirofilaria immitis) in Malaysia. Vet Rec. 1976;98:68–9. doi: 10.1136/vr.98.4.68. [DOI] [PubMed] [Google Scholar]
- 35.Duscher G, Peschke R, Wille-Piazzai W, Joachim A. Parasites on paper--The use of FTA Elute (R) for the detection of Dirofilaria repens microfilariae in canine blood. Vet Parasitol. 2009;161:349–51. doi: 10.1016/j.vetpar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Borthakur SK, Deka DK, Islam S, Sarma DK, Sarmah PC. Prevalence and molecular epidemiological data on Dirofilaria immitis in dogs from Northeastern States of India. Sci World J. 2015;2015:265385. doi: 10.1155/2015/265385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atwell R. Heartworm disease and research in Australia. Br Vet J. 1989;145:301. doi: 10.1016/0007-1935(89)90026-2. [DOI] [PubMed] [Google Scholar]
- 38.Hii SF, Lawrence AL, Cuttell L, Tynas R, Abd Rani PAM, Slapeta J, Traub RJ. Evidence for a specific host-endosymbiont relationship between 'Rickettsia sp genotype RF2125' and Ctenocephalides felis orientis infesting dogs in India. Parasit Vectors. 2015;8:169. doi: 10.1186/s13071-015-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence AL, Hii SF, Jirsova D, Panakova L, Ionica AM, Gilchrist K, Modry D, Mihalca AD, Webb CE, Traub RJ, Slapeta J. Integrated morphological and molecular identification of cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) vectoring Rickettsia felis in central Europe. Vet Parasitol. 2015;210:215–23. doi: 10.1016/j.vetpar.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Changbunjong T, Buddhirongawatr R, Suwanpakdee S, Siengsanan J, Yongyuttawichai P, Cheewajorn K, Jangjaras J, Sangloung C, Ratanakorn P. A survey of ectoparasitic arthropods on domestic animals in Tak province, Thailand. Southeast Asian J Trop Med Public Health. 2009;40:435–42. [PubMed] [Google Scholar]
- 41.Kernif T, Socolovschi C, Wells K, Lakim MB, Inthalad S, Slesak G, Boudebouch N, Beaucournu JC, Newton PN, Raoult D, Parola P. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp Immunol Microb. 2012;35:51–7. doi: 10.1016/j.cimid.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hii SF, Abdad MY, Kopp SR, Stenos J, Rees RL, Traub RJ. Seroprevalence and risk factors for Rickettsia felis exposure in dogs from Southeast Queensland and the Northern Territory, Australia. Parasit Vectors. 2013;6:159. doi: 10.1186/1756-3305-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in South-Central Cambodia. Am J Trop Med Hyg. 2012;86:246–53. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Földvári G, Hell E, Farkas R. Babesia canis canis in dogs from Hungary: detection by PCR and sequencing. Vet Parasitol. 2005;127:221–6. doi: 10.1016/j.vetpar.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Inokuma H, Okuda M, Ohno K, Shimoda K, Onishi T. Analysis of the 18S rRNA gene sequence of a Hepatozoon detected in two Japanese dogs. Vet Parasitol. 2002;106:265–71. doi: 10.1016/S0304-4017(02)00065-1. [DOI] [PubMed] [Google Scholar]
- 46.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. 2003;93:307–17. doi: 10.1016/S0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 47.Brown GK, Martin AR, Roberts TK, Aitken RJ. Detection of Ehrlichia platys in dogs in Australia. Aust Vet J. 2001;79:554–8. doi: 10.1111/j.1751-0813.2001.tb10747.x. [DOI] [PubMed] [Google Scholar]
- 48.Paris DH, Blacksell SD, Stenos J, Graves SR, Unsworth NB, Phetsouvanh R, Newton PN, Day NP. Real-time multiplex PCR assay for detection and differentiation of rickettsiae and orientiae. Trans R Soc Trop Med Hyg. 2008;102:186–93. doi: 10.1016/j.trstmh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. 2006;135:303–14. doi: 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]


