Abstract
Background
The aim was to develop and validate the quality of life scale for nasopharyngeal carcinoma (NPC) patients, the QOL-NPC (version 2), a specific instrument to measure quality of life for NPC patients.
Methods
The QOL-NPC was developed and validated according to standard procedures. The patients were assessed using the QOL-NPC, FACT-G, and FACT-H&N. Classical test theory was used to evaluate the reliability, validity, and responsiveness of the QOL-NPC.
Results
A total of 487 patients (97.4 %) completed the questionnaire. The QOL-NPC comprised four domains, as follows: physical function (eight items); psychological function (five items); social function (five items); and side effects (eight items). All of the items had a lower proportion of missing data. Cronbach's alpha values of the domains ranged from 0.72 to 0.84. The split-half reliability coefficients ranged from 0.77 to 0.84. All of the intra-class correlation coefficients were > 0.8. The normed fit index, non-normed fit index, and comparative fit index were >0.89. The root mean square error of approximation was 0.097, with a 90 % confidence interval (0.093, 0.100). The domain scores of the QOL-NPC were significantly correlated with the FACT-G and FACT-H&N (P < 0.05). All of the domain scores of patients using different amounts of radiotherapy were significantly different (P < 0.001). All domain scores decreased at the completion of radiotherapy, with effect sizes ranging from −0.82 to −0.22.
Conclusions
The QOL-NPC is valid for measuring QOL with good reliability, validity, and responsiveness. The QOL-NPC is recommended to measure the QOL for Chinese NPC patients.
Keywords: Health-related quality of life, QOL-NPC, Reliability, Validity, Responsiveness
Background
Nasopharyngeal carcinoma (NPC) is a malignancy with a high incidence in several geographic areas, especially southern China and Hong Kong [1, 2]. Because NPC is located in close proximity to the base of the skull and is sensitive to radiotherapy (RT), the primary treatment is RT alone or combined with chemotherapy [3, 4]. RT causes various side effects, such as xerostomia, dysphagia, and hearing loss. These side effects obviously have a serious impact on the health-related quality of life (QOL) in NPC patients. The QOL of the NPC patients has been widely studied with the following inventories: the European Organization for the Research and Treatment of Cancer Core QOL questionnaire (QLQ-C30) and head and neck module (QLQ-H&N35) [4, 5]; the MOS 36-item short-form health survey (SF-36) [6, 7]; The University of Washington Quality of Life [8]; the Functional Assessment of Cancer Therapy-General Scale (FACT-G) and head and neck module (FACT-H&N) [9, 10]; and the functional assessment of cancer therapy-nasopharyngeal (FACT-NP) [11].
The quality of life scale of nasopharyngeal carcinoma patients (QOL-NPC, version 1 [V1]) is an NPC-specific scale, which was used to assess the physical functioning and health status of the NPC people in the past 2 weeks [12–14]. The QOL-NPC was widely used to evaluate the QOL of Chinese NPC patients. Based on the application there were some problems. (1) The item of the QOL-NPC (V1) was rated on a 0–10 numeric visual analogue scale (VAS). Some patients reported that it was difficult to understand. For example, some patients with poorer reading skills were not able to distinguish score 5 from score 6. Most studies have reported that VAS and Likert responses have few differences in reliability and responsiveness, and are highly correlated [15–17]. Because the Likert responses are easier to administer, compute, and interpret for the patients, Likert responses are most often applied [15–18]. (2) Some items had problems. The previous patients reported that they were worried about the infection of the disease due to a lack of medical knowledge. Therefore, the item “worried about the infection of the disease” was applied in V1. Some important symptoms were missing in V1, such as pain in the throat and cough when swallowing food.
The purpose of this study was to develop and assess the QOL-NPC (version 2 [V2]) according to a set of standardized procedures of instrument development.
Methods
Development of the QOL-NPC
The standard development and validation procedures were followed to develop and validate the QOL-NPC [19–23]. The procedures are shown in Fig. 1, which included construct definition, item generation, language testing and content validity, pilot study, and validation study.
Fig. 1.
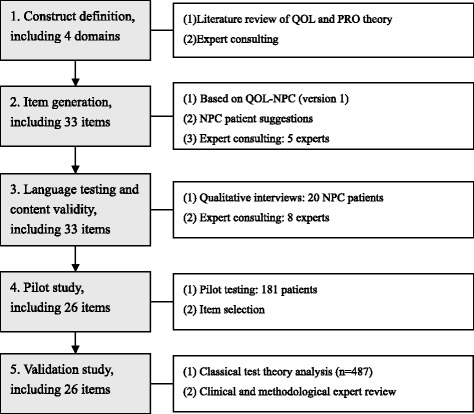
Steps towards development and validation procedure
Construct definition and item generation
The QOL-NPC (V1) contains 30 items in four domains: physical function (PH, seven items); psychological function (PS, six items); social function (SO, five items); and side effect (SE, 12 items).
The domains of the QOL-NPC (V2) were sourced from V1. The items of the V1 were carefully discussed and revised by five experts. For example, the item “worried about the infection of the disease” was revised to “worried about the inheritance of the disease.” According to suggestions from NPC patients and clinical professionals, the following three items were added: have a pain in your throat (PH domain); cough when swallowing food (PH domain); and feel difficult to communicate with your family and friends (SO domain). Finally, a total of 33 items were generated. The VAS scale of the item was revised into a 1–5 Likert scale. The 1–5 Likert scales were expressed as not at all (excellent), a little bit (very good), moderate (good), quite a bit (fair), and extreme (poor).
Language testing and content validity
All of the items were tested in a convenience sample of 20 NPC patients from different educational levels. The patients were asked whether or not they could understand the meaning of the items. Problematic items were revised according to the comments of the patients.
Eight experts were asked to assess the content validity. Expert consulting was available to evaluate whether or not the items of the QOL-NPC could represent the most relevant and important aspects of NPC patients [24]. Minor revisions and rewording of some items were performed until content validity was achieved.
Pilot testing
A cross-sectional study (pilot testing) was conducted to select the items. A total of 181 NPC patients were enrolled. The Research Ethics Committee of the Cancer Center at Sun Yat-Sen University provided ethical approval. The sample size was 5–10 times the item number for the pilot test. The items were screened and selected using the floor and ceiling method, coefficient of variation, correlation analysis, internal consistency coefficients, and confirmatory factor analysis. According to item selection, seven items were deleted, five of which were deleted from the SE domain. Due to the popularity of intensity-modulated radiotherapy (IMRT), the NPC patients had fewer side effects, such as dysphonia, alopecia (hair loss), dizziness, and decreased vision due to RT.
After the item selection, the QOL-NPC (V2) contained 26 items in four domains: PH (eight items); PS (five items); SO (five items); and SE domain (eight items). Each item scored 1 to 5 points. Each domain was transformed into a 0–100 score. A higher score indicated a better QOL. The scale was self-administered by the patients. The scale was showed in Appendix 1.
Validation study
A cross-sectional study (validation study) was conducted to assess the psychological characteristics of the QOL-NPC V2. The Research Ethics Committee of the Cancer Center at Sun Yat-Sen University provided ethical approval. The study was conducted between 1 July 2013 and 31 May 2014. Eligibility criteria included the following: (1) pathologically-proven NPC in the Cancer Center of Sun Yat-Sen University; (2) ≥16 years of age; and (3) able to provide informed consent to participate. The patients were excluded if diagnosed with another cancer, NPC relapse, or unconscious, confused, or cognitively impaired. The cognitively impaired were diagnosed by a psychologist.
The investigators included two medical post-graduates and three physicians, who were trained before the survey. The investigators explained the aim of our study before obtaining informed consent from the patients. If the patients agreed to participate in the survey, a questionnaire was given to them. The questionnaire included a socio-demographic sheet, QOL-NPC V2, FACT-G, and FACT-H&N. The socio-demographic sheet covered gender, educational degree, marriage status, dialect (Cantonese, Hakka, Chaoshanese, and others), pathologic type, Union for International Cancer Control (UICC) stage, methods of RT, RT stage, and other disease. The patients completed the questionnaire without assistance. If the patients did not understand the items on the questionnaire, the investigators explained them. If the questionnaire had missing data, the questionnaire was immediately returned to the patient for completion.
Terwee et al. considered a sample size of at least 50 patients to be adequate for the assessment of retest reliability and responsiveness [25]. Eighty inpatients were required to complete the QOL-NPC V2 within 2–3 days, which was used for the retest-test. A short interval (2–3 days) was chosen for the following reasons: (1) The NPC in-patients were all treated with RT, which had an obvious influence on QOL, especially for the long interval. (2) Marx et al. reported no significant differences for the test-retest reliability of 2 day and 2 week intervals [26]. The newly-diagnosed patients (60 patients), who had not been previously treated by RT, were required to finish the QOL-NPC after 50 ± 2 days of RT treatment. The data were used for the responsiveness test. These patients completed the scale by themselves in the retest and responsiveness tests. The investigators, the setting environment, and the investigation procedure were the same as the first test.
Data analysis
Classical test theory (CTT) was used to assess the scale. SPSS 21.0 (Chicago, IL, USA) and Lisrel software (version 8.7) were performed [27]. The percentage of missing data, and the time to complete the instrument was calculated. Internal consistency reliability and split-half reliability were assessed using Cronbach's alpha value and Pearson's correlation coefficients between two halves of the items, respectively. Test-retest reliability was evaluated using an intra-class correlation coefficient (ICC) and the 95 % confidence interval (CI) of the two scores within 2–3 days. The correlation coefficients of the item-own domain (the item and its own domain) and the item-other domains (the item and other domains) were calculated. Construct validity was evaluated by the normed fit index (NFI), non-normed fit index (NNFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA) based on confirmatory factor analysis (CFA) [28–30]. The correlation coefficients between the QOL-NPC and the FACT (FACT-G and FACT-H&N) were calculated to assess criterion validity. Discriminant validity was assessed by comparing the domain scores of the patients among different RT stages and different RT methods (analysis of variance). A paired samples t-test was used to analyze the score changes over time. Effect size was calculated as the change in scores divided by the standard deviation of the baseline score [31].
Results
A sample of 500 patients was enrolled in the study. Thirteen patients (2.6 %) did not complete the questionnaire. Thus, 487 patients were included for the analysis (Table 1). The mean age was 47.0 ± 11.1 years (range, 16.1–78.1 years). There were 341 male and 146 female patients. Of the patients, 93.0 % were married, 68.8 % were Cantonese, 88.9 % were the undifferentiated type, 46.6 % were III stage, and 79.9 % did not have another disease.
Table 1.
Characteristics of the patients
| Number (%, n = 487) | Number (%, n = 80)a | Number (%, n = 60)b | |
|---|---|---|---|
| Gender | |||
| Male | 341 (70.0) | 58 (72.5) | 43 (71.7) |
| Female | 146 (30.0) | 22 (27.5) | 17 (28.3) |
| Educational degree | |||
| ≤9 years | 272 (55.9) | 60 (75.0) | 45 (75.0) |
| ~12 years | 180 (37.0) | 14 (17.5) | 12 (20.0) |
| >12 years | 35 (7.2) | 6 (7.5) | 3 (5.0) |
| Marriage stage | |||
| Unmarried | 34 (7.0) | 12 (15.0) | 9 (15.0) |
| Married | 453 (93.0) | 68 (85.0) | 51 (85.0) |
| Dialect | |||
| Cantonese | 335 (68.8) | 53 (66.3) | 38 (63.3) |
| Hakka | 87 (17.9) | 14 (17.5) | 12 (20.0) |
| Chaoshanese | 48 (9.9) | 5 (6.3) | 5 (8.3) |
| Others | 17 (3.5) | 8 (10.0) | 5 (8.3) |
| Source of the patients | |||
| In-patients | 80 (16.4) | 80 (100.0) | 60 (100.0) |
| Out-patients | 407 (83.6) | 0 (0.0) | 0 (0.0) |
| Pathological type | |||
| Squamous cell | 22 (4.5) | 6 (7.5) | 5 (8.3) |
| Differentiation type | 32 (6.6) | 9 (11.3) | 5 (8.3) |
| Undifferentiated type | 433 (88.9) | 65 (81.3) | 50 (83.3) |
| UICC stage | |||
| I stage | 22 (4.5) | 0 (0.0) | 0 (0.0) |
| II stage | 78 (16.0) | 12 (15.0) | 4 (6.7) |
| III stage | 227 (46.6) | 36 (45.0) | 28 (46.7) |
| IV stage | 160 (32.9) | 32 (40.0) | 28 (46.7) |
| Radiotherapy | |||
| IMRT | 294 (60.4) | 72 (90.0) | 60 (100.0) |
| Three-dimensional RT | 47 (9.7) | 2 (2.5) | 0 (0.0) |
| Conventional RT | 86 (17.7) | 4 (5.0) | 0 (0.0) |
| No | 60 (12.3) | 2 (2.5) | 0 (0.0) |
| Other disease | |||
| Yes | 98 (20.1) | 60 (75.0) | 47 (78.3) |
| No | 389 (79.9) | 20 (25.0) | 13 (21.7) |
aThe patients were used for the retest test
bThe patients were used for the responsiveness test
The average time to complete the instrument was 8.4 ± 4.6 min, ranging from 3.8 to 16.3 min. Ten patients did not understand certain items, such as the item “mental stress”. They completed the items with the help of the investigators. The scores of all the items ranged from 1 to 5 (Table 2). Item SE8 scored the highest (3.90), while item PS2 scored the lowest (2.46). Item SE8 had 3.7 % missing data. Other items had a lower proportion of missing data.
Table 2.
Missing data, mean and SD for each item (n = 487)
| Score 1 | Score 2 | Score 3 | Score 4 | Score 5 | Missing (%) | Mean | SD | |
|---|---|---|---|---|---|---|---|---|
| PH1 | 13 | 92 | 195 | 170 | 13 | 4(0.8) | 3.16 | 0.86 |
| PH2 | 24 | 105 | 170 | 161 | 20 | 7(1.4) | 3.10 | 0.96 |
| PH3 | 28 | 184 | 191 | 60 | 10 | 14(2.9) | 2.66 | 0.85 |
| PH4 | 14 | 63 | 155 | 166 | 82 | 7(1.4) | 3.50 | 1.02 |
| PH5 | 13 | 40 | 96 | 209 | 125 | 4(0.8) | 3.81 | 1.00 |
| PH6 | 20 | 60 | 101 | 171 | 129 | 6(1.2) | 3.68 | 1.12 |
| PH7 | 14 | 56 | 83 | 213 | 117 | 4(0.8) | 3.75 | 1.04 |
| PH8 | 16 | 70 | 129 | 183 | 86 | 3(0.6) | 3.52 | 1.05 |
| PS1 | 5 | 30 | 146 | 236 | 63 | 7(1.4) | 3.67 | 0.82 |
| PS2 | 33 | 281 | 101 | 59 | 12 | 1(0.2) | 2.46 | 0.88 |
| PS3 | 48 | 235 | 64 | 113 | 23 | 4(0.8) | 2.64 | 1.09 |
| PS4 | 35 | 207 | 168 | 59 | 16 | 2(0.4) | 2.62 | 0.91 |
| PS5 | 10 | 26 | 116 | 293 | 39 | 3(0.6) | 3.67 | 0.78 |
| SO1 | 8 | 17 | 122 | 281 | 55 | 4(0.8) | 3.74 | 0.77 |
| SO2 | 5 | 40 | 86 | 261 | 92 | 3(0.6) | 3.82 | 0.87 |
| SO3 | 38 | 158 | 63 | 160 | 67 | 1(0.2) | 3.12 | 1.23 |
| SO4 | 34 | 217 | 95 | 107 | 31 | 3(0.6) | 2.76 | 1.07 |
| SO5 | 5 | 46 | 184 | 220 | 28 | 4(0.8) | 3.46 | 0.79 |
| SE1 | 32 | 117 | 182 | 136 | 17 | 3(0.6) | 2.98 | 0.96 |
| SE2 | 31 | 76 | 141 | 147 | 87 | 5(1.0) | 3.38 | 1.14 |
| SE3 | 11 | 41 | 122 | 205 | 98 | 10(2.1) | 3.71 | 0.96 |
| SE4 | 7 | 38 | 111 | 184 | 138 | 9(1.8) | 3.85 | 0.98 |
| SE5 | 11 | 62 | 105 | 192 | 105 | 12(2.5) | 3.67 | 1.03 |
| SE6 | 14 | 62 | 126 | 181 | 93 | 11(2.3) | 3.58 | 1.04 |
| SE7 | 9 | 55 | 138 | 168 | 102 | 15(3.1) | 3.63 | 1.01 |
| SE8 | 6 | 34 | 96 | 197 | 136 | 18(3.7) | 3.90 | 0.94 |
PHx the xth item of the PH domain, score 1 lowest QOL, score 5 highest QOL, SD standard deviation
The mean score of the SE domain was the maximum (64.5), and the mean score of the PS domain was the minimum (50.3; Table 3). The Cronbach's alpha value of the domain ranged from 0.72 to 0.84. The split-half coefficients of the domain ranged from 0.77 to 0.84. The SE domain had a maximum Cronbach's alpha value and split-half coefficient. All of the ICCs were >0.8, and of all the coefficients were significantly different.
Table 3.
Descriptive statistics and reliability of the QOL-NPC (n = 487)
| Domain | No. of items | Mean ± SD | Range of score | Cronbach’s alpha | Split-half coefficient | ICC (95 % CI) |
|---|---|---|---|---|---|---|
| PH | 8 | 60.0 ± 16.9 | (6.3, 100.0) | 0.80 | 0.83 | 0.87 (0.85, 0.89) |
| PS | 5 | 50.3 ± 15.5 | (0.0, 100.0) | 0.72 | 0.77 | 0.88 (0.86, 0.90) |
| SO | 5 | 59.4 ± 17.2 | (0.0, 100.0) | 0.76 | 0.81 | 0.82 (0.78, 0.86) |
| SE | 8 | 64.5 ± 17.6 | (3.1, 100.0) | 0.84 | 0.84 | 0.88 (0.84, 0.92) |
All items correlated more strongly with their own domain than the other domains (Table 4). For example, the correlation coefficients of the items in the PH domain and PH ranged from 0.47 to 0.77, which were greater than the other domains.
Table 4.
Items-domains correlation analysis of QOL-NPC (n = 487)
| Correlation with item | Factor loading of CFA | ||||
|---|---|---|---|---|---|
| PH | PS | SO | SE | ||
| PH1 | 0.54 | 0.31 | 0.27 | 0.29 | 0.67 |
| PH2 | 0.72 | 0.33 | 0.34 | 0.39 | 0.72 |
| PH3 | 0.64 | 0.30 | 0.34 | 0.39 | 0.66 |
| PH4 | 0.63 | 0.15 | 0.29 | 0.45 | 0.59 |
| PH5 | 0.48 | 0.09 | 0.23 | 0.29 | 0.51 |
| PH6 | 0.77 | 0.29 | 0.22 | 0.55 | 0.65 |
| PH7 | 0.47 | 0.22 | 0.19 | 0.32 | 0.51 |
| PH8 | 0.64 | 0.20 | 0.21 | 0.44 | 0.55 |
| PS1 | 0.36 | 0.61 | 0.42 | 0.35 | 0.53 |
| PS2 | 0.22 | 0.79 | 0.35 | 0.26 | 0.72 |
| PS3 | 0.17 | 0.74 | 0.25 | 0.20 | 0.57 |
| PS4 | 0.37 | 0.80 | 0.40 | 0.37 | 0.81 |
| PS5 | 0.22 | 0.53 | 0.27 | 0.16 | 0.54 |
| SO1 | 0.21 | 0.26 | 0.58 | 0.17 | 0.47 |
| SO2 | 0.26 | 0.24 | 0.61 | 0.31 | 0.47 |
| SO3 | 0.19 | 0.31 | 0.80 | 0.21 | 0.63 |
| SO4 | 0.38 | 0.45 | 0.78 | 0.28 | 0.74 |
| SO5 | 0.41 | 0.43 | 0.71 | 0.33 | 0.70 |
| SE1 | 0.52 | 0.32 | 0.22 | 0.65 | 0.63 |
| SE2 | 0.71 | 0.33 | 0.24 | 0.74 | 0.76 |
| SE3 | 0.46 | 0.26 | 0.24 | 0.71 | 0.66 |
| SE4 | 0.45 | 0.25 | 0.28 | 0.70 | 0.65 |
| SE5 | 0.31 | 0.16 | 0.21 | 0.69 | 0.56 |
| SE6 | 0.25 | 0.20 | 0.26 | 0.63 | 0.50 |
| SE7 | 0.47 | 0.35 | 0.34 | 0.73 | 0.66 |
| SE8 | 0.41 | 0.29 | 0.27 | 0.68 | 0.59 |
The results of the CFA analysis showed that the RMSEA was equal to 0.097 with a 90 % CI (0.093, 0.100). Both the NFI and NNFI were equal to 0.89. The CFI was equal to 0.90. The factor loadings of CFA are shown in Table 4. The minimum factor loading was 0.47 (SO1 and SO2). The structure diagram is shown in Fig. 2.
Fig. 2.
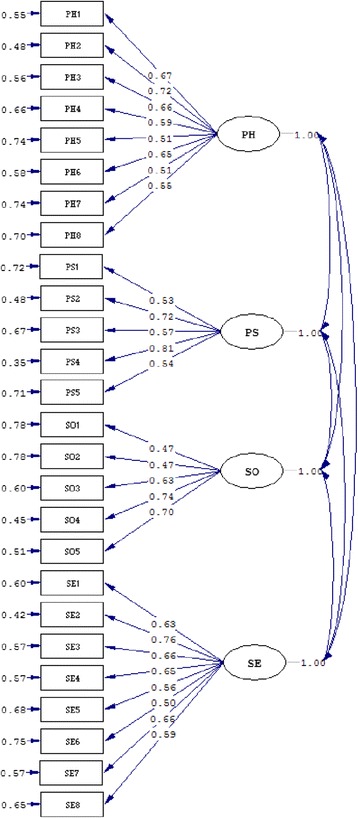
Results of CFA
The PH, PS, and SO domains of the QOL-NPC had a positive correlation with physical, emotional, and social/family well-being of the FACT-G with coefficients of 0.71, 0.63, and 0.56, respectively. The correlation coefficient between the SE domain of the QOL-NPC and FACT-H&N was 0.51, which was significantly different.
The PH, PS, and SE domain scores of patients in different RT stages were significantly different (P <0.001; Table 5). The patients who were receiving RT had the lowest scores in the PH, PS, and SE domains. The patients before RT and >5 years after RT had the highest scores. The SO domain scores of patients in different RT stages were not significantly different (P >0.05). All the domain scores of patients using different RT methods were significantly different (P <0.001; Table 5). The patients who did not receive RT had the highest scores, followed by those receiving intensity-modulated radiotherapy (IMRT).
Table 5.
The domain scores (mean ± SD) of patients among different RT stages and RT method
| n | PH | PS | SO | SE | |
|---|---|---|---|---|---|
| RT stages | |||||
| Before RT | 60 | 65.9 ± 15.5 | 54.5 ± 15.8 | 60.8 ± 17.6 | 71.6 ± 17.0 |
| During RT | 171 | 54.0 ± 14.9 | 47.4 ± 13.6 | 58.3 ± 15.7 | 59.2 ± 17.9 |
| ≤1 year after RT | 145 | 59.3 ± 14.2 | 48.5 ± 16.5 | 57.5 ± 15.6 | 62.6 ± 16.3 |
| ~5 years after RT | 65 | 63.5 ± 14.1 | 55.1 ± 16.3 | 63.8 ± 17.6 | 70.5 ± 15.9 |
| >5 years after RT | 46 | 72.3 ± 17.4 | 54.3 ± 14.7 | 61.3 ± 24.2 | 73.0 ± 15.4 |
| P-value | <0.001 | <0.001 | 0.100 | <0.001 | |
| RT method | |||||
| IMRT | 294 | 61.3 ± 15.7 | 51.5 ± 15.2 | 61.8 ± 16.4 | 66.8 ± 16.7 |
| Three-dimensional RT | 46 | 56.8 ± 13.2 | 44.5 ± 14.1 | 56.7 ± 17.1 | 60.6 ± 17.5 |
| Conventional RT | 86 | 53.4 ± 16.1 | 46.3 ± 15.6 | 51.8 ± 17.7 | 53.9 ± 16.6 |
| No RT | 60 | 65.9 ± 15.5 | 54.5 ± 15.8 | 60.8 ± 17.6 | 71.6 ± 17.0 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
RT radiotherapy, IMRT intensity-modulated radiotherapy
Sixty patients before RT were enrolled to test the responsiveness of the QOL-NPC over time. At the completion of RT, all domain scores decreased (Table 6). The score changes for all domains were significantly different, with effect sizes ranging from −0.82 (SE domain) to −0.22 (SO domain).
Table 6.
Change in scores and effect size from baseline to the end of radiotherapy (n = 60)
| Baseline score | Change scoresa | 95 % CI of change scores | Effect sizeb | |
|---|---|---|---|---|
| PH | 65.9 ± 15.5 | −4.9 ± 6.4 | (3.2, 6.5) | −0.31 |
| PS | 54.5 ± 15.8 | −3.8 ± 5.3 | (2.4, 5.2) | −0.24 |
| SO | 60.8 ± 17.6 | −3.9 ± 6.0 | (2.4, 5.5) | −0.22 |
| SE | 71.6 ± 17.0 | −5.9 ± 5.4 | (4.5, 7.3) | −0.82 |
Change scoresa, the score at the end of radiotherapy minus the baseline, −100 (maximum worsening) to +100 (maximum improvement)
Effect sizeb, calculated as the change in scores divided by the SD of the baseline score
Discussion
The most commonly specific instruments used to assess QOL of NPC patients include QLQ-C30, QLQ-H&N35 [4, 32, 33], and FACT-NP, which consists of FACT-G and NPC subscale [11]; however, due to cultural differences, we developed the QOL-NPC to assess the QOL of Chinese NPC patients.
The QOL-NPC (V2) had good content validity according to the suggestions of experts. The QOL-NPC was broadly defined as the endpoint directly derived from the patient, which included symptoms, health status, adherence, and side effect [34]. The QOL-NPC included the most important aspects characterizing specific aspects of NPC patients, which is structurally made up of physical function, psychological function, social function, and side effects. For example, physical function included feeling tired, losing weight, having a headache, nasal tampon or nasal bleeding, satisfied with appearance, and coughing when swallowing food. Side effects included dry mouth (xerostomia), pain in the throat, difficulty in opening the mouth, memory decline, skin injuries in the head and neck, and damaged teeth due to RT. It is well-accepted that dry mouth is the most significant morbidity during and following RT, which causes serious disorders in tasting, chewing, and swallowing, as well as sleeping disorders [35].
The QOL-NPC (V2) had good reliability and validity based on the results of CTT. All of the domains had moderate or high Cronbach's alpha coefficients (0.72–0.84), and split-half reliability coefficients (0.77–0.84). The researchers gave a positive rating for internal consistency when Cronbach's alpha was >0.70 [25]. All of the domains had high intra-class correlation coefficients (0.82–0.88), which indicated that the QOL-NPC (V2) can evaluate the QOL of patients. Based on the results of CFA, RMSEA was equal to 0.097 with a 90 % CI (0.093, 0.100); NFI, NNFI, and CFI approached 0.90. The factor loadings of CFA were >0.47. These results showed that the QOL-NPC had good construct validity. The corresponding domains of the QOL-NPC and the FACT-G and FACT-H&N were significantly related to each other. For example, the PH domain of the QOL-NPC had a significantly positive correlation with physical well-being of the FACT-G.
The PH, PS, and SE domain of the QOL-NPC were sensitive to discriminate the QOL of NPC patients in different RT stages. The QOL of the NPC patients during RT were the lowest. These results were consistent with our hypothesis. It is known that RT has a serious impact on the health status of the patients [4, 36]. All the domain scores of patients using different RT methods were significantly different. The patients who did not receive RT had the highest scores. The patients who receive IMRT had the higher scores than those receiving other RT methods. Our results were consistent with other studies, which showed that IMRT played a significant role in improving the QOL of NPC patients [37, 38].
The QOL-NPC (V2) had good responsiveness based on the results of CTT. After the newly diagnosed NPC patients received RT treatment, they had numerous side effects, especially head and neck symptoms, such as pain in the mouth and throat, dry mouth, and difficulties in speaking. Therefore, the domain scores of the QOL-NPC decreased. The effect sizes of these domains ranged from −0.82 to −0.22. The effect sizes of the SE domain were greater than the other domains. Similar decreases in QOL were observed in the Quality Of Life Radiation Therapy Instrument and the Head & Neck Module [39].
The QOL-NPC had good operability. Of the patients, 97.4 % completed the questionnaire. Most of the items had a lower proportion of missing data. The patients completed the QOL-NPC in an average time of 8.4 min.
There were some study limitations. (1) All of the patients in the study were enrolled from the Cancer Center of Sun Yat-Sen University. The QOL-NPC (V2) should be further evaluated by the data from other centers. (2) Only 60 NPC patients were used to test the responsiveness of the QOL-NPC (V2). The responsiveness of the scale should be further assessed in a larger sample of patients. (4) Some patients completed the QOL-NPC with the help of the investigators. It was a limitation of the study, for item explanation by a third party can generate application bias.
Conclusions
The QOL-NPC (V2) is valid for measuring QOL with good reliability, validity, and responsiveness. We recommend the application of the QOL-NPC (V2) for measuring QOL in the Chinese NPC patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81272575, 81403296), the Science and Technology Planning Project of Guangdong Province (2012B031800301), the Medical Scientific Research Foundation of Guangdong Province (B2013163), the Outstanding Youth Foundation of Guangdong Province colleges and universities (YQ2015041), the Young Talents Foundation of Guangzhou University of Chinese medicine (QNYC20140101), and the Torch Plan of Guangzhou University of Chinese Medicine (No: XH20140105). The authors thank all patients for their participation in the study and the hospital staff for assistance with the data collection.
Abbreviations
- CI
confidence interval
- CFA
confirmatory factor analysis
- CFI
comparative fit index
- CTT
classical test theory
- ICC
intra-class correlation coefficient
- NFI
normed fit index
- NNFI
non-normed fit index
- RMSEA
root mean square error of approximation
- FACT-G
Functional Assessment of Cancer Therapy-General Scale
- FACT- H&N
Functional Assessment of Cancer Therapy head and neck module
- FACT-NP
the functional assessment of cancer therapy-nasopharyngeal
- IMRT
intensity-modulated radiotherapy
- QOL-NPC
quality of life scale of nasopharyngeal carcinoma patients
- RT
radiotherapy
- NPC
nasopharyngeal carcinoma
- QOL
quality of life
- QLQ-C30
the research and treatment of cancer core QOL questionnaire
- QLQ-H&N35
the research and treatment of cancer questionnaire head and neck module
- PH
physical function
- PS
psychological function
- SO
social function
- SE
side effect
- UICC
Union for International Cancer Control
- VAS
visual analogue scale
- V1
version 1
- V2
version 2
Appendix 1
Quality of life scale of nasopharyngeal carcinoma patients: The QOL-NPC (version 2).
The Quality of life scale of nasopharyngeal carcinoma patients (version 2) includes 26 items, which is presented as a five-point scale. Please respond to each item according to your feeling in the past 2 weeks. The following items are about your physical function, psychological function and social function related to nasopharyngeal carcinoma.
| PH1. Sleep | poor | fair | good | very good | excellent |
| PH2. Appetite | poor | fair | good | very good | excellent |
| PH3. Satisfaction with appearance | not at all | a little bit | moderate | quite a bit | extreme |
| PH4. Weight loss | extreme | quite a bit | moderate | a little bit | not at all |
| PH5. Tiredness | extreme | quite a bit | moderate | a little bit | not at all |
| PH6. Headache | extreme | quite a bit | moderate | a little bit | not at all |
| PH7. Nasal tampon or nasal bleeding | extreme | quite a bit | moderate | a little bit | not at all |
| PH8. Coughing while swallowing | extreme | quite a bit | moderate | a little bit | not at all |
| PS1. Feeling lives to be meaningful | not at all | a little bit | moderate | quite a bit | extreme |
| PS2. Satisfaction with treatment effect | not at all | a little bit | moderate | quite a bit | extreme |
| PS3. Worries about impact of disease | extreme | quite a bit | moderate | a little bit | not at all |
| PS4. Worries about inheritance of disease | extreme | quite a bit | moderate | a little bit | not at all |
| PS5. Mental stress | extreme | quite a bit | moderate | a little bit | not at all |
| SO1. Satisfaction with interpersonal relations | not at all | a little bit | moderate | quite a bit | extreme |
| SO2. Satisfaction with ability of work and daily life | not at all | a little bit | moderate | quite a bit | extreme |
| SO3. Difficulty in communication | extreme | quite a bit | moderate | a little bit | not at all |
| SO4. Influence of role in family and work | extreme | quite a bit | moderate | a little bit | not at all |
| SO5. Influence of social activities | extreme | quite a bit | moderate | a little bit | not at all |
The following items were about the side effects due to radiotherapy.
| SE1. Dry mouth | extreme | quite a bit | moderate | a little bit | not at all |
| SE2. Throat pain | extreme | quite a bit | moderate | a little bit | not at all |
| SE3. Harmfulness in head-neck skin | extreme | quite a bit | moderate | a little bit | not at all |
| SE4. Difficulty in opening mouth | extreme | quite a bit | moderate | a little bit | not at all |
| SE5. Loosed and damaged teeth | extreme | quite a bit | moderate | a little bit | not at all |
| SE6. Hearing loss | extreme | quite a bit | moderate | a little bit | not at all |
| SE7. Radioactive rhinitis | extreme | quite a bit | moderate | a little bit | not at all |
| SE8. Memory loss | extreme | quite a bit | moderate | a little bit | not at all |
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YS was involved in the study design, construct definition, item generation and validation study; and drafted the manuscript. CWM was involved in language testing and content validity, and drafted the manuscript. WQC and LW collected the data and drafted the manuscript. QX analyzed the data and discussed the results. ZCW and ZLW interpreted the data. LZL was involved in the recruitment of patients. XLC was involved in the study design, pilot testing and validation study; and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. 2014;33(8):381–7. doi: 10.5732/cjc.014.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie SH, Yu IT, Tse LA, Mang OW, Yue L. Sex difference in the incidence of nasopharyngeal carcinoma in Hong Kong 1983–2008: suggestion of a potential protective role of oestrogen. Eur J Cancer. 2013;49(1):150–5. doi: 10.1016/j.ejca.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, Chan AT, Huang PY, Benhamou E, Zhu G, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 4.Bian X, Song T, Wu S. Outcomes of xerostomia-related quality of life for nasopharyngeal carcinoma treated by IMRT: based on the EORTC QLQ-C30 and H&N35 questionnaires. Expert Rev Anticancer Ther. 2015;15(1):109–19. doi: 10.1586/14737140.2015.961427. [DOI] [PubMed] [Google Scholar]
- 5.Tsai WL, Chien CY, Huang HY, Liao KC, Fang FM. Prognostic value of quality of life measured after treatment on subsequent survival in patients with nasopharyngeal carcinoma. Qual Life Res. 2013;22(4):715–23. doi: 10.1007/s11136-012-0213-8. [DOI] [PubMed] [Google Scholar]
- 6.Gu MF, Su Y, Chen XL, He WL, He ZY, Li JJ, Chen MQ, Mo CW, Xu Q, Diao YM. Quality of life and radiotherapy complications of Chinese nasopharyngeal carcinoma patients at different 3DCRT. Asian Pac J Cancer Prev. 2012;13(1):75–9. doi: 10.7314/APJCP.2012.13.1.075. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Hu WH, Xia YF, Ma J, Liu MZ, Cui NJ. Quality of life of nasopharyngeal carcinoma survivors in Mainland China. Qual Life Res. 2007;16(1):65–74. doi: 10.1007/s11136-006-9113-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen AM, Farwell DG, Luu Q, Vazquez EG, Lau DH, Purdy JA. Intensity-modulated radiotherapy is associated with improved global quality of life among long-term survivors of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):170–5. doi: 10.1016/j.ijrobp.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Yu CL, Fielding R, Chan CL, Sham JS. Chinese nasopharyngeal carcinoma patients treated with radiotherapy: association between satisfaction with information provided and quality of life. Cancer. 2001;92(8):2126–35. doi: 10.1002/cncr.1554. [DOI] [PubMed] [Google Scholar]
- 10.Teckle P, McTaggart-Cowan H, Van der Hoek K, Chia S, Melosky B, Gelmon K, Peacock S. Mapping the FACT-G cancer-specific quality of life instrument to the EQ-5D and SF-6D. Health Qual Life Outcomes. 2013;11:203. doi: 10.1186/1477-7525-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong MC, Lo PS, Wong KH, Yeung RM, van Hasselt CA, Eremenco S, Cella D. Development and validation of the functional assessment of cancer therapy nasopharyngeal cancer subscale. Head Neck. 2009;31(6):738–47. doi: 10.1002/hed.21023. [DOI] [PubMed] [Google Scholar]
- 12.Gu MF, Du YZ, Chen XL, Li JJ, Zhang HM, Tong Q. Item selection in the development of quality of life scale for nasopharyngeal carcinoma patients. Ai Zheng. 2009;28(1):82–5. [PubMed] [Google Scholar]
- 13.Chen XL, Gu MF, Du YZ, Li JJ, Zhang HM. Evaluating the validity and reliability of quality of life among nasopharyngeal carcinoma patients. Chin J Cancer Prev Treat. 2010;17(14):1045–8. [Google Scholar]
- 14.Chen XL, Gu MF, He WL, Liu H, Li JJ, Mo CW, Xu Q. Evaluation of quality of life for nasopharyngeal carcinoma patients using item response theory. Chin J Cancer Prev Treat. 2013;20(18):1380–4. [Google Scholar]
- 15.Bolognese JA, Schnitzer TJ, Ehrich EW. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthritis Cartilage. 2003;11(7):499–507. doi: 10.1016/S1063-4584(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 16.Celenza A, Rogers IR. Comparison of visual analogue and Likert scales in evaluation of an emergency department bedside teaching programme. Emerg Med Australas. 2011;23(1):68–75. doi: 10.1111/j.1742-6723.2010.01352.x. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis. 1987;40(12):1129–33. doi: 10.1016/0021-9681(87)90080-4. [DOI] [PubMed] [Google Scholar]
- 18.Harland NJ, Dawkin MJ, Martin D. Relative utility of a visual analogue scale vs. a six-point Likert scale in the measurement of global subject outcome in patients with low back pain receiving physiotherapy. Physiotherapy. 2015;101(1):50–4. doi: 10.1016/j.physio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 19.The WHOQOL Group The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403–9. doi: 10.1016/0277-9536(95)00112-K. [DOI] [PubMed] [Google Scholar]
- 20.Power MJ, Green AM, WHOQOL-Dis Group Development of the WHOQOL disabilities module. Qual Life Res. 2010;19(4):571–84. doi: 10.1007/s11136-010-9616-6. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprangers MA, Cull A, Groenvold M, Bjordal K, Blazeby J, Aaronson NK. The European Organization for Research and Treatment of Cancer approach to developing questionnaire modules: an update and overview. EORTC Quality of Life Study Group. Qual Life Res. 1998;7(4):291–300. doi: 10.1023/A:1008890401133. [DOI] [PubMed] [Google Scholar]
- 23.The WHOQOL Group The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–85. doi: 10.1016/S0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 24.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Snyder C, Boers M, Cella D. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21(5):739–46. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 25.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Marx RG, Menezes A, Horovitz L, Jones EC, Warren RF. A comparison of two time intervals for test-retest reliability of health status instruments. J Clin Epidemiol. 2003;56(8):730–5. doi: 10.1016/S0895-4356(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 27.Park Y-J, Ryu H, Han K, Kwon JH, Kim HK, Kang HC, Yoon J-W, Cheon S-H, Shin H. Suicidal ideation in adolescents: an explanatory model using LISREL. West J Nurs Res. 2010;32(2):168–84. doi: 10.1177/0193945909349115. [DOI] [PubMed] [Google Scholar]
- 28.Jöreskog KG, Goldberger AS. Estimation of a model with multiple indicators and multiple causes of a single latent variable. J Am Stat Assoc. 1975;70(351):631–9. doi: 10.2307/2285946. [DOI] [Google Scholar]
- 29.Thompson B, Daniel LG. Factor analytic evidence for the construct validity of scores: a historical overview and some guidelines. Educ Psychol Meas. 1996;56(2):197–208. doi: 10.1177/0013164496056002001. [DOI] [Google Scholar]
- 30.Lix LM, Osman BA, Adachi JD, Towheed T, Hopman W, Davison KS, Leslie WD. Measurement equivalence of the SF-36 in the canadian multicentre osteoporosis study. Health Qual Life Outcomes. 2012;10(1):29. doi: 10.1186/1477-7525-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005;58(12):1217–9. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JCJM. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 33.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, Biorklund A, de Leeuw JR, Fayers PM, Jannert M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17(3):1008–19. doi: 10.1200/JCO.1999.17.3.1008. [DOI] [PubMed] [Google Scholar]
- 34.Willke RJ, Burke LB, Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004;25(6):535–52. doi: 10.1016/j.cct.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Liu F, Lan X, Yu L, Wu W, Wu X, Xiao F, Li S. Clinical observation of submandibular gland transfer for the prevention of xerostomia after radiotherapy for nasopharyngeal carcinoma: a prospective randomized controlled study of 32 cases. Radiat Oncol. 2014;9:62. doi: 10.1186/1748-717X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alon EE, Lipschitz N, Bedrin L, Gluck I, Talmi Y, Wolf M, Yakirevitch A. Delayed Sino-nasal complications of radiotherapy for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2014;151(2):354–8. doi: 10.1177/0194599814530858. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Hu W, Wang W, Chen P, Ding W, Luo W. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(1):e47–54. doi: 10.1016/j.ijrobp.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 38.Jang-Chun L, Jing-Min H, Yee-Min J, Dai-Wei L, Chang-Ming C, Chun-Shu L, Wen-Yen H, Yu-Fu S, Kuen-Tze L, Chao-Yueh F, et al. Comparisons of quality of life for patients with nasopharyngeal carcinoma after treatment with different RT technologies. Acta Otorhinolaryngol Ital. 2014;34(4):241–6. [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XL, Qiu ZW, Gu MF, Su Y, Liu LZ, Liu Y, Mo CW, Xu Q, Sun J, Li DH. Translation and validation of the Chinese version of the Quality OF Life Radiation Therapy Instrument and the Head & Neck Module (QOL-RTI/H&N) Health Qual Life Outcomes. 2014;12:51. doi: 10.1186/1477-7525-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]


