Atherosclerosis is an inflammatory disease driven by hyperlipidemia.1 There is accumulating evidence that lipid metabolism and inflammation are closely linked.2 However, the crosstalk between these processes in the development and progression of atherosclerosis are not fully defined. Macrophages mediate tissue innate immune response and lipid metabolism, and therefore act as a key player at the crossroads of innate immunity and lipid homeostasis in atherosclerosis.1
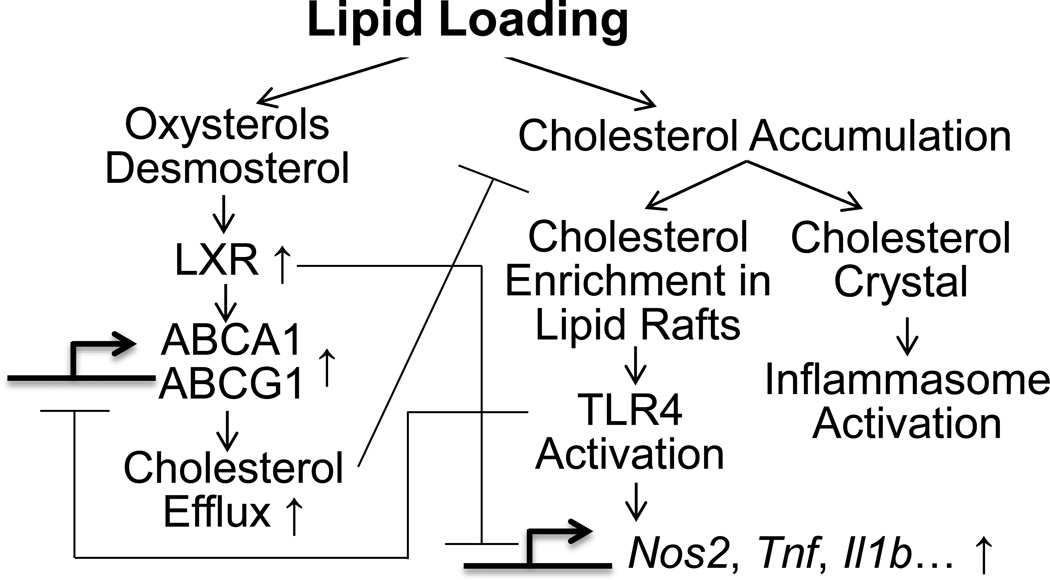
In lipid-loaded macrophages, cholesterol biosynthetic intermediates, such as desmosterol, and oxysterols derived from cholesterol, activate the transcription factor, liver X receptors (LXR). LXRs forms heterodimers with retinoid X receptors (RXRs) on the promoters of many genes involved in cholesterol metabolism, e.g., ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1), to upregulate their expression and enhance cholesterol efflux.3 When exceeding capacity, cholesterol begins to accumulate and macrophages become lipid-enriched. Cholesterol enrichment in the plasma membrane promotes the formation of toll-like receptor 4 (TLR4)-MD2 complexes,4 which enhance macrophage response to TLR4 ligands such as lipopolysaccharide (LPS). Excessive free cholesterol can trigger crystal formation and inflammasome activation, further exacerbates macrophage inflammation and cholesterol accumulation. Beyond promotion of cholesterol homeostasis, ligand-dependent conjugation of SUMO2/3 to LXRs targets them to promoters of TLR target genes, where they preserve NCoR corepressor complexes and thus maintain suppression of target gene transcription.5 Conversely, through activation of interferon-regulatory factor 3 (IRF3), TLR3 and TLR4 ligands inhibit the transcriptional activity of LXR on its target genes including ABCA1 and ABCG1, and disrupt lipid homeostasis.2, 3 Thus, crosstalk between inflammation and lipid metabolism in macrophages can synergistically exacerbate the pathological progress in atherosclerosis.3 Elucidation of the functional denominators linking macrophage activation and derailed cholesterol metabolism may facilitate the development of novel therapeutics in atherosclerosis. (Figure 1)
Figure 1. Schematic figure of crosstalk between cholesterol metabolism and inflammatory signaling in macrophages.
LXR, liver X receptor; ABCA1 and ABCG1, ATP-binding cassette transporters A1 and G1; TLR, toll like receptor.
In a recent issue of Circulation Research, Chen et al. report that interferon regulatory factor 2 binding protein 2 (IRF2BP2) is a novel regulator of macrophage inflammation and lipid homeostasis that acts by maintaining anti-inflammatory transcription factor Krüppel-like factor 2 (KLF2) expression, therefore reducing susceptibility to atherosclerosis.6 (Figure 2) IRF2BP1 and IRF2BP2 are transcriptional corepressors binding to the C-terminal repression domain of IRF2, a negative regulator of many interferon (IFN)-responsive genes.7 IRF2BP2 also represses the nuclear factor of activated T cells (NFAT1)-dependent transactivation of NFAT-responsive genes, such as IL-2 and IL-4.8 Human genome wide association studies (GWAS) identified genetic variants near IRF2BP2 associated with elevated plasma total cholesterol and LDL-C,9, 10 although no association with coronary heart diseases (CHD) has been revealed in current GWASs.9 Besides, in a classical in vitro model of macrophage polarization using human monocyte-derived macrophage, IRF2BP2 mRNA was massively suppressed by M1 polarization (LPS and IFNγ) and induced by M2 polarization (IL-4).11 These studies suggested the potential role of IRF2BP2 in macrophage polarization and lipid metabolism, but the mechanisms and impact on disease remained undetermined, prompting Chen et al. to pursue their novel studies.
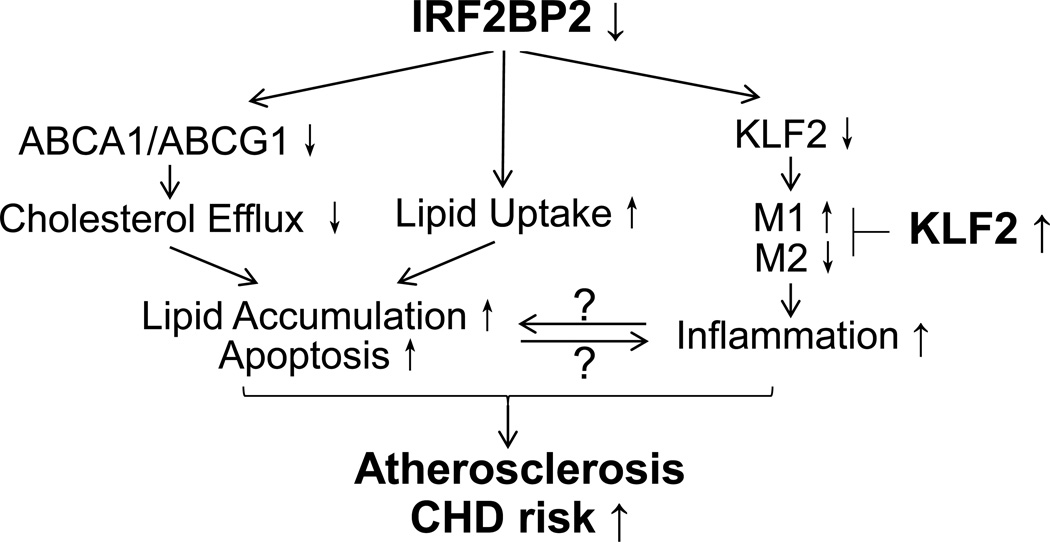
Figure 2. The role of IRF2BP2 in macrophage inflammation and atherosclerosis.
Macrophage deficiency in IRF2BP2 leads to increased lipid accumulation through increased lipid uptake and reduced efflux, accompanied by increased LPS-induced M1 activation and reduced IL-4 induced M2 activation. IRF2BP2 is required for the expression of the anti-inflammatory transcription factor KLF2. Restoring KLF2 in IRF2BP2 deficient macrophages attenuates inflammatory M1 activation and rescues anti-inflammatory M2 activation, and improves cholesterol handling. Ablation of IRF2BP2 in macrophages worsens atherosclerosis in mice and a deletion variant that lowers expression of IRF2BP2 and its target gene KLF2 predisposes to CHD in humans.
Chen et al.6 present a combination of cell and mouse studies, mechanistic molecular experiments, and human genetics that advance our understanding of IFR2BP2 in regulating inflammation and lipid metabolism in macrophages and modulating atherosclerosis in rodent models and humans. First, the authors described that IRF2BP2 regulates macrophage inflammation and lipid homeostasis in vitro. Bone marrow derived macrophages (BMDM) derived from myeloid-specific Irf2bp2 KO mice (LysMCre/−/Irf2bp2flox/flox, abbreviated as KO) showed higher inflammatory M1 markers (IL1b, Tnf, Ccl2 and Nos2) under basal condition and during stimulation, but reduced IL-4 induced M2 markers (Arginase1, Retnlb, Mgl1 and Mrc1). In wild type (WT, Irf2bp2flox/flox) BMDM, modified-LDL loading upregulated Abca1 and Abcg1 expression. The effects were markedly impaired in Irf2bp2 KO BMDM. This was accompanied by increased [3H]-cholesterol labeled acetylated-LDL uptake and reduced [3H]-cholesterol efflux to lipoprotein acceptors as well as increased apoptosis. As a result of increased uptake and reduced efflux, total cholesterol mass, including both free and esterified cholesterol was increased.
The authors then revealed that Irf2bp2-deficient macrophages exacerbated atherosclerosis in murine models.6 Western diet-fed Ldlr−/− recipients of KO bone marrow (DKO) showed increased lesion area, M1 macrophage infiltration and apoptosis, accompanied by increased CD68+ macrophage infiltration and ventricular hypertrophy, suggesting the profound inflammatory effects of Irf2bp2 deficiency in macrophages. The inflammatory phenotype of BMDM and more extensive atherosclerosis were also observed in Irf2bp2 KO in ApoE−/− background. The translational relevance was illustrated by the finding that a deletion variant that lowers IRF2BP2 expression predisposes to CHD in humans. A 9-nucleotide deletion at the 3’-UTR of IRF2BP2 was associated with increased CHD risk in a recessive model in a relatively small case-control study of angiographic coronary disease. Luciferase reporter assay indeed suggested that the deletion mutation leads to reduced luciferase translation. Importantly, carriers for homozygous deletion polymorphism showed reduced IRF2BP2 expression in peripheral blood mononuclear cells (PBMC).
The authors performed mRNA microarray to determine differentially expressed (DE) genes between WT and KO BMDM.6 One of the top DE genes suppressed in KO BMDM is Klf2, an anti-inflammaotry transcription factor. KLF2 inhibits the transcriptional activity of both NF-κB and activator protein 1, in part by means of recruitment of transcriptional coactivator p300/CBP-associated factor. Klf2 protein levels were barely detectable in atherosclerosis lesion of DKO mice. To establish the functional requirement of KLF2 in IRF2BP2-mediated effects, lentivirus mediated Klf2 overexpression in Irf2bp2 KO BMDM attenuated inflammation and improved cholesterol handling. Critically, the authors showed that IRF2BP2 is required for myocyte enhancer factor-2 (MEF2)-mediated KLF2 transcriptional activation, and that homozygous IRF2BP2 mutation carriers also showed lower KLF2 protein levels in PBMC.
These mechanistic and translational studies define a novel molecular pathway controlled by IRF2BP2 in atherosclerosis. Yet, as with any new molecular targets with translational and therapeutic potential, many questions remain to be answered. Perhaps of greatest clinical importance is the need to clarify the human genetics at the IRF2BP2 locus. The initial GWAS discoveries were for total and LDL cholesterol yet large CHD datasets (e.g., CARDIoGRAM) have not identified a convincing CHD signal for these same variants.9, 10 This might be a matter of sample size/power for CHD or more likely lack of coverage of the 3’UTR deletion polymorphism on the GWAS SNP array. The authors do provide evidence for an association of the 3’UTR deletion with CHD but this is a relatively small study without replication and, in this dataset, there was no association with plasma lipids. Thus, it is important that large-scale studies replicate the CHD association and, if confirmed, define whether this is likely to be mediated through plasma lipids. In clinical context, it is noteworthy that all in vitro macrophage work was in mouse BMDM and human studies were limited to PBMC. Whether monocyte-derived macrophages of mutation carriers show reduced IRF2BP2 (e.g., via expression quantitative trait loci or allele specific expression) and enhanced inflammatory response, or deficiency in cholesterol metabolism remain an open question. Because of functional and transcriptomic differences between mouse and human macrophages particularly in innate immune12 and lipid responses13, functional genomic studies in human macrophages, either primary monocyte-derived macrophages or human induced pluripotent stem cell derived macrophages14 (which permit gene-editing) are required to confirm the clinical relevance of the Irf2bp2 rodent macrophage phenotype. These studies are required to provide convincing evidence for the role and likely intermediate human mechanism in CHD as well as provide a compelling rationale for investment in translational therapeutics.
The role of IRF2BP2 is not simply to increase KLF2 expression. BMDM isolated from LyzM-Cre-mediated myeloid-specific Klf2 knockout mice showed enhanced adhesion to endothelial cells, but were similar to WT macrophages in response to polarization and lipid accumulation.15 In this context, although Klf2 overexpression rescued the phenotype of Irf2bp2 KO in BMDM, it is unlikely that direct activation of macrophage Klf2 expression will reproduce all Irf2bp2 effects. Macrophage might not be the only effector cell of IRF2BP2 function. In the current study, cell-specific Irf2bp2 KO mice were generated by LyzM-Cre/loxP-mediated recombination, which will delete the target gene in monocytes, mature macrophages and granulocytes. The broader relevance of IRF2BP2 in other myeloid cells, e.g., neutrophils, with inflammatory action in atherosclerosis should be considered. In addition, LysM-Cre transgene is expressed in Kupffer cells. As the author demonstrated, the LyzM-Cre Irf2bp2 KO mice fed a high fat diet (HFD) showed elevated serum total cholesterol and altered FPLC lipoprotein profile compared to WT mice, possibly due to the ablated Irf2bp2 in liver Kupffer cells. Since no difference in lipid profiles was seen between Irf2bp2 KO Ldlr−/− and HET Ldlr−/− mice, the enhanced atherosclerosis observed in the DKO mice were not likely to be attributable to plasma lipid profile or liver function, but relative to humans, this may be an artifact of the extreme hyperlipidemia in the atherosclerosis mouse models. Given that human GWAS revealed a relationship to plasma lipids, the effects of IRF2BP2 in Kupffer cells and in hepatic lipoprotein metabolism should not be overlooked.
Chen et al.6 have advanced the field by identifying the impact and the mechanisms of the anti-inflammatory actions of IRF2BP2 in atherosclerosis. The most important advance is the insight into new mechanisms of IRF2BP2 function at the crossroads of innate immunity and lipid homeostasis. This may provide opportunities for the development of novel anti-inflammatory therapeutics, but much remains to be determined. Although ablation of Irf2bp2 worsens atherosclerosis, whether overexpression of Irf2bp2 is beneficial remains unclear. As overexpression of Klf2 induces marked hepatic triglycerides accumulation in mice,16 it is uncertain what the adverse effects of IRF2BP2 activation could be in humans. Indeed, the actions of IRF2BP2 across multiple cells and tissues raise concerns for specificity of targeting in chronic disease as well as for limiting adverse effects. If successful, however, restoring IRF2BP2 expression and signaling in human macrophages could become a promising strategy to limit inflammatory response and lipid dysregulation in cardiometabolic diseases, but extent of IRF2BP2 activation may be critical.
In summary, this intriguing target that functions at the junction of lipid metabolism and inflammation in atherosclerosis underscores the importance of cross-talk between lipid metabolism and immunity in cardiovascular pathology. Undoubtedly, this knowledge will advance our understanding of macrophages in complex disease and provide greater opportunity to target macrophage phenotypes and functions in atherosclerosis and cardiometabolic disorders.
Acknowledgments
Funding Sources
This work was supported by R01-HL-113147 and K24-HL-107643 to MPR. MPR is also supported by R01-HL-111694, R01-DK-090505 and U01-HL-108636. H.Z. is supported by the American Heart Association Postdoctoral Fellowship 15POST25620017.
Footnotes
Disclosures
None
References
- 1.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nature reviews. Immunology. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 3.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between lxr and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Molecular cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 4.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel sumoylation-dependent pathways mediate gene- and signal-specific transrepression by lxrs and ppargamma. Molecular cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HH, Keyhanian K, Zhou X, Vilmundarson RO, Almontashiri NA, Cruz S, Pandey NR, Yap NL, Ho T, Stewart CA, Huang H, Hari A, Geoffrion M, McPherson R, Rayner KJ, Stewart AF. Irf2bp2 reduces macrophage inflammation and susceptibility to atherosclerosis. Circulation research. 2015;117 doi: 10.1161/CIRCRESAHA.114.305777. xxx-xxx [in this issue] [DOI] [PubMed] [Google Scholar]
- 7.Childs KS, Goodbourn S. Identification of novel co-repressor molecules for interferon regulatory factor-2. Nucleic acids research. 2003;31:3016–3026. doi: 10.1093/nar/gkg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carneiro FR, Ramalho-Oliveira R, Mognol GP, Viola JP. Interferon regulatory factor 2 binding protein 2 is a new nfat1 partner and represses its transcriptional activity. Molecular and cellular biology. 2011;31:2889–2901. doi: 10.1128/MCB.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lettre G, Palmer CD, Young T, et al. Genome-wide association study of coronary heart disease and its risk factors in 8,090 african americans: The nhlbi care project. PLoS genetics. 2011;7:e1001300. doi: 10.1371/journal.pgen.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. Journal of immunology (Baltimore, Md.: 1950) 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 12.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. Journal of immunology (Baltimore, Md. 1950) 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 13.Kritharides L, Christian A, Stoudt G, Morel D, Rothblat GH. Cholesterol metabolism and efflux in human thp-1 macrophages. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1589–1599. doi: 10.1161/01.atv.18.10.1589. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Xue C, Shah R, Bermingham K, Hinkle CC, Li W, Rodrigues A, Tabita-Martinez J, Millar JS, Cuchel M, Pashos EE, Liu Y, Yan R, Yang W, Gosai SJ, VanDorn D, Chou ST, Gregory BD, Morrisey EE, Li M, Rader DJ, Reilly MP. Functional analysis and transcriptomic profiling of ipsc-derived macrophages and their application in modeling mendelian disease. Circulation research. 2015;117:17–28. doi: 10.1161/CIRCRESAHA.117.305860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lingrel JB, Pilcher-Roberts R, Basford JE, Manoharan P, Neumann J, Konaniah ES, Srinivasan R, Bogdanov VY, Hui DY. Myeloid-specific kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circulation research. 2012;110:1294–1302. doi: 10.1161/CIRCRESAHA.112.267310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JL, Lu XJ, Zou KL, Ye K. Kruppel-like factor 2 promotes liver steatosis through upregulation of cd36. Journal of lipid research. 2014;55:32–40. doi: 10.1194/jlr.M039453. [DOI] [PMC free article] [PubMed] [Google Scholar]