Abstract
We analyzed the only two sudden unexpected death in epilepsy (SUDEP) cases from 320 prospectively recruited patients in the three-year Prevention and Risk Identification of SUDEP Mortality (PRISM) Project. Both patients had surgically refractory epilepsy, evidence of left insular damage following previous temporal/temporo-insular resections, and progressive changes in Heart Rate Variability (HRV) in monitored evaluations prior to death. Insular damage is known to cause autonomic dysfunction and increased mortality in acute stroke. This report suggests a possible role for the insula in the pathogenesis of SUDEP. The presence of intrinsic insular lesions or acquired insular damage in refractory epilepsy patients may be an additional risk factor for SUDEP.
Keywords: SUDEP, insula, heart rate variability, autonomic, parasympathetic, post-ictal bradycardia
Introduction
Sudden unexpected death in epilepsy (SUDEP) incidence ranges in general epilepsy populations from 0.9–2.3 per 1000 person-years in the wider epilepsy population to 1.1–5.9 per 1000 person-years in those with chronic refractory epilepsy [1]. Evidence points to fatal seizure related phenomena [2] although precise agonal pathophysiological pathways are yet to be elucidated. A variety of heart rate variability (HRV), cardiac conduction and rhythm abnormalities occur in refractory epilepsy patients [3] but very few near-SUDEP [4] or SUDEP cases [5] are attributable to these. The combination of bradycardia/asystole and apnea/hypopnea has been more frequently observed [2]. The role of cortical autonomic and respiratory control centers in driving such dysfunction is unknown. The insula, one potential such center is implicated in cardiac autonomic control [6] as well as stroke mortality [7]. We report two SUDEP cases with acquired insular damage from the NINDS Prevention and Risk Identification of SUDEP Mortality (PRISM) Project. Sequential EEG and EKG monitoring records were analyzed before and after insular damage with subsequent progressive cardiac conduction and rhythm disturbances. Progressive cardiac changes in these patients indicate a causal cardiac mechanism for death, possibly driven by insular dysfunction.
Patients and Methods
All intractable epilepsy patients aged ≥18 years were enrolled into the NINDS Prevention and Risk Identification of SUDEP Mortality (PRISM) Project, a SUDEP study tasked with establishing infrastructure and feasibility for a SUDEP Center Without Walls. 246 patients were recruited at University Hospitals Case Medical Center in Cleveland, Ohio for prospective follow up. Detailed phenotypic and electroclinical seizure data were collected in the Epilepsy Monitoring Unit as part of the study. We retrospectively analyzed HRV during interictal periods in different video-EEG admissions using an in- house MATLab software program.
Case 1
A 28 year-old, left-handed male with medically intractable, putatively left temporal epilepsy (due to anoxic brain damage in early childhood) of 26 years duration, was referred for pre-surgical evaluation after a failed left temporal resection 11 years previously. He had habitual, daily automotor seizures, followed by right mouth twitching, altered awareness and rare secondary generalization. At evaluation in 2012, he was on Valproic acid (2000mg/day), Lacosamide (400mg/day) and Ativan (2mg/day). He had severe learning difficulties but otherwise no physical neurological abnormalities. MRI brain scan showed gliosis surrounding the left anterior temporal resection cavity, extending into the anterior inferior insula (previous pre-operative MRI scan of the brain showed a small left hippocampus without abnormal signal and no insular abnormality) (Figure 1A). He had undergone several video-EEG assessments (because of failure to capture seizures when admitted in three of these) after failed surgery and then unexpectedly died 21 months after his last assessment. Autopsy failed to show any significant pathologic findings other than sclerosis in the left hippocampal remnant, confirming definite SUDEP. There was no family history of sudden death. Serial video-EEG studies were retrospectively scrutinized for any clues indicating predisposition to sudden death. HRV was analyzed at similar inter-ictal periods (both during the afternoon, being the patient awake lying in bed and with no seizures) in the v- EEG evaluations in 2009 and 2012. At evaluation in 2009 the patient was on Lacosamide (200mg/day) and (Depakote 1000/day).
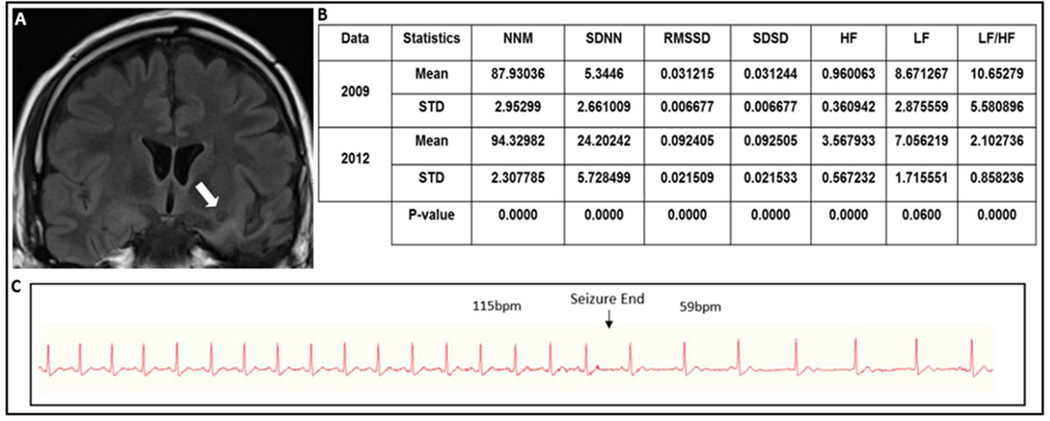
Figure 1.
(A) FLAIR coronal MRI showing evidence of left inferior temporal resection with surrounding gliosis and left anterior inferior insular gliosis and cavitation (arrow). (B) Heart rate time and frequency domain parameters calculated during 2009 and 2012 evaluations and results from GEE analysis. (C) Continuous EKG recording showing tachycardia during the seizure and suddenly resolution with abrupt onset of sinus bradycardia lasting for 13 seconds.
Legend: MNN (Mean of Normal to Normal heart beats), SDNN (Standard deviation of Normal to Normal heart beats), RMSSD (Root Mean Square of Successive Differences), SDSD (Standard Deviation of Successive Differences), HF (High Frequency), LF (Low Frequency), STD (Standard Deviation).
Analysis
We retrospectively analyzed HRV during interictal periods in different video-EEG admissions while the patient was awake and lying in bed in successive video-EEG evaluations when no seizures were recorded. Several time-domain parameters were calculated including MNN (Mean of Normal to Normal heart beats), RMSSD (Root Mean Square of Successive Differences), SDNN (Standard deviation of Normal to Normal heart beats) and SDSD (Standard Deviation of Successive Differences). In addition, frequency-domain parameters were calculated including normalized low frequency (LF) power (0.04–0.15 Hz), and normalized HF power (.15–.4 Hz) and LF/HF power ratio. These were calculated over 5-minute periods during 30 minutes of interictal periods.
Generalized Estimating Equations (GEE) were used to compare heart rhythm and HRV measures between interictal periods. A model was fit for each measure, based on data from a single subject. These models include an intercept and a factor variable denoting the two time periods. An autoregressive, AR(1) working correlation matrix was used to account for within subject correlation of the observations across each of the 5-minute periods. The p-values given in the tables are associated with testing the null hypothesis that there are no period effects. Bonferroni correction for multiple comparisons was adopted, so that p-values less than 0.0056 (.05 divided by 9) are considered as significant.
Interictal phase (baseline)
HRV was compared over 5-minute epochs in 30-minute interictal awake state records between most recent (2012) and earliest available EMU evaluations (2009) (Fig.1C). An increase of MNN (7.28%), SDNN (352.84%), RMSSD (196.03%) and HF (271.64%), and decreased LF (18.63%) and LF/HF ratio (80.26%) indicating a statically significant increased HRV in the most current evaluation in comparison with the previous one (Figure 1 B).
Ictal phase
Three habitual partial seizures, each of ~1 minute duration, were recorded. In the one seizure, the habitual ictal sinus tachycardia (115bpm) was followed by an immediate and abrupt sinus bradycardia (59bpm) for 13 seconds, after which HR returned to baseline (Figure 1C).
Case 2
A 33 year-old, right handed male with medically intractable left hemisphere epilepsy of 6 years duration was referred for repeat pre-surgical evaluation in 2011. He had previously undergone an unsuccessful left posterior insula and peri-opercular resection following an invasive evaluation in 2007(Figure 2A). Neuropathology showed focal cortical dysplasia (FCD) Palmini Type II A. His habitual seizures remained unchanged. At evaluation, he was on Phenytoin (400mg/day), Levetiracetam (4000mg/day), Oxcarbazepine (900mg/day), and Pregabalin (1200mg/day). He had 2–3 seizures/week, characterized by right or left face somatosensory aura, right version, followed by clonic secondary generalization. A habitual seizure was captured on surface EEG with left temporo-parietal onset. The patient subsequently suddenly and unexpectedly died at home. Autopsy failed to show any significant causative pathological findings, fulfilling criteria for definite SUDEP. There was no family history of sudden death. At evaluation in 2006 the patient was on Phenytoin (500mg/day), Oxcarbazepine (300/day) and Levetiracetam (2000/day).
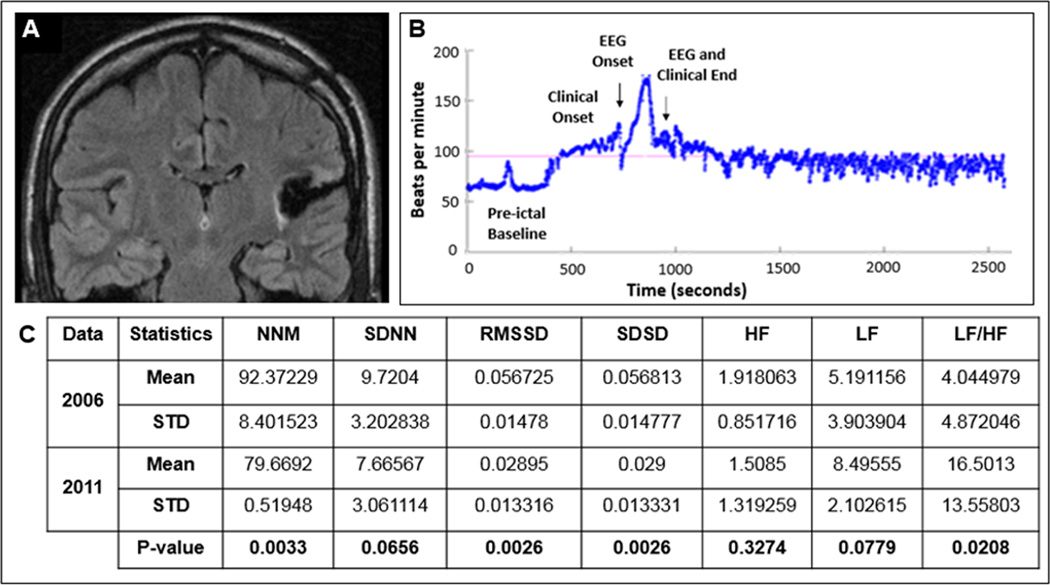
Figure 2.
(A) Flair coronal post-operative MRI showed evidence of a left posterior temporo-insular resection cavity with surrounding gliosis. (B) Heart rate plots show ictal sinus tachycardia, followed by sustained absolute and relative post-ictal sinus tachycardia lasting at least 25 minutes after a non-fatal secondary generalized clonic seizure (C) Heart rate time and frequency domain parameters calculated during the pre (2006) and post-surgery (2011) EMU evaluations and the results from GEE analysis.
Legend: MNN (Mean of Normal to Normal heart beats), SDNN (Standard deviation of Normal to Normal heart beats), RMSSD (Root Mean Square of Successive Differences), SDSD (Standard Deviation of Successive Differences), HF (High Frequency), LF (Low Frequency), STD (Standard Deviation).
Analysis
Ictal and interictal heart rhythms and HRV during 2006 (presurgical) and 2011 (postoperative) EMU admissions were analyzed using exactly the same methodology as in Patient 1.
Interictal phase
A statistically significant decrease of NNM (13.75%), SDNN (21.14%), RMSSD (48.96%) and HF (21.35%), and an increase in LF (63.65%) and LF/HF ratio (307.95%) was noted (Figure 2C).
Ictal phase
In the post-surgical EMU evaluation in 2011, pre-ictal bradycardia (HR 55- 60/minute) was noted. He had a secondary generalized clonic seizure of left temporo-parietal onset of ~2 minute duration. Ictal sinus tachycardia was followed by sustained absolute and relative post-ictal sinus tachycardia lasting at least 25 minutes, beyond which point the file had been clipped for archiving (Figure 2B).
Discussion
Precise SUDEP pathomechanisms are as yet unknown. Death appears to follow a variable pattern of cardiac autonomic and respiratory dysfunction [2]. Observations from monitored SUDEP and near-SUDEP cases suggest some phenomenological heterogeneity [2, 4, 8]. The role of autonomic cortical control structures in SUDEP is an intriguing unknown, especially since several such structures (insula, amygdala, hippocampus, orbitofrontal cortex) are often either primary epileptogenic zones or part of putative seizure networks. Electrical stimulation of the human insula produced cardiac chronotropic and blood pressure responses in 5 epilepsy patients [7]; left-sided insular dominance for parasympathetic cardiovascular effects was concluded [7]. The insula has been directly or indirectly implicated in both cardiac autonomic control and mortality in stroke patients. Prolonged direct electrical stimulation of the rat insular cortex produces lethal cardiac arrhythmia and sudden death [6]. Insular damage in patients with cerebral infarction is associated with increased sympathetic activity, cardiac arrhythmias [9], conduction blocks and mortality, including due to sudden death [10, 11]. Non-fatal ictal bradycardia and asystole have been reported in insular epilepsy [12, 13]. Insular seizures have been implicated in just one previous case of SUDEP. [14
In our patients, pre and post-operative MRI scans indicated acquired insular damage from planned insular resection in Patient 2, as well as from inevitable peri-resective area gliosis in Patient 1 extending into the anterior inferior insula. Both deaths were unwitnessed but the possibility of a cardiac contribution is significant. HRV changes were demonstrably progressive over time. Patient 1 demonstrated an unequivocal increase in vagal tone as manifested by increased HRV and HF during the most current assessment, post-ictal period and a consequent postictal bradycardia. Neither patient was on medication that could have influenced autonomic tone (Lacosamide-induced bradycardia has been reported, although Patient 1 this is unlikely as his dosage was stable for several years [15]). In Patient 1, increased vagal tone was progressive in comparisons between two consecutive, both post-operative studies, suggesting that neuronal responses to injury and autonomic alterations thus produced took place over years rather than immediately after surgery. This is strikingly similar to a report of left hemisphere epilepsy patient who developed a progressive, seven month increase in HRV and vagal tone leading to an in-hospital monitored SUDEP [16]. Patient 2 conversely demonstrated increased sympathetic tone and abnormal, prolonged recovery from post-ictal tachycardia. The patient was on a lower dose of oxcarbazepine (from 900mg to 300mg) and a higher dose of phenytoin (from 400mg to 500mg) by the time of the second evaluation, and although HRV may have been affected by these changes, we view this as relatively unlikely to be solely responsible for all the changes noted. A previous study documented very similar changes in HRV in 18 patients with intractable temporal lobe epilepsy in comparison to 18 patients with well controlled seizures over a mean follow up period of 6.1 years. [17] Another report of SUDEP in a patient with Bitemporal epilepsy similarly described progressive decrease in HRV (a 42.4% decrease in SDNN) over approximately 9 months. This evidence may represent a significant predisposition to fatal ventricular arrhythmia although this is speculative. [18] HRV is a standard measure of cardiac autonomic function which noninvasively reflects sympathetic and parasympathetic balance using EKG recordings. A close relationship is known to exist between increased sympathetic activity and/or decreased parasympathetic activity with a consequent tendency to fatal arrhythmia [19]. The high frequency (HF) domain is a marker of parasympathetic activity whereas the low frequency (LH) domain is thought to represent both sympathetic and parasympathetic activity. Nocturnal HRV is significantly reduced in epilepsy and decreased HRV accompanies increased sudden cardiac death risk [20]. The role of excitation produced by seizure discharge in a damaged left insula (Patient 1), as opposed to loss of homeostatic correction due to a mostly absent left insula (Patient 2) may explain the divergent cardiac responses to seizures seen in the two cases. Alternatively or additionally, differing handedness and hemispheric dominance may have played a role since lateralization of insula function is well described [21].
The two SUDEP cases reported here present reasonable circumstantial evidence, for the first time, of an insular contribution to SUDEP. The presence of intrinsic insular lesions or acquired insular damage in refractory epilepsy patients may be an additional risk factor for SUDEP.
Highlights.
The insula is a cortical structure involved in autonomic function
Insular damage is associated with mortality through autonomic dysfunction
In patients with refractory epilepsy and SUDEP, insular damage and autonomic dysfunction may play a role
Acknowledgments
This work is supported by the Center for SUDEP Research NIH/NINDS U01-NS090405-01 and NIH/NINDS U01-NS090407-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378(9808):2028–2038. doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- 2.Ryvlin P, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet neurology. 2013;12(10):966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 3.van der Lende M, et al. Cardiac arrhythmias during or after epileptic seizures. Journal of neurology, neurosurgery, and psychiatry. 2015 doi: 10.1136/jnnp-2015-310559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlisi M, et al. Seizure induced ventricular fibrillation: a case of near-SUDEP. Seizure : the journal of the British Epilepsy Association. 2013;22(3):249–251. doi: 10.1016/j.seizure.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Dasheiff RM, Dickinson LJ. Sudden unexpected death of epileptic patient due to cardiac arrhythmia after seizure. Archives of neurology. 1986;43(2):194–196. doi: 10.1001/archneur.1986.00520020080028. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer SM, Cechetto DF, Hachinski VC. Cerebrogenic cardiac arrhythmias. Cerebral electrocardiographic influences and their role in sudden death. Archives of neurology. 1990;47(5):513–519. doi: 10.1001/archneur.1990.00530050029008. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer S, Hachinski V. Complications of acute stroke. Lancet. 1992;339(8795):721–724. doi: 10.1016/0140-6736(92)90607-5. [DOI] [PubMed] [Google Scholar]
- 8.Lhatoo SD, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68(6):787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- 9.Sander D, Klingelhofer J. Changes of circadian blood pressure patterns after hemodynamic and thromboembolic brain infarction. Stroke. 1994;25(9):1730–1737. doi: 10.1161/01.str.25.9.1730. [DOI] [PubMed] [Google Scholar]
- 10.Abboud H, et al. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Annals of neurology. 2006;59(4):691–699. doi: 10.1002/ana.20806. [DOI] [PubMed] [Google Scholar]
- 11.Christensen H, et al. Insular lesions, ECG abnormalities, and outcome in acute stroke. Journal of neurology, neurosurgery, and psychiatry. 2005;76(2):269–271. doi: 10.1136/jnnp.2004.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tayah T, et al. Ictal bradycardia and asystole in an adult with a focal left insular lesion. Clinical neurology and neurosurgery. 2013;115(9):1885–1887. doi: 10.1016/j.clineuro.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Seeck M, et al. Ictal bradycardia in a young child with focal cortical dysplasia in the right insular cortex. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2003;7(4):177–181. doi: 10.1016/s1090-3798(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 14.Ryvlin P. Avoid falling into the depths of the insular trap. Epileptic disorders : international epilepsy journal with videotape. 2006;8(Suppl 2):S37–S56. [PubMed] [Google Scholar]
- 15.Chinnasami S, Rathore C, Duncan JS. Sinus node dysfunction: an adverse effect of lacosamide. Epilepsia. 2013;54(6):e90–e93. doi: 10.1111/epi.12108. [DOI] [PubMed] [Google Scholar]
- 16.Jeppesen J, et al. Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia. 2014;55(7):e67–e71. doi: 10.1111/epi.12614. [DOI] [PubMed] [Google Scholar]
- 17.Suorsa E, et al. Heart rate dynamics in temporal lobe epilepsy-A long-term follow-up study. Epilepsy research. 2011;93(1):80–83. doi: 10.1016/j.eplepsyres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Rauscher G, et al. Sudden unexpected death in epilepsy associated with progressive deterioration in heart rate variability. Epilepsy & behavior : E&B. 2011;21(1):103–105. doi: 10.1016/j.yebeh.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European heart journal, Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 20.Stein PK, Fauchier L, Babuty D. Sudden death, arrhythmic events and measurements of heart rate variability. J Am Coll Cardiol. 1999;34(7):2148–2149. doi: 10.1016/s0735-1097(99)00478-7. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheimer SM, et al. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]