Abstract
Background
Ticks are among the most important vectors of pathogens affecting companion animals, and also cause health problems such as tick paralysis, anaemia, dermatitis, and secondary infections. Twenty ixodid species have previously been recorded on dogs, cats, and horses in Australia, including Rhipicephalus sanguineus, Ixodes holocyclus and Haemaphysalis longicornis, which transmit tick-borne diseases. A survey of hard ticks (Acari: Ixodidae) was conducted during 2012–2015 to investigate tick species that infest dogs, cats, and horses in Australia.
Methods
Individual tick specimens were collected from dogs, cats and horses across Australia and sample collection locations were mapped using QGIS software. Ticks were morphologically examined to determine species, instar and sex. The companion animal owners responded to questionnaires and data collected were summarised with SPSS software.
Results
A total of 4765 individual ticks were identified in this study from 7/8 states and territories in Australia. Overall, 220 larvae, 805 nymphs, 1404 males, and 2336 females of 11 tick species were identified from 837 companion animal hosts. One novel host record was obtained during this study for Ixodes myrmecobii, which was found on Felis catus (domestic cat) in the town of Esperance, Western Australia. The most common tick species identified included R. sanguineus on dogs (73 %), I. holocyclus on cats (81 %) and H. longicornis on horses (60 %).
Conclusions
This study is the first of its kind to be conducted in Australia and our results contribute to the understanding of the species and distribution of ticks that parasitise dogs, cats, and horses in Australia. Records of R. sanguineus outside of the recorded distribution range emphasise the need for a systematic study of the habitat range of this species. Several incomplete descriptions of ixodid species encountered in this study hindered morphological identification.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1480-y) contains supplementary material, which is available to authorized users.
Keywords: Ticks, Companion animals, Dogs, Cats, Horses, Tick-borne diseases, Australia
Background
As haematophagous obligatory parasites of reptiles, birds, and mammals, ticks are among the most important vectors of pathogens affecting livestock, companion animals, and humans worldwide [1, 2]. Ticks transmit viruses, bacteria, and protozoa during blood feeding, which can compromise the health of the vertebrate host [3]. A variety of factors influence the susceptibility of companion animals to TBD, including exposure to questing ticks, the pet’s lifestyle, and ectoparasite control [4]. Some TBD of companion animals are zoonotic [5, 6], which in some circumstances may also place human owners at risk of infection. Furthermore, companion animals can act as sentinels for emerging TBD [7–9]. In 2013, it was estimated that there are a total of 4.2 million pet dogs, and 3.3 million pet cats in Australia [10].
Of the 896 recognised tick species worldwide [11] there are 70 species endemic to Australia: 14 soft tick (family Argasidae) and 56 hard tick (family Ixodidae) species [12]. While the majority of these ticks are unique to Australia, there are five species that have been introduced since European colonisation in the last 250 years with poultry (e.g. Argas persicus), horses (e.g. Otobius megnini), cattle (e.g. Haemaphysalis longicornis and Rhipicephalus australis), and dogs (e.g. Rhipicephalus sanguineus) [12]; however, R. sanguineus may have been introduced earlier than this [13].
To date, 20 ixodid species have been recorded on dogs, cats and horses in Australia (Table 1). Dogs are the primary hosts of R. sanguineus; however, native ticks such as Ixodes cornuatus, Ixodes holocyclus, and Ixodes tasmani are known to parasitise domestic dogs, as well as cats. Ixodids that usually feed on cattle (H. longicornis and R. australis) also feed on horses but, as with dogs and cats, horses can also be parasitised by native Australian ticks [14].
Table 1.
Ticks (Acari: Ixodidae) previously recorded on dogs (Canis lupus familiaris), cats (Felis catus), and horses (Equus ferus caballus) in Australia
| Host species | ||||
|---|---|---|---|---|
| Tick species | Canis lupus familiaris | Felis catus | Equus ferus caballus | References |
| Amblyomma moreliae | ✗ | ✗ | ✓ | [46] |
| Amblyomma triguttatum ornatissimum | ✗ | ✗ | ✓ | [47] |
| Amblyomma triguttatum queenslandense | ✓ | ✗ | ✗ | [47] |
| Amblyomma triguttatum triguttatum | ✓ | ✓ | ✓ | [47–49] |
| Bothriocroton auruginans | ✓ | ✗ | ✗ | [46] |
| Bothriocroton fimbriatum | ✗ | ✗ | ✓ | [46] |
| Bothriocroton hydrosauri | ✗ | ✗ | ✓a | [14] |
| Haemaphysalis bancrofti | ✓ | ✓ | ✓ | [50, 51] |
| Haemaphysalis bremneri | ✗ | ✗ | ✓ | [50] |
| Haemaphysalis longicornis b | ✓ | ✓ | ✓ | [14] |
| Haemaphysalis novaeguineae | ✗ | ✗ | ✓ | [50] |
| Ixodes australiensis | ✓ | ✗ | ✗ | [52] |
| Ixodes cornuatus | ✓ | ✓ | ✗ | [52] |
| Ixodes fecialis | ✗ | ✓ | ✗ | [52] |
| Ixodes hirsti | ✗ | ✓ | ✗ | [52] |
| Ixodes holocyclus | ✓ | ✓ | ✓ | [52] |
| Ixodes myrmecobii | ✓ | ✗ | ✓ | [14] |
| Ixodes tasmani | ✓ | ✓ | ✓ | [52, 53] |
| Rhipicephalus australis b | ✓ | ✓ | ✓ | [45, 54] |
| Rhipicephalus sanguineus b | ✓ | ✓ | ✗ | [54] |
As a result of its geographical isolation and robust biosecurity regulations, Australia is considered free of many of the TBD endemic to countries overseas. There are currently two TBD of dogs recognised in Australia; canine infectious cyclic thrombocytopenia (CICT) and canine babesiosis. Anaplasma platys is the causative agent of CICT and was detected in dogs in central Australia in the early 2000s [15, 16]. Canine babesiosis is caused by Babesia vogeli and Babesia gibsoni in Australia. Babesia vogeli has been detected in dogs from northern Australia [17, 18] and New South Wales [18], and is transmitted by R. sanguineus [19, 20]. Babesia gibsoni has been detected in dogs from south-eastern Australia [21]. Evidence in Japan suggests that H. longicornis, which is also distributed in Australia, is a vector of B. gibsoni [22], and there is also one report of direct transmission of the piroplasm between dogs in Australia [23].
Although cytauxzoonosis is a major TBD of cats in the United States [24, 25], neither cytauxzoonosis nor any other TBD of cats are known to occur in Australia. Equine piroplasmosis was first diagnosed in Australia in 1976 [26]. The disease was later confirmed to be caused by the protozoan pathogen Babesia equi [27], which has since been redescribed as Theileria equi [28]. The presence of T. equi in horses in Australia was considered to have occurred due to the importation of infected horses during the twentieth Century [29], however, the disease remained localised and Australia is now free of equine piroplasmosis [29, 30].
Tick infestations can cause other health problems in companion animals. Tick paralysis manifests as ascending paralysis and local neurological deficits [31–33]. The Australian tick species known to frequently cause tick paralysis in eastern and south-eastern Australia are I. cornuatus and I. holocyclus [34]. Tick paralysis caused by Ixodes hirsti has also been reported in cats [35]. Additionally, heavy or repeated infestations of ticks can cause anaemia in the host animal, which is associated with blood loss during tick feeding [36]. Immunosuppression [37], secondary infections at the bite site [38], and localised dermatitis [39] can also result from tick infestations.
The present study aimed to determine the tick species that are associated with dogs, cats and horses in Australia, and is part of broader research investigating tick-borne pathogens.
Methods
Sample collection
Individual ticks (n = 4765) were collected during 2012–2015 from a total of 837 companion animal hosts (n = 4191 from 643 dogs; n = 345 from 42 horses; n = 229 from 152 cats) from New South Wales (NSW), the Northern Territory (NT), Queensland (QLD), South Australia (SA), Tasmania (TAS), Victoria (VIC), and Western Australia (WA). Ticks were removed from animals by staff at veterinary clinics, and by various persons throughout Australia in response to a nationwide advertising campaign. The ticks were preserved in 70 % ethanol and were sent to Murdoch University for analysis. For each submission received, the source, approximate geographic location of collection site, host, and date of collection was recorded.
Ethics statement
The Murdoch University Animal Ethics Committee sanctioned the opportunistic removal of ticks from animal hosts. The use of questionnaires was approved by the Murdoch University Human Research Ethics Committee (Permit No. 2011/005).
Tick identification
Individual ticks were examined with an Olympus SZ61 stereomicroscope (Olympus, Center Valley, PA, USA) with a Schott KL 1500 LED light source (Schott AG, Mainz, Germany). Photographs were taken with an Olympus SC30 digital camera and analysis getIT software (Olympus, Center Valley, PA, USA). The instar, sex, and species were morphologically identified [14, 40] and the data were recorded with Microsoft® Excel® for Mac 2011, version 14.5.2.
Sample mapping
The sample collection locations were geo-referenced using the open source software QGIS, version 2.10.1 [41] with the latest Australian coordinate system: Geocentric Datum of Australia 1994 (GDA94) [42]. Layers were styled with a categorised renderer, with layer symbology classified according to tick species, and a point displacement renderer was used to visualise overlapping points around a centre symbol on rendering circles [43].
Questionnaire design
A questionnaire was designed in conjunction with Bayer Australia Ltd to obtain information about the age, sex, weight, habitat, use of tick control products and clinical signs of tick paralysis [44] of dogs, cats and horses that were presented to veterinary clinics (Additional file 1). The companion animal owners completed the questionnaires while at the veterinary clinic. A total of 433 questionnaires from 30 veterinary clinics were collected by Bayer Australia Ltd area managers, and sent to Murdoch University.
Statistical analysis
The database of tick identification results, sample information, and questionnaire data was generated and summarised with Microsoft® Excel® for Mac 2011, version 14.5.2, and IBM® SPSS® Statistics 2013 software, version 22 (Armonk, NY, USA). During the analysis, dogs were considered small if their weight was ≤ 10 kg, medium if 11–19 kg, and large if ≥ 20 kg, and the scale of tick paralysis was recoded into a binary variable (present or absent).
Results
Morphological identification of Ixodidae
Overall, 220 larvae, 805 nymphs, 1404 males, and 2336 females were identified from 837 companion animals in 7/8 Australian states and territories. The number and location (state) of ixodids that were identified on dogs, cats, and horses are presented in Tables 2, 3 and 4. Photographs of a single female for each species identified, except for Bothriocroton sp., are displayed in Additional file 2.
Table 2.
Tick species, location and number of instars collected from dogs
| Number of instars | |||||||
|---|---|---|---|---|---|---|---|
| Species | State | Larvae | Nymphs | Males | Females | Instar total | Number of hosts |
| Amblyomma triguttatum triguttatum | WA | 0 | 5 | 0 | 5 | 10 | 8 |
| Bothriocroton sp. | TAS | 0 | 1 | 0 | 0 | 1 | 1 |
| VIC | 11 | 0 | 1 | 1 | 13 | 5 | |
| Haemaphysalis bancrofti | NSW | 0 | 2 | 0 | 2 | 4 | 3 |
| QLD | 0 | 0 | 0 | 1 | 1 | 1 | |
| Haemaphysalis longicornis | NSW | 0 | 127 | 0 | 61 | 188 | 35 |
| QLD | 2 | 23 | 0 | 0 | 25 | 4 | |
| Ixodes cornuatus | TAS | 0 | 7 | 0 | 6 | 13 | 11 |
| VIC | 0 | 2 | 0 | 0 | 2 | 2 | |
| Ixodes holocyclus | NSW | 0 | 39 | 78 | 373 | 490 | 193 |
| QLD | 0 | 47 | 4 | 226 | 277 | 205 | |
| TAS | 0 | 0 | 0 | 2 | 2 | 2 | |
| WA | 0 | 0 | 0 | 1 | 1 | 1 | |
| Ixodes myrmecobii | WA | 0 | 0 | 0 | 4 | 4 | 4 |
| Ixodes tasmani | NSW | 0 | 0 | 0 | 1 | 1 | 1 |
| QLD | 0 | 0 | 0 | 4 | 4 | 2 | |
| TAS | 16 | 4 | 6 | 57 | 83 | 49 | |
| VIC | 0 | 0 | 0 | 2 | 2 | 2 | |
| Rhipicephalus australis | QLD | 0 | 1 | 0 | 0 | 1 | 1 |
| Rhipicephalus sanguineus | NSW | 0 | 0 | 0 | 6 | 6 | 3 |
| NT | 31 | 240 | 927 | 858 | 2056 | 76 | |
| QLD | 0 | 12 | 22 | 32 | 66 | 15 | |
| SA | 5 | 55 | 231 | 119 | 410 | 15 | |
| WA | 132 | 168 | 115 | 116 | 531 | 46 | |
| Total | 197 | 733 | 1384 | 1877 | 4191 | 685a | |
aThe total number of dogs sampled in this study was n = 643. The total number of host records presented in Table 2 (n = 685) is inflated due repeated measures that occurred in cases where more than one tick species was identified on a host
Table 3.
Tick species, location and number of instars collected from cats
| Number of instars | |||||||
|---|---|---|---|---|---|---|---|
| Species | State | Larvae | Nymphs | Males | Females | Instar total | Number of hosts |
| Haemaphysalis bancrofti | NSW | 0 | 0 | 0 | 1 | 1 | 1 |
| Ixodes cornuatus | TAS | 0 | 0 | 0 | 1 | 1 | 1 |
| Ixodes hirsti | TAS | 0 | 0 | 0 | 1 | 1 | 1 |
| Ixodes holocyclus | NSW | 0 | 22 | 9 | 88 | 119 | 77 |
| QLD | 0 | 2 | 0 | 64 | 66 | 59 | |
| Ixodes myrmecobii | WA | 0 | 0 | 0 | 1 | 1 | 1 |
| Ixodes tasmani | TAS | 23 | 8 | 0 | 7 | 38 | 11 |
| VIC | 0 | 1 | 0 | 0 | 1 | 1 | |
| Rhipicephalus sanguineus | NSW | 0 | 0 | 0 | 1 | 1 | 1 |
| Total | 23 | 33 | 9 | 164 | 229 | 153a | |
aThe total number of cats sampled in this study was n = 152. The total number of host records presented in Table 3 (n = 153) is inflated due repeated measures that occurred in cases where more than one tick species was identified on a host
Table 4.
Tick species, location and number of instars collected from horses
| Number of instars | |||||||
|---|---|---|---|---|---|---|---|
| Species | State | Larvae | Nymphs | Males | Females | Instar total | Number of hosts |
| Amblyomma triguttatum triguttatum | NSW | 0 | 0 | 0 | 1 | 1 | 1 |
| QLD | 0 | 0 | 0 | 4 | 4 | 1 | |
| WA | 0 | 1 | 0 | 9 | 10 | 8 | |
| Haemaphysalis bancrofti | NSW | 0 | 1 | 2 | 10 | 13 | 3 |
| QLD | 0 | 2 | 1 | 6 | 9 | 5 | |
| Haemaphysalis longicornis | NSW | 0 | 27 | 0 | 176 | 203 | 14 |
| QLD | 0 | 2 | 0 | 2 | 4 | 2 | |
| Ixodes holocyclus | NSW | 0 | 5 | 2 | 33 | 40 | 11 |
| QLD | 0 | 0 | 6 | 51 | 57 | 10 | |
| Ixodes tasmani | QLD | 0 | 0 | 0 | 1 | 1 | 1 |
| Rhipicephalus australis | QLD | 0 | 1 | 0 | 2 | 3 | 1 |
| Total | 0 | 39 | 11 | 295 | 345 | 57a | |
a The total number of horses sampled in this study was n = 42. The total number of host records presented in Table 4 (n = 57) is inflated due repeated measures that occurred in cases where more than one tick species was identified on a host
Host records
One novel host record was obtained for I. myrmecobii; one female I. myrmecobii was collected from Felis catus (domestic cat) in the town of Esperance, WA (Additional file 3). All other host records of dogs, cats, and horses for the various tick species identified were consistent with previous host records (Table 1).
Ixodidae collection locations
The collection locations for each ixodid species identified from companion animal hosts are presented in Fig. 1.
Fig. 1.

Collection locations of ticks removed from dogs, cats, and horses in Australia. Each point represents a unique collection location for the corresponding tick species. Overlapping points were displaced with a point displacement renderer around a centre symbol (denoted in legend); point displacement distance was defined by number of map units (kilometres)
The individual geographic collection locations, including the coordinates that were geo-referenced and displayed in Fig. 1, for the instars identified on dogs, cats, and horses are listed in Additional file 3. Several collection locations occurred outside of the previously recorded distribution ranges for the following species: H. longicornis (one in the suburb of Sancrox, NSW) [14]; I. holocyclus (two in TAS, and one in WA) [40]; and R. sanguineus (72 in southwest WA, 17 canine hosts; 410 in SA, 15 canine hosts) [45].
Questionnaires
The majority of samples were received in the years 2013 (28 %) and 2014 (65 %), and most were collected from companion animal hosts during the months of spring (September-November) and summer (December-February) (Fig. 2). The data gained from responses to questionnaires is summarised in Table 5.
Fig. 2.
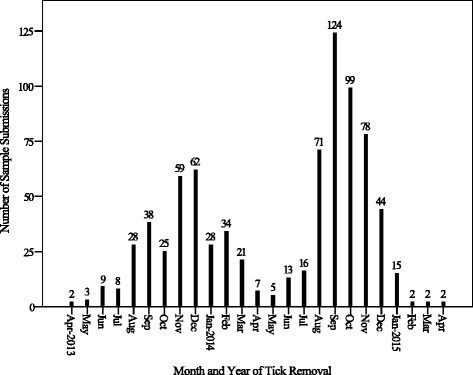
Number of sample submissions in each month from 2013–2015
Table 5.
Summary of questionnaire responses
| Host factors | Hosts | |||
|---|---|---|---|---|
| Dogs | Cats | Horses | ||
| Sex | Female | 152 | 47 | 3 |
| Male | 167 | 55 | 4 | |
| Total | 319 | 102 | 7 | |
| Age (months) | Median | 60 | 36 | 127 |
| Interquartile range | 24–108 | 19.5–96 | 90–240 | |
| Minimum | 2 | 1 | 72 | |
| Maximum | 240 | 244 | 300 | |
| Total | 311 | 98 | 7 | |
| Clinical signs of tick paralysis | Absent | 65 | 9 | 5 |
| Present | 153 | 77 | 2 | |
| Total | 218 | 86 | 7 | |
| Are tick control products used? | No | 106 | 56 | 2 |
| Yes | 168 | 42 | 4 | |
| Total | 274 | 98 | 6 | |
| Dog Size | Small | 139 | N/A | N/A |
| Medium | 53 | N/A | N/A | |
| Large | 120 | N/A | N/A | |
| Total | 312 | N/A | N/A | |
| Habitat | Indoors | 114 | N/A | N/A |
| Outdoors | 135 | N/A | N/A | |
| Total | 249 | N/A | N/A | |
| Where is dog walked? | Confined to home | 29 | N/A | N/A |
| Urban parks | 73 | N/A | N/A | |
| Semi-urban bush | 38 | N/A | N/A | |
| Countryside/remote bush/farmland | 35 | N/A | N/A | |
| Urban parks and semi-urban bush | 15 | N/A | N/A | |
| Urban parks, semi-urban bush and countryside/remote bush/farmland | 8 | N/A | N/A | |
| Semi-urban bush and countryside/remote bush/farmland | 18 | N/A | N/A | |
| Urban parks and countryside/remote bush/farmland | 1 | N/A | N/A | |
| Total | 217 | N/A | N/A | |
In the vast majority of cases where signs of tick paralysis were reported in companion animals, I. holocyclus was identified from the host (97 %; 226/232) (data not shown). In one case, R. sanguineus was removed from a cat with tick paralysis. The remaining five cases of tick paralysis were reported in dogs that were infested with Bothriocroton sp. (n = 1), I. tasmani (n = 1), Haemaphysalis bancrofti (n = 1) and R. sanguineus (n = 2) (data not shown).
Discussion
This report describes the first comprehensive nationwide survey of ticks associated with companion animals in Australia and the results are generally consistent with the individual geographical distributions and host records [14, 40, 46–54], with a few exceptions. Interestingly, one novel host record was obtained in this study for I. myrmecobii on F. catus in Esperance, WA. Although native Australian ticks primarily feed on native wildlife species [14], they also feed on a variety of introduced mammals and birds [46–58]. The primary hosts of the introduced species H. longicornis and R. australis are cattle, but these ticks have been recorded on other livestock, introduced and native wildlife, and companion animals [14, 54, 58].
The collection locations obtained for the vast majority of ticks in this study adhered to previously described Australian distribution ranges, or to previous collection locations [14, 40, 45, 47, 52, 54, 59]. The records of two I. holocyclus in TAS, and one I. holocyclus in the city of Wagga Wagga, NSW, most likely occurred due to travel to I. holocyclus endemic areas [40] prior to tick removal, which was documented by the companion animal owners. Given that the distribution of ticks is affected by climate, vegetation, and the presence of the primary host species [60], it is also likely that the single I. holocyclus recorded from a dog in southwest WA is a result of interstate travel from I. holocyclus endemic areas. The collection locations that occurred outside of the previously recorded distribution ranges for H. longicornis and R. sanguineus [14, 45] may also be attributable to travel, since people and their companion animals can readily travel with, and potentially disperse, ticks outside of their endemic range.
It is probable that the distribution of R. sanguineus has extended further south of the NT border into northern and central SA, which is comprised of the same terrestrial ecoregion as southern and central NT (deserts and xeric shrub lands) [61]. Investigations of R. sanguineus group ticks overseas have found two paraphyletic lineages of R. sanguineus: the tropical (northern) lineage [R. sanguineus (sensu lato)]; and the temperate (southern) lineage [possibly R. sanguineus (sensu stricto)] [62–66], and these lineages may represent two different species [66]. These paraphyletic groupings remain to be investigated across different climatic regions of Australia.
The collection localities of I. myrmecobii along the southern coastline of WA obtained in this study are novel. The information pertaining to the distribution range of this enigmatic tick species is limited, with very few studies of I. myrmecobii conducted [14, 67, 68]. Formal geographical distribution data for many of the Australian tick species we report in this study is either non-existent, or requires a systematic study.
Bothriocroton ticks collected from dogs in TAS and VIC (n = 10) could not be identified to the species level as the morphological features were too damaged in the male and female specimens, and there is currently no key for the identification of Bothriocroton nymphal and larval species. These specimens are likely Bothriocroton auruginans, which is distributed in TAS and VIC [14, 40], and is the only species of Bothriocroton that parasitises dogs in Australia. The current Australian tick morphology keys [14, 40] also lack a complete description of I. cornuatus instars; therefore, the I. cornuatus nymphs examined in this study have been only tentatively identified, pending further species confirmation by molecular techniques.
There were no male H. longicornis ticks identified in this study, which was expected, as the populations of H. longicornis in Australia (as well as in north-eastern Russia, northern Japan, New Zealand, New Caledonia, and Fiji) are parthenogenetic [69], and represent the only known example of triploidy in ticks [70]. In Australia, very few males have ever been reported [50, 71].
The use of standard Australian tick morphology keys to identify ticks collected in Australia seems appropriate given the context of the study, however, there are species found elsewhere with similar morphology to those that are present in Australia. It is possible that other tick species could be inadvertently introduced into this country as a result of international movements of animals and humans, thus future studies could include molecular phylogenetic analyses of genetic markers (e.g. mitochondrial cytochrome c oxidase subunit 1 (cox1), 12S ribosomal RNA (rRNA), and 16S rRNA genes) to increase the confidence and accuracy of tick identification.
As expected, the majority of the ticks examined in this study were collected during the warmer months of spring and summer, when ixodids are generally more abundant [72–76] (Fig. 2). There is limited data pertaining to ownership of companion animals in Australia. A 2013 survey of 1089 pet owners reported that 76 % of dogs are kept exclusively or partly indoors [10]. Conversely in this study, 54 % of dogs usually lived outdoors (i.e. in a kennel), and 13 % were confined to the home. Increased exposure to tick habitats likely increases the chance of tick attachment, which could explain our observations, as only dogs with ticks were sampled in this study. Overseas studies have reported that factors such as host species, breed, and habitat significantly affect the likelihood of tick species attachment [75, 77]. Explanatory variables for tick species attachment in this study could not be fairly assessed, as questionnaire data was skewed towards companion animals that were infested with I. holocyclus on the eastern coast of Australia.
Several tick species identified in this study are of potential concern to the health of companion animals according to the current literature. Importantly, R. sanguineus is a well-known vector of B. vogeli [78], the cause of canine babesiosis. Most of the animals that were infested with I. holocyclus had clinical signs of tick paralysis (77 %; 226/293) (data not shown), and this condition can be fatal [79, 80]. The reports of tick paralysis in one cat infested with R. sanguineus, and in five dogs infested with Bothriocroton sp., I. tasmani, H. bancrofti and R. sanguineus are unusual. These may have been reported erroneously on the questionnaire, or Ixodes spp. known to cause tick paralysis might have attached to the animal, but were not collected.
There are few reports of TBD associated with I. cornuatus [81] and virtually nothing is known about pathogens transmitted by I. myrmecobii and I. hirsti. Although Amblyomma triguttatum triguttatum, H. bancrofti, H. longicornis, I. tasmani and R. australis have been associated with TBD in other host species [40, 82–87], it remains to be investigated whether these species carry pathogens that could impact the health of companion animals.
Conclusions
This first nationwide study of ticks on companion animals in Australia has provided a comprehensive snapshot of the current tick-host associations in dogs, cats, and horses that should be of interest to pet owners and carers, veterinarians, and manufacturers of ectoparasiticides. The species that were most commonly found on these animals are well-known vectors of pathogens, or cause neurological disease. However, the vector competency of several species identified has not been widely investigated. Such knowledge is required to better understand the risks of TBD transmission to pets and potentially, to their owners. Further investigations are required to establish the environmental and host factors that influence tick species infestations on companion animals, which may help to develop prevention strategies against tick infestations.
Acknowledgements
This study was funded by the Australian Research Council (Linkage Project 130100050), Bayer Australia Ltd and Bayer AG (Germany). The authors wish to acknowledge the assistance of the many companion animal owners who provided tick specimens and answered the questionnaires, and of the veterinary staff from the many veterinary clinics and hospitals around Australia. Additionally, we thank staff from the following organisations for providing and curating tick samples: Animal Management in Rural & Remote Indigenous Communities (AMRRIC), The Animal Protection Society of WA Inc. (APS), Dermcare-Vet, the Parasitology Section within the School of Veterinary and Life Sciences at Murdoch University, and Wollongbar Primary Industries Institute. We wish to thank the area managers of Bayer Australia Ltd for their assistance in distributing to- and collecting the sampling kits from veterinary clinics around Australia. The authors would also like to thank Prof. Guadalupe Miró for her guidance and expert advice on morphological tick identification, and Dr. Margaret Andrew for her advice on, and provision of, ecogeographical datasets.
Additional files
Questions answered by companion animal owners at veterinary clinics. (DOCX 55 kb)
Dorsal and ventral photographs of female ixodids. A) Dorsal view of Amblyomma triguttatum triguttatum. B) Ventral view of A. t. triguttatum. C) Dorsal view of Haemaphysalis bancrofti. D) Ventral view of H. bancrofti. E) Dorsal view of Haemaphysalis longicornis. F) Ventral view of H. longicornis. G) Dorsal view of Ixodes cornuatus. H) Ventral view of I. cornuatus. I) Dorsal view of Ixodes hirsti. J) Ventral view of I. hirsti. K) Dorsal view of Ixodes myrmecobii. L) Ventral view of I. myrmecobii. M) Dorsal view of Ixodes holocyclus. N) Ventral view of I. holocyclus. O) Dorsal view of Ixodes tasmani. P) Ventral view of I. tasmani. Q) Dorsal view of Rhipicephalus australis. R) Ventral view of R. australis. S) Dorsal view of Rhipicephalus sanguineus. T) Ventral view of R. sanguineus. Individual tick specimens were collected from the following localities: the township of Gidgegannup, WA (A-B); the town of Missabotti, NSW (C-D); the town of Bellingen, NSW (E-F); the city of Devonport, TAS (G-J, O-P); the town of Esperance, WA (K-L); the town of Byangum, NSW (M-N); the town of Sarina, QLD (Q-R); the Indigenous Australian community of Mutitjulu, NT (S-T). (PDF 1501 kb)
Collection localities and number of ticks (Acari: Ixodidae) recorded on dogs (Canis lupus familiaris), cats (Felis catus), and horses (Equus ferus caballus). (PDF 241 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TLG participated in the design of the study, performed tick identification and data analysis, and wrote the manuscript. CLO contributed to the design of the study, and preparation of the manuscript. AWG contributed to the design of the study, manuscript preparation, and coordinated sample collection and curation. RLR contributed to conceiving, designing, and coordinating the study, tick collection, and preparation of the manuscript. UMR contributed to the design of the study, and preparation of the manuscript. PJI conceived, designed and coordinated the study, and contributed to preparation of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Telleasha L. Greay, Email: t.greay@murdoch.edu.au
Charlotte L. Oskam, Email: c.oskam@murdoch.edu.au
Alexander W. Gofton, Email: a.gofton@murdoch.edu.au
Robert L. Rees, Email: bob.rees@bayer.com
Una M. Ryan, Email: una.ryan@murdoch.edu.au
Peter J. Irwin, Email: p.irwin@murdoch.edu.au
References
- 1.de la Fuente J, Estrada- Peña A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008;1:6938–46. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 2.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28:437–46. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Beugnet F, Marié JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305. doi: 10.1016/j.vetpar.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Day MJ. One health: the importance of companion animal vector-borne diseases. Parasit Vectors. 2011;4:49. doi: 10.1186/1756-3305-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cito F, Rijks J, Rantsios AT, Cunningham AA, Baneth G, Guardabassi L, et al. Prioritization of companion animal transmissible diseases for policy intervention in Europe. J Comp Pathol. 2015. In press. [DOI] [PubMed]
- 6.Rijks JM, Cito F, Cunningham AA, Rantsios AT, Giovannini A. Disease risk assessments involving companion animals: an overview for 15 selected pathogens taking a European perspective. J Comp Pathol. 2015 (In press). [DOI] [PubMed]
- 7.Falco RC, Smith HA, Fish D, Mojica BA, Bellinger MA, Harris HL, et al. The distribution of canine exposure to Borrelia burgdorferi in a Lyme-disease endemic area. Am J Public Health. 1993;83:1305–10. doi: 10.2105/AJPH.83.9.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merino FJ, Serrano JL, Saz JV, Nebreda T, Gegundez M, Beltran M. Epidemiological characteristics of dogs with Lyme borreliosis in the province of Soria (Spain) Eur L Epidemiol. 2000;16:97–100. doi: 10.1023/A:1007690807637. [DOI] [PubMed] [Google Scholar]
- 9.Hamer SA, Tsao JI, Walker ED, Mansfield LS, Foster ES, Hickling GJ. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res. 2009;70:49–56. doi: 10.2460/ajvr.70.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Richmond R. Pet ownership in Australia. Aust Vet J. 2013;91:2. doi: 10.1111/j.1751-0813.2013.000109.GRP.x. [DOI] [PubMed] [Google Scholar]
- 11.Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa. 2010;2528:1–28. [Google Scholar]
- 12.Barker SC, Walker AR, Campelo D. A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int J Parasitol. 2014;44:941–53. doi: 10.1016/j.ijpara.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Oskarsson MC, Klutsch CF, Boonyaprakob U, Wilton A, Tanabe Y, Savolainen P. Mitochondrial DNA data indicate an introduction through Mainland Southeast Asia for Australian dingoes and Polynesian domestic dogs. Proc R Soc B. 2012;279:967–74. doi: 10.1098/rspb.2011.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts FHS. Australian ticks. Melbourne: Commonwealth Scientific and Industrial Research Organization; 1970. [Google Scholar]
- 15.Brown GK, Martin AR, Roberts TK, Aitken RJ. Detection of Ehrlichia platys in dogs in Australia. Aust Vet J. 2001;79:554–8. doi: 10.1111/j.1751-0813.2001.tb10747.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown GK, Martin AR, Roberts TK, Dunstan RH. Molecular detection of Anaplasma platys in lice collected from dogs in Australia. Aust Vet J. 2005;83:101–2. doi: 10.1111/j.1751-0813.2005.tb12208.x. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies R, Ryan UM, Muhlnickel CJ, Irwin PJ. Two species of canine Babesia in Australia: detection and characterization by PCR. J Parasitol. 2003;89:409–12. doi: 10.1645/0022-3395(2003)089[0409:TSOCBI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Brown GK, Canfield PJ, Dunstan RH, Roberts TK, Martin AR, Brown CS, et al. Detection of Anaplasma platys and Babesia canis vogeli and their impact on platelet numbers in free-roaming dogs associated with remote Aboriginal communities in Australia. Aust Vet J. 2006;84:321–5. doi: 10.1111/j.1751-0813.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- 19.Hill MWM, Bolton BL. Canine babesiosis in Queensland. Aust Vet J. 1966;42:391–2. doi: 10.1111/j.1751-0813.1966.tb04631.x. [DOI] [Google Scholar]
- 20.Irwin PJ, Hutchinson GW. Clinical and pathological findings of Babesia infection in dogs. Aust Vet J. 1991;68:204–9. doi: 10.1111/j.1751-0813.1991.tb03194.x. [DOI] [PubMed] [Google Scholar]
- 21.Muhlnickel CJ, Jefferies R, Morgan-Ryan UM, Irwin PJ. Babesia gibsoni infection in three dogs in Victoria. Aust Vet J. 2002;80:606–10. doi: 10.1111/j.1751-0813.2002.tb10961.x. [DOI] [PubMed] [Google Scholar]
- 22.Hatta T, Matsubayashi M, Miyoshi T, Islam K, Alim MA, Anisuzzaman, et al. Quantitative PCR-based parasite burden estimation of Babesia gibsoni in the vector tick, Haemaphysalis longicornis (Acari: Ixodidae), fed on an experimentally infected dog. J Vet Med Sci. 2013;75:1–6. doi: 10.1292/jvms.12-0175. [DOI] [PubMed] [Google Scholar]
- 23.Jefferies R, Ryan UM, Jardine J, Broughton DK, Robertson ID, Irwin PJ. Blood, bull terriers and babesiosis: further evidence for direct transmission of Babesia gibsoni in dogs. Aust Vet J. 2007;85:459–63. doi: 10.1111/j.1751-0813.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 24.Birkenheuer AJ, Marr H, Alleman AR, Levy MG, Breitschwerdt EB. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet Parasitol. 2006;137:144–9. doi: 10.1016/j.vetpar.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 25.MacNeill AL, Barger AM, Skowronski MC, Lanka S, Maddox CW. Identification of Cytauxzoon felis infection in domestic cats from southern Illinois. J Feline Med Surg. 2015;17:1069–72. doi: 10.1177/1098612X14567158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchill RC, Best DR. Babesiosis of a horse in Australia. Aust Vet J. 1976;52:487. doi: 10.1111/j.1751-0813.1976.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney D, Wright I, Frerichs W, Groenendyk S, O’Sullivan B, Roberts M, et al. The identification of Babesia equi in Australia. Aust Vet J. 1977;53:461–4. doi: 10.1111/j.1751-0813.1977.tb05459.x. [DOI] [PubMed] [Google Scholar]
- 28.Mehlhorn H, Schein E. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitol Res. 1998;84:467–75. doi: 10.1007/s004360050431. [DOI] [PubMed] [Google Scholar]
- 29.Martin R. Equine piroplasmosis: the temporary importation of seropositive horses into Australia. Aust Vet J. 1999;77:308–9. doi: 10.1111/j.1751-0813.1999.tb10269.x. [DOI] [PubMed] [Google Scholar]
- 30.Exotic animal diseases bulletin: equine piroplasmosis. In: Emergency and Exotic Animal Diseases - Bulletins and Alerts. Department of Agriculture and Water Resources. http://www.agriculture.gov.au/pests-diseases-weeds/animal/ead-bulletin/exotic_animal_diseases_bulletin_-_no_103. Accessed 14 Jan 2016.
- 31.Atwell R, Campbell F. Megaoesophagus in dogs with tick paralysis (Ixodes holocyclus) Aust Vet Pract. 2001;31:75–9. [Google Scholar]
- 32.Campbell F, Atwell R. Heart failure in dogs with tick paralysis caused by the Australian paralysis tick, Ixodes holocyclus. Int J Appl Res Vet M. 2003;1:148–62. [Google Scholar]
- 33.Holland CT. Asymmetrical focal neurological deficits in dogs and cats with naturally occurring tick paralysis (Ixodes holocyclus): 27 cases (1999–2006) Aust Vet J. 2008;86:377–84. doi: 10.1111/j.1751-0813.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 34.Jackson J, Beveridge I, Chilton NB, Andrews RH. Distributions of the paralysis ticks Ixodes cornuatus and Ixodes holocyclus in south-eastern Australia. Aust Vet J. 2007;85:420–4. doi: 10.1111/j.1751-0813.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 35.Roberts FHS. Tick paralysis in South Australia. Aust Vet J. 1961;37:440. doi: 10.1111/j.1751-0813.1961.tb03832.x. [DOI] [Google Scholar]
- 36.Riek R. Studies on the reactions of animals to infestation with ticks. I. Tick anaemia. Aust J Agr Res. 1957;8:209–14. doi: 10.1071/AR9570209. [DOI] [Google Scholar]
- 37.Barriga OO. Evidence and mechanisms of immunosuppression in tick infestations. Genet Anal-Biomol E. 1999;15:139–42. doi: 10.1016/S1050-3862(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 38.Ogden NH, Woldehiwet Z, Hart CA. Granulocytic ehrlichiosis: an emerging or rediscovered tick-borne disease? J Med Microbiol. 1998;47:475–82. doi: 10.1099/00222615-47-6-475. [DOI] [PubMed] [Google Scholar]
- 39.Riek RF. Allergic reaction in the horse to infestation with larvae of Boophilus microplus (Canes). (Ixodides: Acarina) Aust Vet J. 1954;30:142–4. doi: 10.1111/j.1751-0813.1954.tb08186.x. [DOI] [Google Scholar]
- 40.Barker SC, Walker AR. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa. 2014;3816:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 41.QGIS. http://www.qgis.org/. Accessed 9 Oct 2015.
- 42.Geoscience Australia. http://www.ga.gov.au/scientific-topics/positioning-navigation/geodesy/geodetic-datums/gda. Accessed 9 Oct 2015.
- 43.Documentation QGIS 2.6. http://docs.qgis.org/2.6/en/docs/user_manual/processing_algs/qgis/vector_geometry_tools/pointsdisplacement.html?highlight=point displacement. Accessed 15 Oct 2015.
- 44.Atwell RB, Campbell FE, Evans EA. Prospective survey of tick paralysis in dogs. Aust Vet J. 2001;79:412–8. doi: 10.1111/j.1751-0813.2001.tb12986.x. [DOI] [PubMed] [Google Scholar]
- 45.Seddon HR. Diseases of domestic animals in Australia: Part 3 tick and mite infestations. Canberra: Department of Health; 1951. [Google Scholar]
- 46.Roberts FHS. Further observations on the Australian species of Aponomma and Amblyomma with descriptions of the nymphs of Amblyomma moreliae (L.Koch) and Amb. loculosum Neumann (Acarina: Ixodidae) Aust J Zool. 1964;12:288–314. doi: 10.1071/ZO9640288. [DOI] [Google Scholar]
- 47.Roberts FHS. On the status of morphologically divergent tick populations of Amblyomma triguttatum Koch (Acarina: Ixodidae) Aust J Zool. 1962;10:367–81. doi: 10.1071/ZO9620367. [DOI] [Google Scholar]
- 48.Waudby HP, Petit S, Dixon B, Andrews RH. Hosts of the exotic ornate kangaroo tick, Amblyomma triguttatum triguttatum Koch, on southern Yorke Peninsula. South Australia Parasitol Res. 2007;101:1323–30. doi: 10.1007/s00436-007-0642-4. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy PH. Observations on the infestation of the larger domestic animals and the kangaroo, by the ornate kangaroo tick (Ambylomma triguttatum) Aust Vet J. 1960;36:436–7. doi: 10.1111/j.1751-0813.1960.tb03730.x. [DOI] [Google Scholar]
- 50.Roberts FHS. A systematic study of the Australian species of the genus Haemaphysalis Koch (Acarina: Ixodidae) Aust J Zool. 1963;11:35–80. doi: 10.1071/ZO9630035. [DOI] [Google Scholar]
- 51.Laan B, Handasyde K, Beveridge I. Occurrence of the tick Haemaphysalis bancrofti Nuttall & Warburton, 1915 in Victoria with additional data on its distribution and with scanning electron micrographs of life cycle stages. Proc R Soc Vic. 2011;123:189–99. [Google Scholar]
- 52.Roberts FHS. A systematic study of the Australian species of the genus Ixodes (Acarina: Ixodidae) Aust J Zool. 1960;8:392–486. doi: 10.1071/ZO9600392. [DOI] [Google Scholar]
- 53.Roberts FHS. The tick fauna of Tasmania. Rec Queen Victoria Mus Launceston New Ser. 1964;17:2–8. [Google Scholar]
- 54.Roberts F. The taxonomic status of the species of the genera Rhipicephalus Koch and Boophilus Curtice (Acarina: Ixodidae) occurring in Australia. Aust J Zool. 1965;13:491–524. doi: 10.1071/ZO9650491. [DOI] [Google Scholar]
- 55.Doube B. Cattle and the paralysis tick Ixodes holocyclus. Aust Vet J. 1975;51:511–5. doi: 10.1111/j.1751-0813.1975.tb06901.x. [DOI] [PubMed] [Google Scholar]
- 56.Guglielmone AA. Sites of attachment in Amblyomma triguttatum triguttatum Koch (Acari: Ixodidae) on natural hosts. Ann Parasitol Hum Comp. 1990;65:145–8. doi: 10.1051/parasite/1990653145. [DOI] [PubMed] [Google Scholar]
- 57.Laan B, Handasyde K, Beveridge I. Observations on the biology and distribution of the tick Ixodes hirsti Hassall, 1931 (Acari: Ixodoidea) Proc R Soc Victoria. 2011;123:200–16. [Google Scholar]
- 58.Green PE. An unusual host for Boophilus microplus. Aust Vet J. 1971;47:179–80. doi: 10.1111/j.1751-0813.1971.tb02139.x. [DOI] [PubMed] [Google Scholar]
- 59.Besier RB, Wroth R. Discovery of the tick Haemaphysalis longicornis in Western Australia. Aust Vet J. 1985;62:205–6. doi: 10.1111/j.1751-0813.1985.tb07304.x. [DOI] [PubMed] [Google Scholar]
- 60.James AM, Burdett C, McCool MJ, Fox A, Riggs P. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med Vet Entomol. 2015;29:178–88. doi: 10.1111/mve.12099. [DOI] [PubMed] [Google Scholar]
- 61.Department of the Environment. http://www.environment.gov.au/. Accessed 23 Oct 2015.
- 62.Walker JB, Keirans JE, Horak IG. The genus Rhipicephalus (Acari, Ixodidae): a guide to the brown ticks of the world. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 63.Burlini L, Teixeira KR, Szabo MP, Famadas KM. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Exp Appl Acarol. 2010;50:361–74. doi: 10.1007/s10493-009-9321-8. [DOI] [PubMed] [Google Scholar]
- 64.Levin ML, Studer E, Killmaster L, Zemtsova G, Mumcuoglu KY. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae) Exp Appl Acarol. 2012;58:51–68. doi: 10.1007/s10493-012-9561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nava S, Mastropaolo M, Venzal JM, Mangold AJ, Guglielmone AA. Mitochondrial DNA analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in the Southern Cone of South America. Vet Parasitol. 2012;190:547–55. doi: 10.1016/j.vetpar.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 67.Austen JM, Ryan UM, Friend JA, Ditcham WG, Reid SA. Vector of Trypanosoma copemani identified as Ixodes sp. Parasitology. 2011;138:866–72. doi: 10.1017/S0031182011000497. [DOI] [PubMed] [Google Scholar]
- 68.Kaewmongkol G, Kaewmongkol S, Burmej H, Bennett MD, Fleming PA, Adams PJ, et al. Diversity of Bartonella species detected in arthropod vectors from animals in Australia. Comp Immunol Microbiol Infect Dis. 2011;34:411–7. doi: 10.1016/j.cimid.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Hoogstraal H, Roberts FH, Kohls GM, Tipton VJ. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae) J Parasitol. 1968;54:1197–213. doi: 10.2307/3276992. [DOI] [PubMed] [Google Scholar]
- 70.Oliver JH, Bremner KC. Cytogenetics of ticks. III. Chromosomes and sex determination in some Australian hard ticks (Ixodidae) Ann Entomol Soc Am. 1968;61:837–44. doi: 10.1093/aesa/61.4.837. [DOI] [Google Scholar]
- 71.Bremner K. Observations on the biology of Haemaphysalis bispinosa Neumann (Acarina: Ixodidae) with particular reference to its mode of reproduction by parthenogenesis. Aust J Zool. 1959;7:7–12. doi: 10.1071/ZO9590007. [DOI] [Google Scholar]
- 72.Doube BM. Seasonal patterns of abundance and host relationships of the Australian paralysis tick, Ixodes holocyclus Neumann (Acarina: Ixodidae), in southeastern Queensland. Aust J Ecol. 1979;4:345–60. doi: 10.1111/j.1442-9993.1979.tb01564.x. [DOI] [Google Scholar]
- 73.Földvári G, Farkas R. Ixodid tick species attaching to dogs in Hungary. Vet Parasitol. 2005;129:125–31. doi: 10.1016/j.vetpar.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Lorusso V, Dantas-Torres F, Lia RP, Tarallo VD, Mencke N, Capelli G, et al. Seasonal dynamics of the brown dog tick, Rhipicephalus sanguineus, on a confined dog population in Italy. Med Vet Entomol. 2010;24:309–15. doi: 10.1111/j.1365-2915.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 75.Smith FD, Ballantyne R, Morgan ER, Wall R. Prevalence, distribution and risk associated with tick infestation of dogs in Great Britain. Med Vet Entomol. 2011;25:377–84. doi: 10.1111/j.1365-2915.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 76.Egyed L, Elo P, Sreter-Lancz Z, Szell Z, Balogh Z, Sreter T. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis. 2012;3:90–4. doi: 10.1016/j.ttbdis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Ogden NH, Cripps P, Davison CC, Owen G, Parry JM, Timms BJ, et al. The ixodid tick species attaching to domestic dogs and cats in Great Britain and Ireland. Med Vet Entomol. 2000;14:332–8. doi: 10.1046/j.1365-2915.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 78.Irwin PJ. Canine babesiosis: from molecular taxonomy to control. Parasit Vectors. 2009;2:S4. doi: 10.1186/1756-3305-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clunies-Ross I. Tick paralysis: a fatal disease of dogs and other animals in eastern Australia. J Counc Sci Ind Res. 1935;8:8–13. [Google Scholar]
- 80.Hall-Mendelin S, Craig SB, Hall RA, O’Donoghue P, Atwell RB, Tulsiani SM, et al. Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Ann Trop Med Parasitol. 2011;105:95–106. doi: 10.1179/136485911X12899838413628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graves SR, Stewart L, Stenos J, Stewart RS, Schmidt E, Hudson S, et al. Spotted fever group rickettsial infection in South-Eastern Australia: isolation of rickettsiae. Comp Immunol Microb and Infect Dis. 1993;16:223–33. doi: 10.1016/0147-9571(93)90149-Y. [DOI] [PubMed] [Google Scholar]
- 82.Weilgama D. Transmission of Theileria peramelis Mackerras, 1959 by Ixodes tasmani. In: Cremin M, Dobson C, Moorhouse DE, editors. Parasite lives. Papers on parasites, their hosts and their associations. To honour J. F. A. Sprent. Brisbane: University of Queensland Press; 1986. pp. 174–8. [Google Scholar]
- 83.Fujisaki K, Kawazu S, Kamio T. The taxonomy of the bovine Theileria spp. Parasitol Today. 1994;10:31–3. doi: 10.1016/0169-4758(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 84.de Castro JJ. Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet Parasitol. 1997;71:77–97. doi: 10.1016/S0304-4017(97)00033-2. [DOI] [PubMed] [Google Scholar]
- 85.Jonsson NN, Bock RE, Jorgensen WK. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet Parasitol. 2008;155:1–9. doi: 10.1016/j.vetpar.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 86.Vilcins IM, Old JM, Deane EM. Detection of a spotted fever group Rickettsia in the tick Ixodes tasmani collected from koalas in Port Macquarie. Australia J Med Entomol. 2008;45:745–50. doi: 10.1603/0022-2585(2008)45[745:DOASFG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 87.Vilcins I-ME, Old JM, Deane E. Detection of a Hepatozoon and spotted fever group Rickettsia species in the common marsupial tick (Ixodes tasmani) collected from wild Tasmanian devils (Sarcophilus harrisii) Tasmania Vet Parasitol. 2009;162:23–31. doi: 10.1016/j.vetpar.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Andrews R, Beveridge I, Bull C, Chilton M, Dixon B, Petney T. Systematic status of Aponomma tachyglossi Roberts (Acari: Ixodidae) from echidnas, Tachyglossus aculeatus, from Queensland. Australia Syst Appl Acarol. 2006;11:23–39. [Google Scholar]


